Abstract
Background
Escalated aggression is a behavioral sign of numerous psychiatric disorders characterized by a loss of control. The neurobiology underlying escalated aggression is unknown and is particularly understudied in females. Research in our laboratory demonstrated that repeated aggressive experience in female hamsters resulted in an escalated response to future aggressive encounters and an increase in dendritic spine density on NAc neurons. We hypothesized that the activation of group I metabotropic glutamate receptors signaling though the Fragile X Mental Retardation Protein (FMRP) pathway may underlie synaptic plasticity associated with aggression escalation.
Methods
Female hamsters were given 5 daily aggression tests with or without prior treatment with the mGluR5 antagonist MPEP. Following aggression testing, mRNA expression and protein levels were measured in the nucleus accumbens for PSD-95 and SAPAP-3, as well as the levels of phosphorylated FMRP.
Results
Experience-dependent escalation of aggression in female hamsters depends on activation of mGluR5 receptors. Furthermore, aggressive experience decreases phosphorylation of FMRP in the NAc which is coupled to a long-term increase in the expression of the synaptic scaffolding proteins, PSD-95 and SAPAP-3. Finally, the experience-dependent increase in PSD-95 is prevented by antagonism of the mGluR5 receptor.
Conclusions
Activation of the FMRP pathway by group I metabotropic glutamate receptors is involved in regulating synaptic plasticity following aggressive experience. The NAc is a novel target for preclinical studies of the treatment of escalated aggression, with the added benefit that emerging therapeutic approaches are likely to be effective in treating pathological aggression in both females and males.
Keywords: Aggression, Fragile X, Plasticity, Nucleus Accumbens, mGluR5, PSD-95
Background
Aggression is a part of many species’ normal repertoire of social behaviors and has enduring consequences in the life of an individual. While aggressive behavior typically serves an adaptive function, it can become maladaptive if it escalates into inappropriate displays of violence [1]. Indeed, aggressive outbursts that result in harm to an individual or the people around them are a major problem in mental health and criminal justice settings [2]. Research on aggression and violence has consistently recognized two subtypes of aggressive behavior: impulsive and pre-meditated [3]. Unlike the purposeful and controlled aggression seen in the pre-mediated type, impulsive aggression is typically described as an escalated aggressive response characterized by a loss of behavioral control or, in layman’s terms, a “short fuse”. Impulsive aggression presents as a symptom of numerous psychiatric disorders as well as without comorbid psychopathology [4]. The neurobiology underlying this inappropriate expression of aggression is poorly understood, and there are no medications currently approved by the FDA specifically for treating impulsive or escalated aggression [5].
Behavioral and neurobiological analyses of aggression in animals have contributed significantly to the understanding of the neural control of aggression and have identified several corticolimbic structures as possible targets for therapeutic interventions [6]. The nucleus accumbens (NAc) in particular has been suggested as a regulator of the reinforcing effects of aggression that make the behavior more likely to persist in the future. Studies in rats and mice have convincingly demonstrated a role for the NAc in regulating the rewarding consequences of male aggressive behavior [7–9] and suggest that aggressive experience leads to plasticity in the NAc that may mediate behavioral changes associated with repeated aggressive experiences [10]. These studies, however, more closely model the pre-meditated rather than the impulsive type of aggression. In addition, like the vast majority of animal studies on aggression, these experiments were conducted only in males despite the obvious fact that intense aggression occurs in females of many species, including humans (see [11] for review). Using Syrian hamsters as our animal model, we have studied the neurobiology of aggression in females. In female hamsters, repeated aggressive experience leads to an escalated aggressive response to future encounters that is primarily manifested as a decrease in attack latency across repeated trials [12], modeling the short fuse seen in impulsive aggressive behavior. Escalated aggression in female hamsters is also coupled with an increase in mature dendritic spines on medium spiny neurons of the NAc core [12]. This structural plasticity in the NAc presumably represents an increase in excitatory inputs [13] that could mediate a heightened response to future activation [14]. The intracellular signaling events leading to this structural plasticity, however, remain unknown.
We tested the novel hypothesis that the Fragile X Mental Retardation Protein (FMRP) signaling pathway mediates structural changes in the NAc following aggressive experience, forming the neural basis for impulsive aggression. FMRP was first characterized in the context of Fragile X syndrome, in which the FMR1 gene is silenced, resulting in the loss of FMRP [15, 16]. Symptoms of the disorder include intellectual disability, autism, as well as heightened aggression [17]. In healthy neurons, however, FMRP is thought to be a key regulator of long-term plasticity via local translation of synaptic proteins in dendritic spines [18]. Specifically, phosphorylation of FMRP inhibits translation, whereas dephosphorylation of FMRP upregulates translation [19, 20]. Changes in FMRP phosphorylation are correlated with increased expression of PSD-95 and SAPAP-3 [21], which regulate the dynamics of dendritic spines and link postsynaptic receptors with their downstream signaling proteins [22]. In addition, the translation of multiple downstream targets of FMRP is regulated by group I metabotropic glutamate receptors [23, 24]. We predicted that repeated aggressive experience in females would activate group I metabotropic glutamate receptors and decrease FMRP phosphorylation, leading to an increase in synaptic scaffold proteins. Furthermore, we predicted that blocking mGluR5 receptors would prevent the behavioral and neural plasticity associated with escalated aggression. To begin to assess potential commonalities between females and males, we also preliminarily examined behavioral and neural plasticity following aggressive experience in male hamsters (see Supplementary Information). Here we report these findings as a model for the neural regulation of escalated aggression and suggest mGluR5 activation of the FMRP signaling pathway as a novel target for therapeutic interventions that could be effective in treating impulsive aggression in both females and males.
Materials and Methods
Animals
Adult female and male Syrian hamsters (Mesocricetus auratus) were purchased from Charles River Laboratories (Wilmington, MA, USA) at approximately 60 days of age. Subject females were housed individually while intruder males were pair-housed in polycarbonate cages (females: 50.8 × 40.6 × 20.3 cm; males: 43.2 × 22.9 × 20.3 cm). These housing arrangements maximize aggression in female subjects, while decreasing aggression in male intruders [25–27]. All animals were maintained on a reversed 14 h light/10 h dark photoperiod (lights off at 1300 h) and all behavioral testing occurred during the dark phase. The animal room was maintained at a controlled temperature of 22° C and food and water were available ad libitum. All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 2011) and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Ovariectomy
To maximize aggressive behavior in females, circulating estradiol levels were maintained at low levels via bilateral ovariectomy [27]. Surgery was conducted under sodium pentobarbital anesthesia (Nembutal; 8.5 mg/100 g body weight, i.p.). Analgesic (Torbugesic, 10 mg, s.c. Fort Dodge Animal Health, Overland Park, KS) and antibiotic (Baytril 2.27% solution, 0.1 ml s.c., Bayer, Shawnee Mission, KS, USA) were administered several hours post-surgery and for the following 3 days.
Behavioral Testing
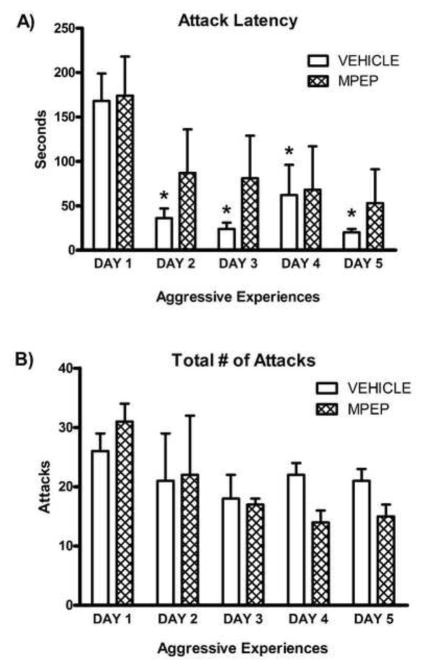
An adult male stimulus hamster was placed into a female subject’s home cage for 5 min on each of 5 consecutive days. In our prior experience with female hamster aggression, this test duration is sufficient to generate measureable levels of aggression without injuring the intruder male. Each session was video recorded and later scored for the latency to the subject’s first attack and the total number of attacks. Attacks were operationally defined as the subject biting, or clearly attempting to bite, the intruder male. Intruder males (n = 24) were used at most every other day and on each test individual females were paired with a different male to minimize the likelihood that submission by the male would influence the behavior of the females [28]. In fact, the greatest change in attack latency occurred between tests 1 and 2 (Figure 1) in which all males were previously untested, meaning that submission by the males was not the basis for the change in attack latency by the females. Control groups of ovariectomized females remained in their home cages for the duration of the experiment and did not receive aggressive experience.
Figure 1. Escalated aggression depends on mGluR5 activation.
A) Females who received repeated aggressive experience (n = 12) had a significant decrease in attack latency 2, 3, 4, and 5 days after the first aggressive experience. This decrease in attack latency was prevented in females who received MPEP (1 mg/kg, n = 12) 30 min prior to behavioral testing. B) The total number of attacks did not differ between vehicle- and MPEP-treated females on any testing day. Error bars represent mean + SEM, * indicates significant difference from day 1, p < 0.05.
MPEP injections
Thirty min prior to each behavioral test, subjects (n = 12) received an injection of the mGluR5 antagonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP, 1 mg/kg body weight, i.p., Tocris Bioscience, Minneapolis, MN, USA), or a 0.9% saline vehicle control (n = 12). This low dose of MPEP used in this study was chosen based on its efficacy in antagonizing dendritic spine formation in prior studies [29]. A control group of females received daily injections of either MPEP (n = 6) or vehicle (n = 6) and remained in their home cages for the duration of the experiment.
Tissue Punches
One or 3 wks following the fifth aggression test, females were given an overdose of sodium pentobarbital (Beuthanasia-D, 0.25 ml i.p./animal, Schering, Union, NJ, USA) and sacrificed by rapid decapitation. Coronal sections containing the NAc and caudate putamen (CP) were taken and bilateral tissue punches (1-mm diameter) were immediately collected from each area. NAc punches included the anterior commissure to bias the punches toward the NAc core, where we found increases in dendritic spine density following aggressive experience [12]. Dorsal medial CP punches were taken to evaluate the regional specificity of the biochemical changes within the striatum following aggressive experience. For each brain area, punches from one hemisphere were stored in RNA-later (Quiagen, Valencia, CA, USA) for qPCR analysis of Psd-95, Sapap-3, and Fmr1, and punches from the opposite hemisphere were flash-frozen for Western blotting. To capture rapid changes in the phosphorylation state of the FMRP protein, separate groups of female hamsters (n = 8) were decapitated without anesthesia 5, 15, or 60 min after the last aggression test and compared with groups of control females (n = 8) sacrificed at the same time. Bilateral tissue punches were immediately collected from the NAc and CP and flash-frozen for Western blotting.
qPCR
Tissue samples were homogenized and RNA was extracted using an RNAeasy Mini Kit (Quiagen) and reverse transcribed using a Transcriptor First-Strand cDNA Synthesis kit (Roche Diagnostics, Indianapolis, Indiana, USA). Because the sequences for many of our genes of interest are unknown for the Syrian hamster, primers were designed using known sequences from the rat or mouse (rat Psd-95 Forward: 5′-GAA-CAC-ATA-TGA-CGT-TGT-GTA-CCT-AAA-3′, Reverse: 5′-TCC-AGG-TGC-TGA-GAA-TAC-GA-3′; mouse Sapap-3 Forward: 3′-GGC-CAA-CAG-CTG-GAA-ACT-C-3′, Reverse: 5′-GCG-AGG-GCT-TCT-TTG-GTA-T-3′; rat Fmr1 Forward 5′-CGC-GGT-CCT-GGA-TAT-ACT-TC-3′, Reverse: 5′-TGG-AGC-TAA-TGA-CCA-ATC-ACT-G-3′).
qPCR amplifications were performed on a LightCycler 480 II (Roche Diagnostics) using the Universal ProbeLibrary Master Mix and probes (Roche Diagnostics). PCR for individual cDNA samples were performed in triplicate. Threshold values were calculated using the second derivative max method (LightCycle 480 software version 1.5) and standardized to the housekeeping gene Gapdh (primers designed from Syrian hamster sequence: Forward: 5′-GTC-TAC-TGG-CGT-CTT-CAC-CAC-3′, Reverse: 5′-ATG-ACC-CTC-TTG-GCT-CCA-C-3′, GenBank Accession ABD77188.1). The thermal cycling program used a pre-incubation step at 95°C for 5 min, followed by 45 cycles consisting of a 10 sec denaturing step at 95°C, an annealing step for 10 sec at 60°C, and an extension step for 10 sec at 72°C. At the end of each cycling program, a measurement of fluorescent intensity was taken and a melting curve was generated. PCR products were sequenced using Sanger Sequencing to confirm amplification of genes of interest. A search using a nucleotide basic local alignment search tool (BLAST, NCBI) revealed that product sequences matched known rat or mouse target sequences with a high degree of homology (Psd-95 97% homology, Sapap-3 92% homology, hamster Gapdh 100% homology) and did not match with other known sequences (no other matches above 50% homology).
Western Blotting
Tissue samples were homogenized in 1% SDS processing buffer and protein was quantified using the Bio-Rad protein DC assay (Bio-Rad Laboratories, Berkeley, CA, USA). Forty μg of total protein was loaded onto a 12–15% polyacylamide gradient gel (Mini-PROTEAN TGX Precast Mini Gel, Bio-Rad Laboratories) and transferred to a nitrocellulose membrane. Membranes were blocked in 5% non-fat dried milk or BSA (phospho-FMRP) in TBS, incubated overnight in primary antibodies against PSD-95 (1:1,000; Cell Signaling, Beverly, MA, USA), SAPAP-3 (1:500, ProteinTech, Chicago, IL, USA), FMRP (1:500, Millipore, Billerica, MA, USA), phospho-FMRP (1:1,000; Abcam, Cambridge, MA, USA), and GAPDH (1:30,000; Millipore) diluted in TBS + 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO, USA). The following day, membranes were incubated for 1 hr in the appropriate secondary antibodies (1:20,000; Li-Cor Biosciences, Lincoln, NE, USA) and then imaged using the Odyssey imaging system (LiCor Biosciences).
Statistical Analysis
Repeated measures ANOVAs with Newman-Keuls post-hoc tests were used to detect significant differences in aggressive behaviors between groups and across testing days, p < 0.05. For gene and protein analyses, significant differences in group means were detected using one-way ANOVAS or t-tests with Bonferroni corrections, p < 0.05.
Results
Escalation of aggressive behavior depends on mGluR5
Repeated aggressive experience resulted in a significant decrease in attack latency across time in control vehicle-treated subjects (F(4, 44) = 8.348, p < 0.0001). Newman-Keuls post-hoc tests indicated a significant decrease in attack latency on day 2, 3, 4, and 5 (all p < 0.01, Figure 1A). Interestingly, this escalation of aggression was independent of aggression intensity, as the total number of attacks did not differ across testing days (Figure 1B). Likewise, in a separate experiment (see supplementary methods), we found that repeated aggressive experience also resulted in the escalation of aggression in male hamsters (supplementary Figure S1).
To test the hypothesis that the effect of experience on aggressive behavior is mediated by mGluR5 receptors, female subjects were treated with MPEP prior to each aggression test. In contrast to vehicle-treated females, females treated with MPEP did not demonstrate a systematic decrease in attack latency (Figure 1A). Blocking mGluR5 receptors did not change attack intensity, as the total number of attacks did not differ across days for either MPEP- or vehicle-treated females (Figure 1B). We did a further analysis of the cumulative distribution of attacks across individual aggression tests. Just as the total number of attacks did not differ across days for the treatment groups, attacks were continuously distributed throughout each aggression test for all groups of animals (data not shown). Thus once attacks were initiated all animals showed the same pattern of attacks regardless of test day or treatment condition.
Aggressive experience decreases the phosphorylation of FMRP protein
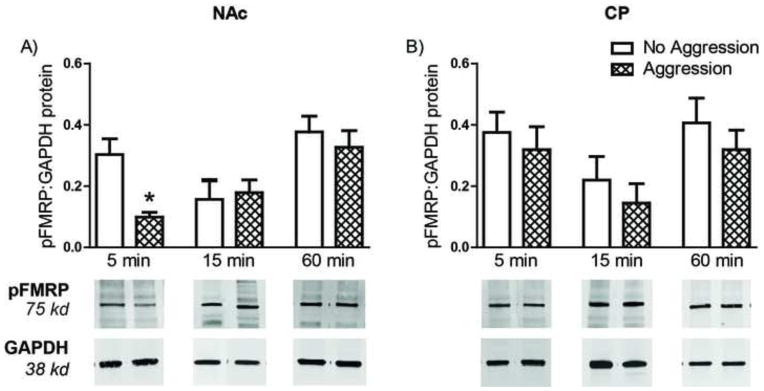
To test the hypothesis that the mGluR5-dependent escalation of aggression may be mediated by FMRP, we examined the expression of Fmr1 mRNA, as well as the phosphorylation state of FMRP, in the NAc following aggressive experience. Neither the expression of Fmr1 mRNA nor FMRP protein changed in the NAc or CP of females one week following the last aggressive experience (data not shown). In contrast, aggressive experience resulted in a rapid and transient decrease in the phosphorylation of FMRP. In females sacrificed 5 min after the last aggressive experience, phosphorylated FMRP was significantly decreased in the NAc (F(2,19)= 9.436, p < 0.01; Figure 2A). This decrease in phosphorylation was absent at 15 min and 60 min following the last aggressive experience (Figure 2A). Again, these changes appear to be specific to the NAc, as the phosphorylation of FMRP did not change in the CP at any time point (Figure 2B). Furthermore, the total amount of FMRP did not differ between behavioral conditions at any time point (data not shown).
Figure 2. Aggressive experience results in a rapid and transient phosphorylation of FMRP.
A) In the Nucleus Accumbens (NAc), levels of phosphorylated FMRP were significantly lower 5 min after the last aggression test in females who received 5 aggressive experiences (n = 8) compared to aggression-naïve control females (n = 8). At 15 min (n = 8) and 60 min (n = 8) following the last aggression test, pFMRP levels had returned to control female levels (n = 8 for each group). B) Aggressive experience had no effect on pFMRP levels in the Caudate (CP). Error bars represent mean + SEM, representative Western blot for each time point shown below quantification, * indicates significant difference between behavioral conditions, p < 0.05.
Aggressive experience increases expression of synaptic scaffolding proteins
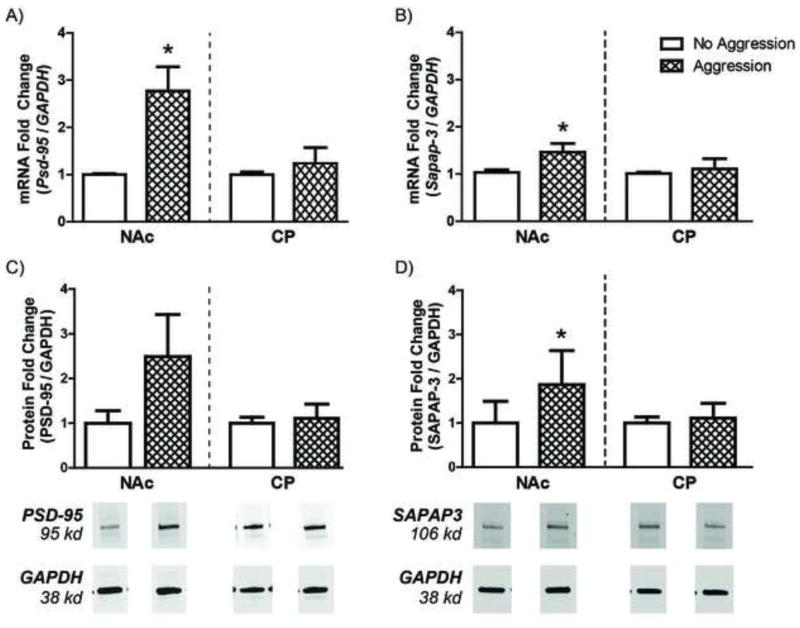
As previous research in our laboratory has demonstrated that aggressive experience increases the density of mature spines on NAc neurons, we hypothesized that aggressive experience would likewise increase the expression of synaptic scaffolding proteins, such as PSD-95 and SAPAP-3. Female subjects who received aggressive experience had a significant increase in Psd-95 mRNA in the NAc 1 wk (t(12) = 2.995, p < 0.01) and 3 wk (t(8) = 3.039, p < 0.05, data not shown) after the last aggressive experience compared to control females (Figure 3A). Aggressive experience also resulted in a smaller, but significant, increase in Sapap-3 mRNA in the NAc 1 wk (t(12) = 1.918, p < 0.05; Figure 3B) after the last aggressive experience; this change was not, however, maintained 3 wk following the last aggressive experience (data not shown). Furthermore, these increases in mRNA levels were paralleled by increases in PSD-95 and SAPAP-3 protein in the NAc compared to control females (Figure 3C and 3D), with a non-significant trend towards an increase for the PSD-95 protein (t(12) = 1.465, p = 0.084) and a significant increase in SAPAP-3 protein (t(12)=4.601, p < .001) 1 wk after the last aggressive experience. Increases in synaptic scaffolding proteins following aggressive experience appear to be specific to the NAc, as aggressive experience did not increase Psd-95 or Sapap-3 mRNA in the CP. Aggressive experience also increased the expression of Psd-95 and Sapap-3 mRNA in male hamsters (Supplementary Figure S2).
Figure 3. Aggressive experience increases the expression of synaptic scaffolding proteins.
A) The expression of Psd-95 mRNA was significantly increased in the Nucleus Accumbens (NAc), but not in the Caudate (CP) of females that received 5 aggressive experiences (n = 8) compared to control females (n = 8) one week following the last aggressive experience. B) Similarly, the expression of Sapap-3 mRNA was also significantly increased in the NAc, but not in the CP, one week following the last aggressive experience. C) PSD-95 protein was increased in the NAc of females that received 5 aggressive experiences compared to control females, although this difference did not reach significance. D) SAPAP-3 protein was significantly increased in the NAc of females that received 5 aggressive experiences compared to control females. Error bars represent mean + SEM, representative Western blots shown below quantification, * indicates significant difference between behavioral conditions, p < 0.05.
Blocking mGluR5 receptors prevents synaptic scaffolding proteins from increasing in the NAc
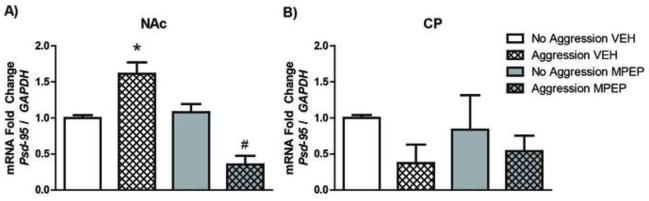
Given our finding that the escalation of aggressive behavior depends on the activation of mGluR5, we hypothesized that blocking mGluR5 may also prevent long-term increases in PSD-95 in the NAc following aggressive experience. To test this hypothesis, a subset female subjects were treated with MPEP or a vehicle control prior to each aggression test and Psd-95 mRNA levels in the NAc and CP were measured 3 weeks after the last aggressive experience. A one-way ANOVA revealed a significant effect of drug treatment on Psd-95 mRNA levels (Figure 4A, F(2,13) = 18.70, p < 0.001). Post-hoc comparisons with Bonferroni corrections indicated that Psd-95 mRNA significantly increased in vehicle-treated females compared to females who received no aggressive experience (t(7) = 3.360, p < .05). In MPEP-treated females, the experience-dependent increase in Psd-95 mRNA was blocked. In fact, Psd-95 mRNA levels were significantly lower MPEP-treated females who received aggressive experience compared to MPEP-treated females who did not receive aggressive experience (t(7) = 7.465, p < .01). There was no difference in Psd-95 mRNA levels between MPEP- and vehicle-treated females who did not receive aggressive experience (p > .05). As in our previous findings, these results appear to be specific to the NAc, as there was no significant effect of drug treatment on Psd-95 mRNA levels in the CP (Figure 4B).
Figure 4. Increases in Psd-95 following aggressive experience depend on mGluR5 activation.
A) Three weeks following the last aggressive experience, vehicle-treated females who received aggressive experience (n = 4) had a significantly higher expression of Psd-95 mRNA in the Nucleus Accumbens (NAc) than did vehicle-treated females who did not receive aggressive experience (n = 3). In contrast, the expression of Psd-95 mRNA was significantly decreased in MPEP-treated females that received aggressive experience (n = 4) compared to MEP-treated females that remained aggressive naïve (n = 3). B) In the Caudate (CP), Psd-95 mRNA expression levels did not change between any drug or behavioral conditions. Error bars represent mean + SEM, * indicates significant difference between vehicle-treated groups, # indicates significant difference between MPEP-treated groups, p < 0.05.
Discussion
Here we report for the first time that neural plasticity following aggressive experience depends on a signaling cascade involving group I mGluRs, FMRP, and PSD-95 in female hamsters. Specifically, aggressive experience results in a rapid, transient dephosphorylation of FMRP in the NAc of female hamsters. This dephosphorylation drives the local increase in synaptic scaffolding proteins such as PSD-95 and SAPAP-3, consistent with a proliferation of excitatory inputs. Furthermore, the escalation of aggressive behavior and the concomitant increase in Psd-95 mRNA each depend on the activation mGluR5 receptors. Taking these findings together, we propose a model in which aggressive encounters activate mGluR5 receptors on medium spiny neurons in the NAc core, leading to a rapid decrease in the phosphorylation of FMRP and a long-lasting increase in the transcription and translation of synaptic scaffolding proteins. With repeated experiences, these changes may result in an increase in AMPA receptor trafficking to the increased density of mature spines in the NAc, mediating the escalated aggression seen in future encounters (Figure 5).
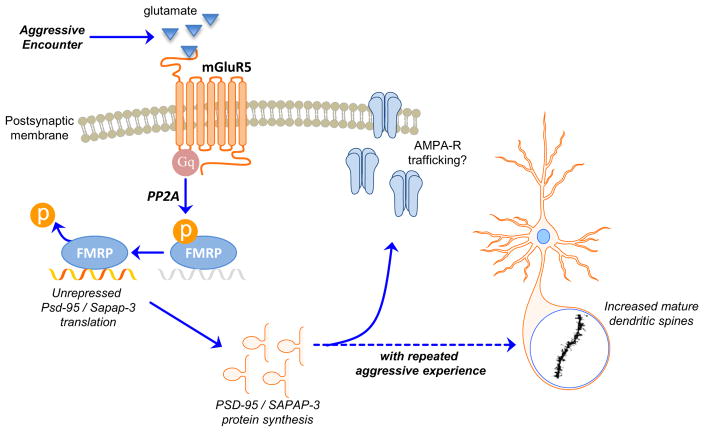
Figure 5. Hypothesized model for the molecular signaling underlying the escalation of female aggression.
Aggressive encounters activate Group I metabotropic glutamate receptors (mGluR5s) on medium spiny neurons in the nucleus accumbens (NAc) core. This activation leads to Gq-mediated signaling that activates PP2A, resulting in the rapid dephosphorylation of FMRP. The dephosphorylation of FMRP allows for de-repression of local translation of synaptic scaffolding proteins, such as PSD-95 and SAPAP-3, in dendrites. Repeated aggressive experience produces a long-term increase in this translation and the resulting increase in synaptic scaffolding proteins is associated with the proliferation of mature dendritic spines in the NAc core. This structural plasticity in the NAc core likely reflects an increase in excitatory inputs to the cell and may mediate a heightened response to future aggressive encounters, leading to escalated aggressive behavior.
The goal in undertaking these studies was to model the pathological escalation of aggressive behavior with an eye towards identifying a neural substrate and molecular target common to both sexes. Spontaneous aggression was identified in female hamsters decades ago, with the original interpretation that hamsters were anomalous as a rodent species in which females are aggressive [30]. In the intervening years, it has become clear that female rodents of many species are aggressive [31]. What makes Syrian hamsters particularly advantageous for studies of aggression is that they are solitary animals that defend individual territories [32]. Thus, unlike in prosocial species, where ecologically valid approaches would require maintaining animals in mixed sex social groups, aggression can be studied in hamsters singly housed in the laboratory.
A role for the NAc in mediating aggressive behavior has been previously demonstrated in male rats [7, 8] and our prior studies of aggressive experience on dendritic spine densities [12] also pointed to the NAc as a putative locus regulating aggression in females. We hypothesized that activation of group I mGluRs in the NAc may be important for mediating behavioral plasticity following aggressive experience in female hamsters. Our approach to blocking mGluR5 receptors was to use a low dose of MPEP to specifically prevent the experience-dependent escalation of female aggression without disrupting the expression of aggression itself. This approach to targeting changes in attack latency contrasts with the use of higher doses of MPEP in male mice, which reduces the overt expression of aggression [33].
As our initial hypothesis was supported, our next goal was to identify the signaling events originating from these cell surface glutamate receptors that could lead to local structural plasticity known to cause intrinsic changes in neuronal excitability. The FMRP signaling pathway was an obvious target, as FMRP is itself found in dendritic spines [34] where it regulates local mRNA translation via changes in its phosphorylation state [19, 20]. Further, the activation of FMRP is associated with increases in postsynaptic scaffolding proteins involved in conferring synaptic stability [21]. Finally, the translation of multiple targets of FMRP, including postsynaptic scaffolding proteins, is regulated by group I mGluRs [23, 24], and mGluR5 in particular has been identified as a key regulator of FMRP-dependent translation [24]. Our finding that aggressive encounters result in a rapid dephosphorylation of FMRP in the NAc, coupled with a long-lasting increase in PSD-95 and SAPAP-3, therefore fits well within the broader literature demonstrating that FMRP phosphorylation regulates local translation of proteins required for stable synaptic plasticity [18].
Importantly, our results add to the small literature examining the role of FMRP in modulating experience-dependent plasticity in healthy neurons [35–37]. Indeed, our finding that blocking mGluR5 receptors prevented Psd-95 from increasing in the NAc following aggressive experience demonstrates that activation of mGluR5 is necessary for the long term increase in synaptic scaffolding proteins following repeated aggressive experience in healthy neurons. These data are in accordance with in vitro studies demonstrating that PSD-95 is rapidly translated in response to stimulation of group I mGluRs [23], and that the phosphorylation of FMRP suppresses the translation of PSD-95 [38]. Although others have demonstrated that pharmacological stimulation of group I mGluRs rapidly upregulates FMRP in primary cortical cultures [23] and in synaptoneurosomes [39], we did not observe any change in overall levels of FMRP following aggressive experience. It is possible that this difference simply reflects the differences in brain regions (cortex vs. NAc) or in methodology (e.g., tissue processing, timecourse) used between studies. It is important to note, however, that the repeated behavioral stimulation used in the current study is more complex than a discrete pharmacological manipulation and therefore may result in different downstream effects.
Aggressive behavior in animal models becomes increasingly applicable to pathology in humans when it differs from the behavioral pattern typical for the that species [40]. By far the most investigated neurochemical system for the control of escalated aggression is the serotonin system (for review see [1]), and preclinical studies demonstrating the serotonergic modulation of aggression led to the development of a class of drugs termed ‘serenics’ that aims to treat pathological aggression [41]. Unfortunately, further development of drugs targeting the serontonergic system for treating aggression has been slowed by the complexities of serotoneric transmission along with multiple interacting factors controlling aggression in both animals and in humans [42, 43]. Moreover, from the few preclinical studies investigating aggression in females, it is clear that serotonergic manipulations that eliminate aggression in males have no effect on aggression in females (e.g., [44]). These data support the importance of investigating other neurochemical systems to develop therapeutic targets that are effective in treating females. Based on the results reported here, group I mGluR regulation of the FMRP signaling pathway within the NAc provides a novel target for preclinical studies of the treatment of escalated aggression, with the added benefit that such therapeutic approaches are more likely to be effective in treating pathological aggression in both females and males.
Supplementary Material
Acknowledgments
The authors wish to thank Kiara Vega, Calyn Maske, Kerry Trotter, and Natalia Rodriguez for their assistance in collecting these data.
Footnotes
Financial Disclosures
Research presented here was supported by NIH R01DA013680 to R.L.M. and also by the National Institute on Drug Abuse of the National Institutes of Health under Award Number T32DA007234 (LB and KM) and T32GM008471 (KM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miczek KA, Faccidomo S, De Almeida RM, Bannai M, Fish EW, Debold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–55. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- 2.Fazel S, Danesh J. Serious mental disorder in 23000 prisoners: a systematic review of 62 surveys. Lancet. 2002;359(9306):545–50. doi: 10.1016/S0140-6736(02)07740-1. [DOI] [PubMed] [Google Scholar]
- 3.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–42. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. J Child Psychol Psychiatry. 2006;47(3–4):262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 5.Kavoussi R, Armstead P, Coccaro E. The neurobiology of impulsive aggression. Psychiatr Clin North Am. 1997;20(2):395–403. doi: 10.1016/s0193-953x(05)70319-1. [DOI] [PubMed] [Google Scholar]
- 6.Miczek KA, Weerts E, Haney M, Tidey J. Neurobiological mechanisms controlling aggression: preclinical developments for pharmacotherapeutic interventions. Neurosci Biobehav Rev. 1994;18(1):97–110. doi: 10.1016/0149-7634(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 7.van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20(24):9320–5. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl) 2008;197(3):449–56. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 9.Fish EW, DeBold JF, Miczek KA. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology (Berl) 2005;182(1):116–27. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17(2):371–8. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- 11.Stockley P, Bro-Jorgensen J. Female competition and its evolutionary consequences in mammals. Biol Rev Camb Philos Soc. 2011;86(2):341–66. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- 12.Staffend NA, Meisel RL. Aggressive experience increases dendritic spine density within the nucleus accumbens core in female Syrian hamsters. Neuroscience. 2012;227:163–9. doi: 10.1016/j.neuroscience.2012.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–8. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 14.Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26(2):82–7. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Lubs HA. A marker X chromosome. Am J Hum Genet. 1969;21(3):231–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 17.Lozano R, Rosero CA, Hagerman RJ. Fragile X spectrum disorders. Intractable Rare Dis Res. 2014;3(4):134–46. doi: 10.5582/irdr.2014.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidorov MS, Auerbach BD, Bear MF. Fragile X mental retardation protein and synaptic plasticity. Mol Brain. 2013;6:15. doi: 10.1186/1756-6606-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12(24):3295–305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- 20.Coffee RL, Jr, Williamson AJ, Adkins CM, Gray MC, Page TL, Broadie K. In vivo neuronal function of the fragile X mental retardation protein is regulated by phosphorylation. Hum Mol Genet. 2012;21(4):900–15. doi: 10.1093/hmg/ddr527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34(4):503–18. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100(24):14374–8. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brain PF. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse) Behav Biol. 1972;7(3):349–57. doi: 10.1016/s0091-6773(72)80106-8. [DOI] [PubMed] [Google Scholar]
- 26.Grelk DF, Papson BA, Cole JE, Rowe FA. The influence of caging conditions and hormone treatments on fighting in male and female hamsters. Horm Behav. 1974;5(4):355–66. doi: 10.1016/0018-506x(74)90021-x. [DOI] [PubMed] [Google Scholar]
- 27.Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm Behav. 1988;22(4):453–66. doi: 10.1016/0018-506x(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 28.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, et al. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44(3):293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36(4):260–9. [PubMed] [Google Scholar]
- 31.DeBold JF, Miczek KA. Aggression persists after ovariectomy in female rats. Horm Behav. 1984;18(2):177–90. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- 32.Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4(3):253–5. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro JF, Postigo D, Martin M, Buron E. Antiaggressive effects of MPEP, a selective antagonist of mGlu5 receptors, in agonistic interactions between male mice. Eur J Pharmacol. 2006;551(1–3):67–70. doi: 10.1016/j.ejphar.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94(10):5395–400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabel LA, Won S, Kawai H, McKinney M, Tartakoff AM, Fallon JR. Visual experience regulates transient expression and dendritic localization of fragile X mental retardation protein. J Neurosci. 2004;24(47):10579–83. doi: 10.1523/JNEUROSCI.2185-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todd PK, Malter JS, Mack KJ. Whisker stimulation-dependent translation of FMRP in the barrel cortex requires activation of type I metabotropic glutamate receptors. Brain Res Mol Brain Res. 2003;110(2):267–78. doi: 10.1016/s0169-328x(02)00657-5. [DOI] [PubMed] [Google Scholar]
- 37.Irwin SA, Swain RA, Christmon CA, Chakravarti A, Weiler IJ, Greenough WT. Evidence for altered Fragile-X mental retardation protein expression in response to behavioral stimulation. Neurobiol Learn Mem. 2000;74(1):87–93. [PubMed] [Google Scholar]
- 38.Muddashetty RS, V, Nalavadi C, Gross C, Yao X, Xing L, Laur O, et al. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42(5):673–88. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci U S A. 1993;90(15):7168–71. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi A, Miczek KA. Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci. 2014;17:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver JE. Aggressiveness, anxiety and drugs. Br J Psychiatry. 1990;157:300. doi: 10.1192/bjp.157.2.300a. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl) 2009;205(3):349–68. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- 43.Duke AA, Begue L, Bell R, Eisenlohr-Moul T. Revisiting the serotonin-aggression relation in humans: a meta-analysis. Psychol Bull. 2013;139(5):1148–72. doi: 10.1037/a0031544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joppa MA, Rowe RK, Meisel RL. Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol Biochem Behav. 1997;58(2):349–53. doi: 10.1016/s0091-3057(97)00277-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.