Abstract
Background
Convergent findings indicate that cortical GABAergic circuitry is altered in schizophrenia. Postmortem studies have consistently found lower levels of GAD67 mRNA in the prefrontal cortex (PFC) of subjects with schizophrenia. At the cellular level, the density of GABA neurons with detectable levels of GAD67 mRNA is ~30% lower across cortical layers. Knowing how this transcript deficit translates to GAD67 protein levels in axonal boutons is important for understanding the impact it might have on GABA synthesis. In addition, because reductions in GAD67 expression prior to, but not after, the maturation of GABAergic boutons results in a lower density of GABAergic boutons in mouse cortical cultures, knowing if GABAergic bouton density is altered in schizophrenia would provide insight into the timing of the GAD67 deficit.
Methods
PFC tissue sections from 20 matched pairs of schizophrenia and comparison subjects were immunolabeled for the vesicular GABA transporter (vGAT) and GAD67.
Results
vGAT+ bouton density did not differ between subject groups, consistent with findings that vGAT mRNA levels are unaltered in the illness, and confirming that the number of cortical GABAergic boutons is not lower in schizophrenia. In contrast, in schizophrenia subjects the proportion of vGAT+ boutons with detectable GAD67 levels (vGAT+/GAD67+ boutons) was 16% lower and mean GAD67 levels were 14% lower in the remaining vGAT+/GAD67+ boutons.
Conclusions
Our findings suggest that GABA production is markedly reduced in a subset of boutons in the PFC of schizophrenia subjects, and that this reduction likely occurs after the maturation of GABAergic boutons.
Keywords: GAD67, vGAT, GABAergic, gamma-aminobutyric acid, quantitative microscopy, development
Introduction
Cognitive deficits, such as impairments in working memory, are recognized as core clinical features in schizophrenia (1), and these impairments are thought to reflect, at least in part, disturbances in gamma aminobutyric acid (GABA)-releasing (GABAergic) neurons within the prefrontal cortex (PFC) (2). Perhaps the most widely and consistently reported finding in postmortem studies of subjects with schizophrenia is lower levels of mRNA for the GABA synthesizing enzyme, glutamic acid decarboxylase 67 (GAD67) (3–8). Although less well-studied, the deficit in GAD67 mRNA has been reported to be accompanied by lower GAD67 protein levels (6, 7, 9). The consistency of these findings suggests that lower GAD67 expression in schizophrenia is a common feature of the illness. Other findings indicate that it is not a consequence of illness chronicity or other factors frequently associated with the illness, such as use of antipsychotic medications (7, 10).
However, not all GABAergic neurons exhibit lower GAD67 mRNA expression in schizophrenia. Specifically, ~30% of GABAergic neurons in schizophrenia PFC lack detectable levels of GAD67 mRNA, whereas the others express GAD67 mRNA at normal levels (3, 5). The subset of GABAergic neurons with markedly lower GAD67 mRNA expression is prominent in PFC layers 2–5 (3, 5). This deficit occurs in the absence of a change in total neuron density (5) or number (11), suggesting that all GABAergic neurons are present but that a subset have a markedly reduced capacity to synthesize GABA. Moreover, expression of some other gene products that can affect GABAergic neurotransmission are reported to be unaffected or only slightly altered in schizophrenia. For example, mRNA for the vesicular GABA transporter (vGAT), which packages GABA into synaptic vesicles, was reported to be unchanged or only modestly lower in the PFC of schizophrenia subjects (10, 12), suggesting that the ability to load GABA into vesicles is preserved in the illness.
Although the timing of onset of the GAD67 mRNA deficit is unknown, dysfunction of the PFC in schizophrenia appears to be a late developmental event. For example, in children who are later diagnosed with schizophrenia, working memory performance appears to be intact until about 9 years of age and then subsequently declines (13). Because working memory relies on the coordinated firing of PFC pyramidal neurons (14) by GABAergic interneurons (15, 16), these findings suggest that the GAD67 deficit may arise during childhood. Knowing if structural alterations in GABAergic axon boutons occur in schizophrenia could inform on the timing of the GAD67 deficit in schizophrenia. For example, genetic reduction of GAD67 expression in parvalbumin basket cells during early stages of development results in fewer boutons, whereas the same reduction later does not alter axonal architecture (17). Thus, it would be expected that a reduction in GAD67 expression during the pre- or perinatal periods in individuals who are later diagnosed with schizophrenia would be accompanied by fewer GABAergic axon boutons.
In concert, these findings suggest the following testable hypotheses: 1) The density of all GABAergic axonal boutons is unaltered in the PFC of subjects with schizophrenia; 2) GAD67 protein levels are markedly lower in a subset of these boutons. To test these hypotheses we immunolabeled PFC tissue sections from 20 matched pairs of schizophrenia and comparison subjects for vGAT and GAD67, and assessed GABAergic bouton density and bouton protein levels across cortical layers using quantitative confocal microscopy techniques.
Methods and Materials
Subjects
Brain specimens from 40 subjects were recovered during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA, USA) after obtaining consent from the next of kin. An independent committee of experienced research clinicians made consensus DSM-IV diagnoses, or confirmed the absence of any diagnoses, for each subject using the results of structured interviews conducted with family members and/or review of medical records (18). To reduce biological variance between groups, and to control for experimental variance, each schizophrenia subject was matched to one comparison subject for sex, and as closely as possible for age and postmortem interval (PMI) (Table 1 and Supplemental Table S1). The length of the PMI can affect protein integrity (7, 19) and aging can differentially affect gene expression (10). Consequently, to reduce the potential effects of these confounding variables we selected all available subjects with PMI < 16 hours and age ≤ 55 years. The University of Pittsburgh’s Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research approved all procedures.
Table 1.
Summary of Demographic and postmortem characteristics of human subjects. There were no diagnostic group differences in age (t19 = 0.541, p = 0.545; t38 = 0.276, p = 0.784), postmortem interval (t19 = 0.532, p = 0.601; t38 = 0.327, p = 0.745), or freezer storage time (t19 = 0.497, p = 0.625; t38 = 0.268, p = 0.790).
| Measure | Comparison Group (n=20) | Schizophrenia Group (n=20) | ||
|---|---|---|---|---|
| n or Mean | % or SD | n or Mean | % or SD | |
| Male | 14 | 70% | 14 | 70% |
| White | 17 | 85% | 18 | 90% |
| Black | 3 | 15% | 2 | 10% |
| Age (years) | 46 | 9 | 45 | 7 |
| Postmortem interval (hours) | 9.9 | 4.0 | 9.5 | 3.4 |
| Tissue storage time (years) | 14 | 5 | 12 | 5 |
The left hemisphere of each brain was blocked coronally at 1–2 cm intervals, immersed in 4% paraformaldehyde for 48 hours at 4°C and then washed in a series of graded sucrose solutions and cryoprotected. Tissue blocks containing the PFC were sectioned coronally at 40 μm on a cryostat and stored in a 30% glycerol/30% ethylene glycol solution at -30° C until processed for immunohistochemistry. Adjacent Nissl- stained sections were used to select sections that were cut perpendicular to the pial surface.
Immunohistochemistry
For each subject, four sections containing PFC area 9 spaced ~500 μm apart were used. To minimize experimental variance within and across subject pairs, two separate experimental runs consisting of two sections per subject (80 sections total per run) were performed with all sections within a run processed simultaneously. Sections were preprocessed (see Supplemental Methods) and then incubated for ~72 hours at 4°C in PBS containing 2% donkey serum and primary antibodies that recognize vGAT (mouse host; 1:500, Synaptic Systems, Goettingen, Germany; product # 131011, Lots 131011/41 and 131011/42) and GAD67 (goat host; 1:100, R&D Systems, Minneapolis, MN, USA; product # AF2086, Lot KRD0110031). The specificity of each antibody was verified by Western blot in our laboratory (data not shown and (20)) or other laboratories (vGAT (21); GAD67 (22, 23)). Sections were then rinsed for 2 hours in PBS and incubated for 24 hours in PBS containing 2% donkey serum and secondary antibodies (donkey host) conjugated to Alexa 488 (vGAT) or 647 (GAD67; Invitrogen, Grand Island, NY, USA; 1:500 for all) at 4°C. After washing, sections were mounted (ProLong Gold antifade reagent, Invitrogen) on slides, which were coded to conceal diagnosis and subject number, and stored at 4°C until imaged.
Microscopy
Data were collected on an Olympus (Center Valley, PA) IX81 inverted microscope equipped with an Olympus spinning disk confocal unit, Hamamatsu EM-CCD digital camera (Bridgewater, NJ), and high precision BioPrecision2 XYZ motorized stage with linear XYZ encoders (Ludl Electronic Products Ltd., Hawthorne NJ) using a 60X 1.40 N.A. SC oil immersion objective. The equipment was controlled by SlideBook 5.0 (Intelligent Imaging Innovations, Inc., Denver, CO), which was the same software used for post-image processing. 3D image stacks (2D images successively captured at intervals separated by 0.25 μm in the z-dimension) that were 512 × 512 pixels (~ 137 × 137 μm) were acquired over 50 percent of the total thickness of the tissue section starting at the coverslip. Importantly, imaging the same percentage of the tissue section thickness rather than the same number of microns controls for the potential confound of storage and/or mounting related volume differences (i.e. z-axis shrinkage). The stacks were collected using optimal exposure settings (i.e., those that yielded the greatest dynamic range with no saturated pixels), with differences in exposures normalized during image processing.
Sampling
As determined by measurements made in Nissl-stained sections (24), the boundaries of the six cortical layers can be estimated based on the distance from the pial surface to the white matter. For the presented studies, the cortical mantle was divided into layers as follows: 1 (pia-10%), 2/superficial 3 (2/3s; 10–35%), deep 3/4 (3d/4; 35–60%), 5 (60–80%), and 6 (80%-gray/white matter border). Ten, systematic randomly sampled image stacks were taken within each of these subdivisions using a sampling grid of 180 × 180 μm2. Running means using pilot data indicated that 10 sites per subdivision were sufficient to limit intra-subject variability for intensity and density measures. The same investigator (BRR), who was blind to subject and diagnosis, collected a total of 8,000 image stacks. Both subjects within a pair were imaged on the same day.
A potential confound of quantitative fluorescence measures in human cortex is lipofuscin autofluorescence. To exclude this potential confound, lipofuscin was imaged using a third channel (equivalent to Alexa 405) at a constant exposure time across all sections. Lipofuscin autofluorescence was masked using a single optimal threshold value for each image stack, and vGAT and GAD67 object masks that overlapped a lipofuscin mask were eliminated from analyses. Importantly, lipofuscin fluorescence intensity did not differ between schizophrenia (648 ± 140 a.u.) and comparison (656 ± 147 a.u.) subjects (paired F1, 19 = 0.08, p = 0.79; unpaired F1, 36 = 0.03, p = 0.86).
Image processing
Each fluorescent channel was deconvolved using Autoquant’s Blind Deconvolution algorithm. Data segmentation was performed as described in Supplemental Methods. The final object masks were then used to collect information on the deconvolved channels.
Definitions of vGAT+ and vGAT+/GAD67+ boutons
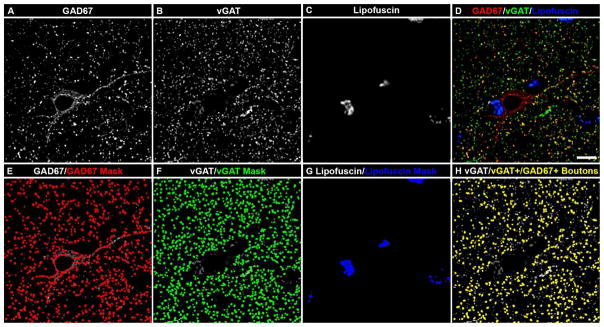
All vGAT-immunoreactive puncta were considered to be boutons (vGAT+ boutons) (25, 26). vGAT+ boutons were classified as containing GAD67 immunoreactivity (vGAT+/GAD67+ boutons) as follows: First, mask operations, which assess the degree of overlap between voxels of different object masks, were used to identify vGAT and GAD67 object masks that overlapped each other’s centers, which at the voxel level overlapped an average of 67% ± 1.2%. Second, for each site the mean fluorescence intensity of GAD67 for all boutons identified in the first step were used as seeds in a K-means cluster analysis to classify vGAT+ boutons as being GAD67+. An example of boutons classified as vGAT+ and vGAT+/GAD67+ is shown in Figure 1.
Figure 1.
vGAT and GAD67 colocalization in human postmortem tissue. (A–H) Projection image (5 z-planes separated by 0.25 μm) of a human PFC tissue section immunolabeled for vGAT and GAD67. Single GAD67 (A) and vGAT (B) immunoreactive channels, and single channel of lipofuscin (C) autofluorescence. (D) Merged GAD67, vGAT, and lipofuscin channels. Single GAD67, vGAT, and lipofuscin channels overlaid with their corresponding object masks (E, F, and G, respectively). Importantly, the object masks in F define the vGAT+ boutons. (H) Single vGAT channel overlaid with vGAT object masks from F that were defined as vGAT+/GAD67+ boutons (yellow object masks) because of the presence of detectable GAD67 immunoreactivity. Bar = 10 μm.
Cartridge identification and analysis
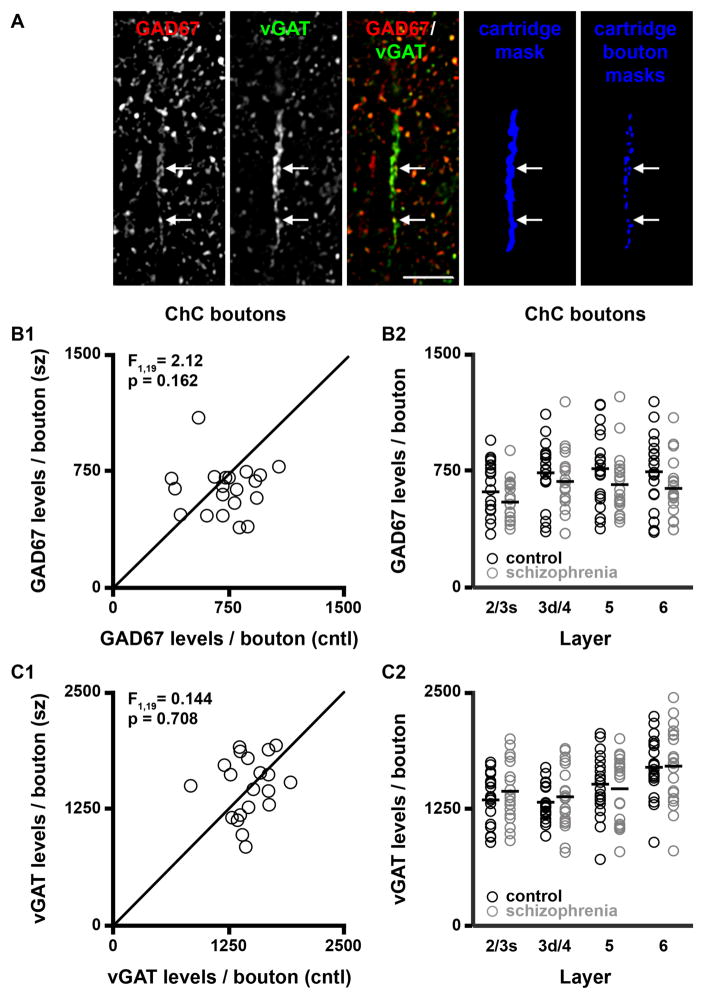
Chandelier (axoaxonic) cells (ChCs) give rise to vertically-arranged clusters of boutons (termed cartridges (27)) that exclusively target the axon initial segment (AIS) of pyramidal cells. Cartridges are easily identified amid the general punctate vGAT labeling in cortical layers 2–6 because of their unique structure and their high levels of vGAT immunoreactivity (Fig. 4). Within each field collected from layers 2–6 under run 1, an unbiased counting frame (~68 × 68 μm2), consisting of two exclusion lines and two inclusion lines placed over the image stack, was used to identify vGAT-IR cartridges for analysis. vGAT-IR cartridges were included for analysis and manually traced only if they were considered to be completely visualized as indicated by (1) continuity across z planes and (2) presence of the entire cartridge within a virtual sampling box. The virtual sampling box started and ended one z-plane from the top and bottom of the image stack, respectively, and had x-y start/end coordinates that were located 20 pixels from any edge. Figure 4 shows a traced vGAT-IR cartridge.
Figure 4.
ChC cartridge bouton GAD67 and vGAT levels. (A) Projection image (7 z-planes separated by 0.25 μm) of a human PFC tissue section immunolabeled for vGAT and GAD67. Bar = 10 μm. (B–C) GAD67 (B1–B2) and vGAT (C1–C2) protein levels per ChC bouton in total gray matter and within individual layers. F-statistics and p-values for B2 and C2 are provided in Supplemental Table S2.
Statistics
A paired samples t-test was used to assess differences in mean age, storage time, and PMI between subject groups; none of these variables were significantly different between groups (Table 1).
Two analyses of covariance (ANCOVA) models were used to analyze the bouton density and protein level data. Because subjects were selected and processed as pairs, the first paired ANCOVA model included bouton density or protein level as the dependent variable, diagnostic group as the main effect, subject pair as a blocking factor, and tissue storage time as a covariate. Subject pairing is an attempt to balance diagnostic groups for sex, age, and PMI, and to account for the parallel processing of tissue samples, and thus is not a true statistical paired design. Consequently, a second unpaired ANCOVA model was performed that included all covariates (i.e., age, sex, PMI, storage time). All statistical tests were conducted with α-level= 0.05.
We also assessed the potential influence of other factors that are frequently comorbid with the diagnosis of schizophrenia using ANCOVA models. For these analyses, we compared subjects with schizophrenia using each variable (sex; diagnosis of schizoaffective disorder; nicotine use at the time of death; use of antipsychotics, antidepressants, or benzodiazepines and/or sodium valproate at the time of death) as the main effect and age, tissue storage time, and PMI as covariates. A Bonferroni-adjusted α-level of 0.05/6 = 0.008 was used to assess significance.
Reported ANCOVA statistics include only those covariates that were statistically significant. As a result, the reported degrees of freedom vary across analyses.
Results
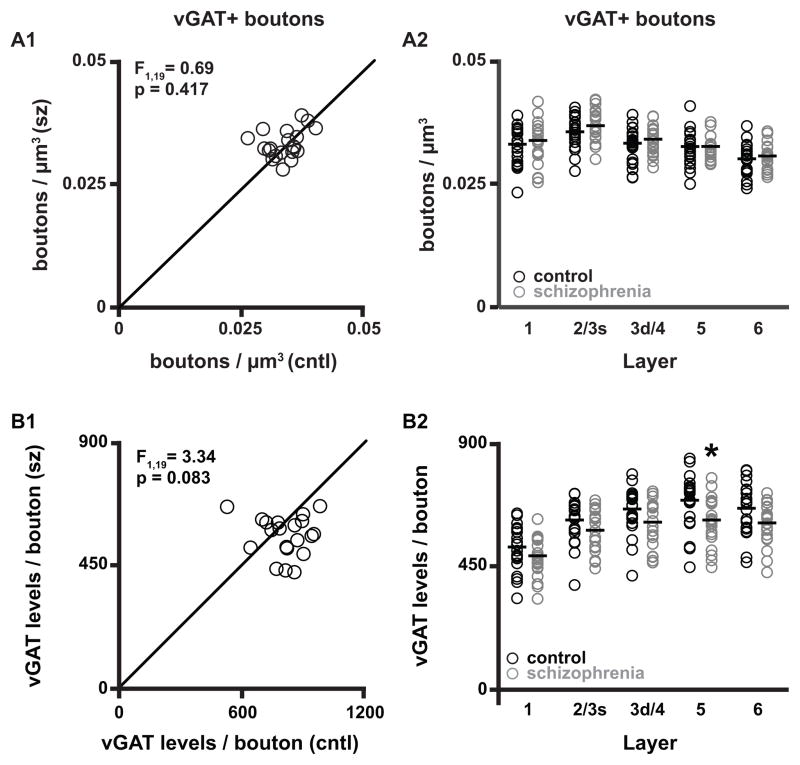
vGAT+ bouton density is unchanged in schizophrenia
Across all layers of the PFC, a total of 968,186 ± 82,032 and 948,895 ± 95,695 vGAT+ boutons for each schizophrenia and comparison subject, respectively, were analyzed. The density of vGAT+ boutons did not differ (paired F1, 19 = 0.69, p = 0.417; unpaired F1, 38 = 0.47, p = 0.497) between subject groups (schizophrenia: 0.033 ± 0.003 boutons/μm3; comparison: 0.032 ± 0.003 boutons/μm3) (Fig. 2A1). Similarly, the density of vGAT+ boutons did not differ between subject groups in any of the cortical layers assessed (Fig. 2A2). Importantly, the rank order of GABAergic bouton density in each cortical layer presented here is similar to what has been published for the PFC in both human (28) and non-human primate (29).
Figure 2.
vGAT+ bouton densities and vGAT protein levels. (A1–A2) Bouton density in total gray matter and within individual layers. (B1–B2) vGAT protein levels per bouton in total gray matter and within individual layers. The data points in each scatterplot (A1 and B1) represent a matched pair of schizophrenia (sz) and comparison (cntl) subject. Points below the unity line reflect pairs in which the measures are lower for the schizophrenia relative to the comparison subject. Asterisk in B2, Layer 5 indicates p < 0.05 for both paired and unpaired analyses. F-statistics and p-values for A2 and B2 are provided in Supplemental Table S2.
Bouton vGAT protein levels are unchanged in schizophrenia
vGAT protein levels in vGAT+ boutons did not differ (paired F1, 19 = 3.34, p = 0.083; unpaired F1, 38 = 3.64, p = 0.064; Fig. 2B1) between schizophrenia (564 ± 72 a.u.) and comparison (611 ± 83 a.u.) subjects in total gray matter. However, an analysis across individual cortical layers found that vGAT bouton levels in layer 5 were 11% lower (paired F1, 19 = 5.09, p = 0.036; unpaired F1, 38 = 5.46, p = 0.025) in schizophrenia subjects (Fig. 2B2).
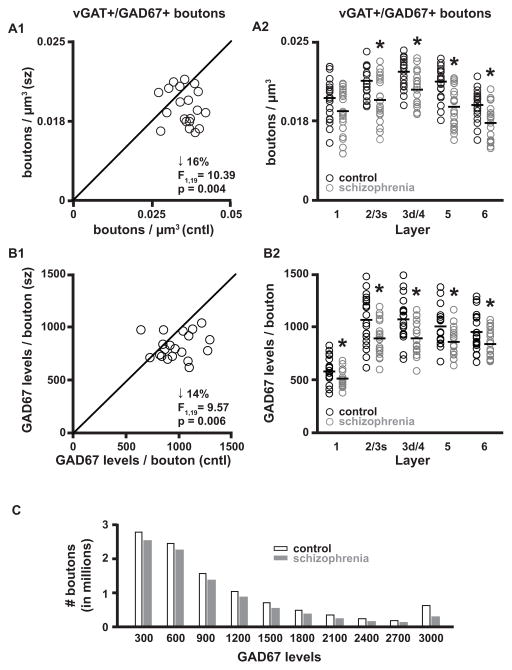
The density of vGAT+/GAD67+ boutons is lower in schizophrenia
Across all cortical layers, the density of vGAT+ boutons with detectable levels of GAD67 (vGAT+/GAD67+ boutons) was 16% lower (paired F1, 19 = 10.39, p = 0.004; unpaired F1, 38 = 12.44, p = 0.001) in schizophrenia (0.015 ± 0.003 boutons/μm3) relative to comparison (0.018 ± 0.002 boutons/μm3) subjects (Fig. 3A1). Similarly, the density of vGAT+/GAD67+ boutons was 13% to 21% lower in cortical layers 2/3s, 3d/4, 5, and 6 (Fig. 3A2).
Figure 3.
vGAT+/GAD67+ bouton densities and GAD67 protein levels. (A1–A2) Bouton density in total gray matter and within individual layers. (B1–B2) GAD67 protein levels per bouton in total gray matter and within individual layers. Asterisks in A2 and B2 indicate p < 0.05 for both paired and unpaired analyses. F-statistics and p-values for A2 and B2 are provided in Supplemental Table S2. (C) To assess if GAD67 levels were 14% lower across all boutons in schizophrenia, the range of GAD67 levels in the comparison group was used to make 10 equally separated bins and each bouton from both diagnosis groups was placed into a bin based on its GAD67 level.
vGAT+/GAD67+ bouton GAD67 protein levels are lower in schizophrenia
vGAT protein levels in vGAT+/GAD67+ boutons did not differ (paired F1, 18 = 0.16, p = 0.69; unpaired F1, 37 = 0.06, p = 0.81) between schizophrenia (822 ± 134 a.u.) and comparison (817 ± 122 a.u.) subjects across all cortical layers (Supplemental Fig. S1A) or in any cortical layer (Supplemental Fig. S1B). In contrast, GAD67 protein levels in vGAT+/GAD67+ boutons were 14% lower (paired F1, 19 = 9.57, p = 0.006; unpaired F1, 38 = 8.33, p = 0.006) in schizophrenia (808 ± 120 a.u.) relative to comparison (942 ± 172 a.u.) subjects in total gray matter (Fig. 3B1), and similar differences, ranging from 11% to 17% lower, were found in each cortical layer (Fig. 3B2). To assess if lower GAD67 levels were present in all boutons with detectable GAD67 across all cortical layers in subjects with schizophrenia, GAD67 levels per bouton in the comparison group were divided into 10 equal bins and each bouton from both subject groups was placed into a bin based on its GAD67 level (Fig. 3C). The presence of boutons in the bin corresponding to the upper 10th percentile suggests that some boutons do not have a reduction in GAD67 levels.
GAD67 and vGAT protein levels in ChC boutons are not altered in schizophrenia
Amid the general vGAT punctate labeling, distinct parallel arrays of IR axonal boutons were readily identified (Fig. 4A). These structures are morphologically identical to the vGAT-IR cartridges previously shown to represent chandelier (axoaxonic) cell (ChC) GABA neuron boutons innervating the axon initial segment of pyramidal cells (30). Changes in pre- and post-synaptic protein levels at the axon initial segment have lead to the hypothesis that GAD67 protein levels are markedly lower in ChC boutons (31). Thus, to determine if boutons arising from ChCs are part of the pool of vGAT boutons with undetectable levels of GAD67 protein identified here, GAD67 levels in ChC boutons were assessed. GAD67 protein levels in ChC boutons did not differ (paired F1, 19 = 2.12, p = 0.162; unpaired F1, 38 = 2.17, p = 0.149; Fig. 4B1) between schizophrenia (639 ± 163 a.u.) and comparison (720 ± 185 a.u.) subjects in total gray matter. Similarly, vGAT ChC bouton protein levels did not differ (paired F1, 19 = 0.144, p = 0.708; unpaired F1, 38 = 0.12, p = 0.730) between schizophrenia (1,501 ± 321 a.u.) and comparison (1,470 ± 236 a.u.) subjects (Fig. 4C1). In addition, ChC bouton GAD67 and vGAT levels did not differ between subject groups in any of the cortical layers assessed (Fig. 4B2 and 4C2, respectively).
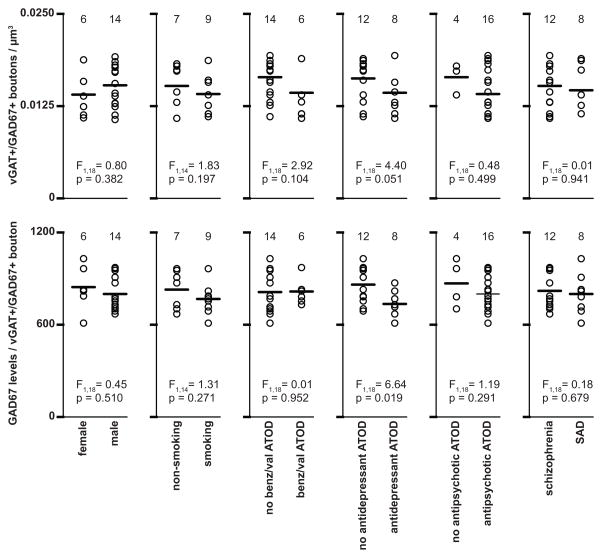
Lower vGAT+/GAD67+ bouton density and GAD67 protein levels are not attributable to comorbid factors
In schizophrenia subjects, GAD67 protein levels in vGAT+/GAD67+ boutons, and the density of vGAT+/GAD67+ boutons did not differ as a function of sex, nicotine use at time of death (ATOD), benzodiazepines and/or sodium valproate ATOD, antidepressants ATOD, antipsychotics ATOD, or diagnosis of schizoaffective disorder. (Fig. 5).
Figure 5.
Effects of comorbid factors on vGAT+/GAD67+ bouton density and levels of GAD67 in subjects with schizophrenia. ATOD, at time of death; benz/val; benzodiazepines/valproic acid; SAD, schizoaffective disorder.
Discussion
In schizophrenia neither neuronal density (5) nor total neuron number (11) are altered in the PFC, suggesting that all GABAergic neurons are present. In support of this interpretation, others have found no difference in the density of GAD65 immunoreactive puncta in the PFC of subjects with schizophrenia relative to comparison subjects (28). However, in primate PFC not all boutons contain detectable levels of GAD65 (20, 32, 33). Here, the presence of vGAT, which is required to package GABA for vesicular release (26) and thus is a marker of all GABAergic terminals, was used to quantify GABAergic bouton density. Importantly, vGAT mRNA (34, 35) levels are not altered in schizophrenia. We found no differences in the density of vGAT+ boutons between schizophrenia and comparison subjects in any layer of the PFC. In concert, these findings support the hypothesis that the density of GABAergic axonal boutons is unaltered in the PFC of subjects with schizophrenia.
Convergent findings suggest that in addition to its role as the primary inhibitory neurotransmitter in the adult brain, GABA signaling is crucial for the maturation of inhibitory synapses (36). For example, genetic knockdown of GAD67 in developing GABAergic neurons results in a cell-autonomous reduction in bouton formation (17). By contrast, reduced GAD67 expression in mature GABAergic neurons does not alter bouton number or morphology. Thus, the observations made here of markedly lower bouton GAD67 levels but a normal complement of vGAT boutons suggests that the GAD67 deficit in schizophrenia occurs after the maturation of the affected GABA neurons. Interestingly, in monkey PFC mature levels of vGAT and GAD67 mRNA are not achieved until the peripubertal period (34), while in human PFC GAD67 mRNA levels progressively increase until the peripubertal stage of development, plateauing or mildly declining in adulthood (37, 38). This interpretation is supported by the finding that in schizophrenia working memory performance appears to be intact prior to 9 years of age, but slowly declines afterwards, suggesting that GAD67 levels within PFC interneurons involved in working memory are reduced later in development (13).
Although there was no difference in vGAT bouton density between schizophrenia and comparison subjects, it is possible a loss of GABAergic neurons and their boutons could be compensated for by sprouting of axon collaterals from the remaining GABAergic neurons. However, neither neuronal density (5) nor total neuron number (11) are altered in the PFC in schizophrenia, suggesting that all GABAergic neurons are present. Alternatively, it is possible that some neurons give rise to a lower than normal number of boutons that is compensated for by collateral sprouting from other GABAergic neurons.
At the cellular level, the density of GABA neurons with detectable levels of GAD67 mRNA is ~30% lower across cortical layers 2–5 in the PFC of subjects with schizophrenia, whereas the levels of GAD67 mRNA within the other neurons do not differ from healthy comparison subjects (3, 5). These findings led us to hypothesize that GAD67 protein levels are markedly lower in a subset of boutons. Our finding that GAD67 protein levels are markedly lower in 16% of GABAergic boutons, such that they are no longer detectable by GAD67 immunoreactivity, supports this hypothesis. However, considering that ~30% of GABA neurons exhibit marked deficits in GAD67 mRNA expression in schizophrenia, it was somewhat surprising that GAD67 levels were not markedly lower in more boutons. We have previously shown a dissociation between the proportions of GABA neuron subpopulations and the proportions of boutons that arise from those subpopulations (29). Thus, it is possible the reduction in the proportion of somas with detectable levels of GAD67 mRNA could differ in magnitude from the reduction in proportion of boutons with detectable levels of GAD67 protein. In addition, we did find that mean GAD67 levels were 14% lower in the remaining vGAT+/GAD67+ boutons. This finding could reflect a general 14% reduction in vGAT+/GAD67+ bouton GAD67 levels. However, some boutons appear to have normal levels of GAD67 protein (Fig. 3C), suggesting that a subset of boutons have lower, but still detectable, GAD67 levels. Although the reduction in bouton GAD67 levels reported here is in line with our previous finding that GAD67 protein levels are 10% lower in total PFC gray matter in schizophrenia (7), others have reported larger deficits in total gray matter GAD67 protein levels (6, 9). In the cortex, GAD67 is readily detectable in neuron cell bodies, dendrites, and throughout the axon (39). Thus, differences in the percent reduction of GAD67 in schizophrenia across the methods used to assess protein levels could reflect changes selective for certain neuronal compartments.
The question becomes: what are the identities of the boutons with undetectable levels of GAD67? In schizophrenia, ~50% of PFC parvalbumin-expressing neurons, a subset of which are ChCs, lack detectable levels of GAD67 mRNA (4), while the remaining PV neurons appear to contain normal levels of GAD67 mRNA (3, 5). Our findings that ChC bouton GAD67 and vGAT levels were not significantly different between schizophrenia and comparison subjects, in concert with previous findings of lower GAD67 levels in the boutons of parvalbumin-expressing basket cells in schizophrenia (7) suggest that the marked GAD67 mRNA reductions in parvalbumin-expressing neurons are restricted to basket cells. In addition to parvalbumin basket cells, GAD67 protein levels might also be lower in at least the subset of calbindin-expressing GABA neurons that also express somatostatin (40). Somatostatin mRNA levels are lower in the PFC of schizophrenia subjects (18, 40–44), and lower somatostatin mRNA expression correlates with lower GAD67 mRNA expression at both the tissue and cellular levels (40, 42). Together, these findings suggest that at least some somatostatin neurons give rise to boutons with lower GAD67 protein levels. Unfortunately, since somatostatin expression is markedly reduced in schizophrenia it will be difficult to directly assess GAD67 levels in boutons arising from somatostatin-expressing neurons in future experiments.
The cause(s) of lower PFC GAD67 levels in schizophrenia is unclear but multiple mechanisms may contribute to the deficit. For example, allelic variants in the gene encoding GAD67, GAD1, are associated with lower GAD67 expression and an increased risk of schizophrenia (45, 46). Dysregulated epigenetic mechanisms, such as altered chromatin-associated histone modifications and higher-order chromatin structure at the GAD1 promoter region are also associated with lower GAD67 expression in the illness (37, 47, 48). Reduced mRNA expression for upstream regulatory factors of the GAD1 gene have been reported in schizophrenia and correlates with reduced levels of GAD67 mRNA (49). However, none of these factors provide an obvious mechanism to account for lower GAD67 mRNA in just a subset of GABA neurons and lower GAD67 protein in just a subset of boutons. Alternatively, lower GAD67 expression may be a compensatory response to lower activity in cortical pyramidal neurons (50) in which case those GABA neurons that receive high levels of excitatory drive from pyramidal neurons (e.g., parvalbumin- and somatostatin- but not calretinin-containing GABA neurons) might be predicted to be preferentially affected. The fact that the number of excitatory inputs to the affected pyramidal neurons is reduced during late childhood and adolescence might account for the late developmental reduction in GAD67 predicted by the results of the present study.
Supplementary Material
Acknowledgments
This work was supported by the NSF (DGE-0549352 to BRR) and NIMH (MH043784 to DAL; MH096985 to KNF).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or the United States Government.
Financial Disclosures
David A. Lewis currently receives investigator-initiated research support from Pfizer and in 2012–2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. Mr. Rocco and Dr. Fish report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 7.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered Cortical Expression of GABA-Related Genes in Schizophrenia: Illness Progression vs Developmental Disturbance. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thune JJ, Uylings HBM, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. JPsychiatrRes. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 12.Fung SJ, Webster MJ, Weickert CS. Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain Res. 2011;1388:22–31. doi: 10.1016/j.brainres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 15.Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nature Neuroscience. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- 16.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA A blockade of prefrontal cortical neurons engaged by working memory. The Journal of Neuroscience. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beneyto M, Sibille E, Lewis D. Human postmortem brain research in mental illness syndromes. In: Charney DS, Nestler E, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2008. pp. 202–214. [Google Scholar]
- 20.Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C, Stella SL, Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol. 2009;512:6–26. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb DI, Chang YC, Schwob JE. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1986;83:8808–8812. doi: 10.1073/pnas.83.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 25.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, et al. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. The Journal of Neuroscience. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- 28.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 29.Rocco BR, Sweet RA, Lewis DA, Fish KN. GABA-Synthesizing Enzymes in Calbindin and Calretinin Neurons in Monkey Prefrontal Cortex. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33:8352–8358. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 32.Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36. doi: 10.1038/mp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocco BR, Sweet RA, Lewis DA, Fish KN. GABA synthesizing enzymes in calbindin and calretinin neurons in monkey prefrontal cortex. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv051. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull. 2015;41:180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2011;69:71–79. doi: 10.1016/j.biopsych.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabriel SM, Davidson M, Haroutunian V, Powchik P, Bierer LM, Purohit DP, et al. Neuropeptide deficits in schizophrenia vs. Alzheimer’s disease cerebral cortex. Biological Psychiatry. 1996;39:82–91. doi: 10.1016/0006-3223(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 44.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 46.Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD(67)), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Molecular Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 47.Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Translational psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, et al. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. J Neurosci. 2013;33:11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimoto S, Bazmi HH, Lewis DA. Lower Expression of Glutamic Acid Decarboxylase 67 in the Prefrontal Cortex in Schizophrenia: Contribution of Altered Regulation by Zif268. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.