Summary
Oxidative damage is a central feature of ulcerative colitis. Here, we tested whether the antioxidant Mesna, when administered alone or in combination with n‐3 polyunsaturated fatty acids (n‐3 PUFAs), affects the outcome of dextran sodium sulphate (DSS)‐induced ulcerative colitis in rats. After the induction of colitis, DSS‐treated rats were further treated orally (p.o), intraperitoneally (i.p) or intrarectally (i.r) for either 7 or 14 days with Mesna, n‐3 PUFAs or both. Rats were euthanized at the end of each treatment period. Clinical disease activity index was recorded throughout the experiment. At necropsy colorectal gross lesions were scored. Colitis was scored histologically, and the expression of myeloperoxidase (MPO), caspase‐3, inducible nitric oxide synthase (iNOS) and nuclear factor κB (NF‐κΒ) in colonic tissue was assessed by immunohistochemistry. Mesna alone was sufficient to significantly reduce colorectal tissue damage when administered orally or intraperitoneally. Orally coadministered n‐3 PUFAs enhanced this effect, resulting in the significant suppression of DSS colitis after 7 days, and a remarkable recovery of colorectal mucosa was evident after 14 days of treatment. The amelioration of colon pathology co‐existed with a significant decrease in MPO expression, overexpression of iNOS and reduction of nuclear NF‐κB p65 in inflammatory cells, and the suppression of apoptosis in colonic epithelial cells. The simultaneous administration of Mesna and n‐3 PUFAs is particularly effective in ameliorating DSS colitis in rats, by reducing oxidative stress, inflammation and apoptosis, probably through a mechanism that involves the inhibition of NF‐κB and overexpression of iNOS.
Keywords: dextran sodium sulphate, iNOS, mesna, n‐3 PUFAs, NF‐κΒ, ulcerative colitis
Ulcerative colitis (UC), a form of inflammatory bowel disease, is a chronic idiopathic immune‐mediated disorder of the colon and rectum. In both humans and experimental models of UC, it is characterized by high levels of inducible nitric oxide synthase (iNOS) activity and increased nitric oxide (NO) and reactive oxygen species (ROS) production in the colon (Boughton‐Smith et al. 1993; Rachmilewitz et al. 1995; Ferreti et al. 1997; Sugimoto et al. 2002; Shusterman et al. 2003; Valko et al. 2007;). The role of iNOS and NO in experimental ulcerative colitis, however, has not been fully elucidated. Depending on the model system used and the chronicity of colitis, both detrimental and beneficial effects have been reported (Boughton‐Smith et al. 1993; Miller et al. 1993; Rachmilewitz et al. 1995; Beckman & Koppenol 1996; Ferreti et al. 1997; McCafferty et al. 1997; Kwiecien et al. 2002). The latter have been attributed, at least in part, to NO‐induced inhibition of nuclear factor‐κB (NF‐κB) translocation into the nuclei of cells and the blockade of superoxide release from neutrophils (Peng et al. 1995).
Current ulcerative colitis treatment schemes aim to reduce the detrimental inflammation in the colonic mucosa. Better known as a chemotherapy adjuvant, 2‐mercaptoethanesulphonic sodium (Mesna) is a drug with well‐known anti‐inflammatory, anti‐oxidative and anti‐apoptotic properties (Kabasakal et al. 2004; Sener et al. 2004, 2005; Ypsilantis et al. 2006, 2008). Mesna has been reported to neutralize ROS through sulphydryl group binding, downregulate NF‐κB and increase the expression of iNOS in the intestine (Shusterman et al. 2003; Ypsilantis et al. 2006, 2008). Although its effects interrelate with critical events of IBD pathogenesis, Mesna has rarely been tested in IBD preclinical models. Specifically, the only study that has used Mesna in this context previously, suggested that it suppresses trinitrobenzene sulphonic acid (TNBS) colitis in rats (Shusterman et al. 2003).
On the other hand, various natural products and ingredients, with antioxidant and anti‐inflammatory properties, including the n‐3 polyunsaturated fatty acids (n‐3 PUFAs), have been extensively used in experimental animals and ex vivo models of IBD (Tjonneland et al. 2009; Jantchou et al. 2010; Hou et al. 2011). According to a large body of preclinical evidence, n‐3 PUFAs scavenge the free radicals and intervene with the expression of several cytokines, transcription factors and enzymes that have been shown to play important roles in UC pathogenesis (Serhan & Savill 2005; Tjonneland et al. 2009; Hou et al. 2011). The clinical benefits of n‐3 PUFAs in human IBD remain largely unproven (MacLean et al. 2005; Ruggiero et al. 2009). Combining n‐3 PUFAs with other more effective treatments of IBD, however, may be a strategy that warrants further investigation.
In this study, we sought to examine the effect of the combined administration of the anti‐oxidant drug Mesna with n‐3 PUFAs (eicosapentaenoic – EPA and docosahexaenoic acid – DHA) on the outcome of dextran sodium sulphate (DSS)‐induced UC in rats. We found that the simultaneous administration of Mesna and n‐3 PUFAs was particularly effective in ameliorating DSS colitis in rats, by reducing oxidative stress, inflammation and colonic epithelial cell apoptosis. This beneficial effect coincided with the inhibition of nuclear NF‐κB p65 translocation and the overexpression of iNOS in inflammatory cells associating with ulcerative lesions in the colon.
Materials and methods
Animals
A total of one hundred (n = 100) 8‐ to 10‐week‐old male Wistar rats, weighing 230–280 gr, were used. The animals were housed in Makrolon cages (four per cage), in a temperature‐controlled environment (20–22°C) with an alternating cycle of 12‐h light and 12‐h dark. The access to standard rat diet (rat chow #510/EL.VI.Z, Platy, Greece) and water was free.
Ethical approval statement
Animal experiments were in accordance with directive 86/609/EEC, approved by the Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, and licenced by the National Veterinary Administration authorities (Licence No. 13/3470/26‐03‐2010). All applicable international, national, local and/or institutional guidelines governing the use of experimental animals were followed. The procedures have been approved by the appropriate regulatory body.
Experimental design
Animals were randomly assigned into 10 groups according to treatment. For the induction of colitis, 5% DSS (MW 36,000–50,000 kDa; MP Biomedicals Inc., Cleveland, OH, USA) was given in drinking water for 7 days. DSS was then removed from drinking water, and rats were further treated orally (PO), intraperitoneally (IP) or intrarectally (IR) for either 7 or 14 days with Mesna (ME), n3 PUFA's (Ω3) or both. PO treatments were performed once daily using an intragastric catheter. Mesna (Uromitexan®; Baxter Oncology Gmbh, Halle/Westfalen, Germany) was given at 400 mg/kg of body weight (BW). n‐3 PUFAs (Maxepa® Oil Seven Seas Limited, Feltham, Middlesex, England) containing 791 mg of eicosapentaenoic acid (EPA)+527 mg of docosahexaenoic acid (DHA)/5 ml were given at 2 g/kg of BW. Intrarectal injections were performed with a 6F catheter. Mesna was administered twice daily, at a dose of 100 mg/kg BW. n‐3 PUFAs were given once daily at a dose of 2 g/kg BW. Intraperitoneal injections of Mesna were performed once daily, using a 26G 5‐ml syringe, at a dose of 300 mg/kg BW. Rats were anesthetized with xylazine (10 mg/Kg BW), atropine (0.05 mg/Kg BW) and ketamine (90 mg/Kg BW) and subsequently euthanized with a sodium pentobarbital i.p. injection (90 mg/Kg BW) at the end of each treatment period at 7 or 14 days after the weekly cycle of DSS. Numbers of rats per experimental group for each time point were as follows: control (n = 5), DSS (n = 5), DSS+Ω3 P.O. (n = 5), DSS+ME P.O. (n = 5), DSS+Ω3 + ME P.O. (n = 5), DSS+Ω3 P.R. (n = 5), DSS+ME P.R. (n = 5), DSS+Ω3 + ME P.R. (n = 5), DSS+ME I.P. (n = 5), DSS+Ω3 P.O.+ME I.P. (n = 5).
Clinical findings and gross pathology assessments of colitis
The body weight of animals and clinical manifestations of colitis, such as diarrhoea and presence of occult blood in stools (Hemoccult® test; Beckman Coulter Ireland Inc., Mervue Business Park, Mervue Galway, Ireland), were recorded daily. The disease activity index (DAI) was calculated three times weekly, according to the methodology of (Cooper et al. 1993). At necropsy, the colon was removed, cut open, rinsed in normal saline and photographed using a digital camera Nikon D3200. The length of the entire colon was measured, and the macroscopic lesions of colonic mucosa were scored according to the criteria of (Morris et al. 1989).
Histopathology, Immunohistochemistry and Morphometry
For histologic evaluation, formalin‐fixed descending colon samples were embedded in paraffin, cut at 5 μm and stained with haematoxylin and eosin or immunohistochemistry (IHC). Mucosal/submucosal inflammation and loss of colonic epithelial integrity were scored histopathologically on a 0–3 ascending scale of extent and severity using the criteria which have been previously described in detail (Mähler et al. 1998). Primary antibodies for IHC included rabbit polyclonal antibodies against myeloperoxidase (ThermoFisher Scientific/Lab Vision, Fremont, CA, USA), iNOS (ThermoFisher Scientific/Lab Vision), cleaved caspase‐3 (Cell Signaling, Beverly, MA, USA) and rabbit monoclonal antibodies against NF‐κΒ p65 (Cell Signaling). Rabbit primary antibody binding was detected with goat anti‐rabbit polymer HRP (ZytoChem Plus, Berlin, Germany). Colour was developed with DAB substrate‐chromogen system (ThermoFisher Scientific/Lab Vision), and tissues were counterstained with haematoxylin. IHC and quantitative histomorphometry were performed as described previously (Karamanavi et al. 2014).
Statistical analyses
Body weights were analysed using one way analysis of variance (anova). Colon length, gross pathology and histopathological scores, and histomorphometrical data were compared with Mann–Whitney U‐analysis. DAI data were analysed by repeated measures anova. Statistical significance was set at P < 0.05. IBM SPSS Statistics (v.200; Chicago, IL, USA) was used for DAI analysis, and graphpad prism version 5.0 for windows (GraphPad software, San Diego, CA, USA) was used for remaining analyses.
Results
Mesna and n‐3 PUFAs improve the clinical outcome of DSS‐induced colitis
To assess whether the various dosing permutations of Mesna and n‐3 PUFAs affect the outcome of DSS colitis, we recorded critical clinical parameters of DSS colitis during the experiments on a daily basis. As expected, colitis had a detrimental effect on the body weight of rats (control vs. DSS, P < 0.0001). By comparing the body weight change of rats at three different time points, we found that various treatments worked to prevent the DSS colitis‐associated body weight loss (Table 1). Mesna given alone, especially via the i.p. route, proved to be particularly efficient in that regard (Table 1). To further elaborate on this result, we also assessed the disease activity (DAI) index, which combines scores of body weight loss, stool consistency and faecal blood (Cooper et al. 1993). We found that all treatments decreased significantly the DAI index at 14 days after DSS insult, with treatments involving p.o, and i.p. administration of Mesna inducing the most profound beneficial effect as early as 2 days after DSS (Table 2).
Table 1.
Effects of treatments on DSS‐induced body weight change. Values represent the mean ± SEM of body weight change. Negative values depict body weight loss. Values with different letters differ significantly (P < 0.05)
| Body weight (BW) change (gram) | |||
|---|---|---|---|
| At the end of DSS treatment | At 7 days after removal of DSS | At 14 days after removal of DSS | |
| Experimental Groups | |||
| DSS | −11.17 ± 1.51a,c | −26.2 ± 2.82a | −37.5 ± 5.08a,f |
| DSS + Ω3 p.o | −11.5 ± 3.17a,b,c,d | −13 ± 3a,b,c | 1 ± 2.92b,c,d,e,f,g |
| DSS + MESNAp.ο | −4.5 ± 2.83a,b,d | −6.5 ± 4.02b,d | 4 ± 3.32c,e,g |
| DSS + Ω3 p.o + MESNA p.o | −16 ± 4.7a,c | −16.5 ± 4.41a,b | 5 ± 3.87c,d,e,g |
| DSS + Ω3 p.r | −15.5 ± 3.02a,c | −11.5 ± 5.17a,b,d | −2 ± 6.44b,c,e,f,g |
| DSS + MESNA p.r | −8 ± 3a,b,c | 0 ± 2.36b,c,d | 7 ± 3.74e,g |
| DSS + Ω3 p.r + MESNA p.r | −19 ± 3.15c | −32 ± 10.55a | −31 ± 14.09f,g |
| DSS + MESNAi.p | 1 ± 3.23b,d | 10.5 ± 4.25d | 20 ± 5.24b, c,e,g |
| DSS + Ω3 p.o + MESNA i.p | −19.5 ± 2.63a,c | −20.5 ± 3.53a,b | −4 ± 1.87 g |
| P value | <0.0001 | <0.0001 | <0.0001 |
Table 2.
Disease activity index (DAI) scores at different time points according to treatment. Values represent mean score. Values with different letters differ significantly (P < 0.05)
| Treatments | Days after removal of DSS | ||||
|---|---|---|---|---|---|
| 2 | 7 | 10 | 12 | 14 | |
| DSS | 10.2e | 8.7e | 8.4c | 7.4c | 6.5b |
| DSS + Ω3 P.O. | 7.7c,d | 4.7b,c | 1.0a | 0.6a | 0.2a |
| DSS + MESNA P.O. | 5.7b | 3.0b | 0.8a | 0.3a | 0.2a |
| DSS + Ω3 P.O. + MESNA P.O. | 8.7d,e | 5.8c,d | 3.0a | 1.6a,b | 0.6a |
| DSS + Ω3 P.R. | 8.3c,d,e | 6.1c,d | 4.2a,b | 3.2a,b | 2.0a |
| DSS + MESNA P.R. | 7.9c,d | 5.2b, c | 2.4a | 0.4a | 0.0a |
| DSS + Ω3 P.R. + MESNA P.R. | 9.0d,e | 7.8d,e | 7.0b, c | 4.5b,c | 2.8a |
| DSS + MESNA I.P. | 2.9a | 0.2a | 0.4a | 0.2a | 0.0a |
| DSS + Ω3 P.O. + MESNA I.P. | 6.4b,c | NA | 2.4a | 2.0a,b | 0.6a |
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Mesna and n‐3 PUFAs decrease gross pathology indices of DSS colitis
Having determined that treatment with Mesna and n‐3 PUFAs accelerated the recovery of rats from clinical symptoms of colitis, we next examined the macroscopical pathology of the colon. Using a well‐accepted gross colonic pathology scoring scheme (Morris et al. 1989) we found that treatments ameliorated the typical macroscopical lesions of DSS colitis, such as oedema, hyperaemia, epithelial erosions, mucosal ulcers and haemorrhages, at 7 and 14 days after DSS. However, this effect reached statistical significant levels only in the regimens that included Mesna (Figure 1). Colon length reduction, a well‐described outcome of DSS colitis, has been widely used as an indirect indicator of chronic colonic injury. Indeed, as early as 7 days after the end of DSS administration, the colon length of DSS‐treated rats different significantly from that of the untreated controls (control vs. DSS, P < 0.05). Analysing the colon length data from all experimental groups suggested that both Mesna and n‐3 PUFAs worked to preserve normal colon length, an outcome that was most evident at day 14 after removing DSS from animals (Figure 1).
Figure 1.
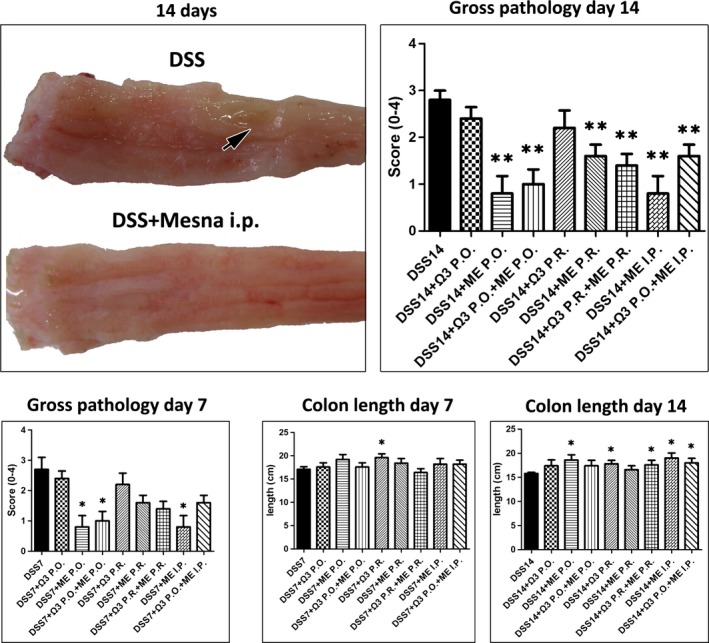
Mesna and n‐3 PUFAs treatment effects on the gross pathology of DSS colitis. Representative macroscopical lesions in the last part of descending colon and rectum of rats at fourteen days after the termination of DSS administration. By contrast to the colonic mucosa from a rat treated with Mesna, the colonic mucosa of the colitic control has a rough appearance and a well‐sized ulcerative lesion (arrow). Treatments including Mesna significantly decrease the gross pathology score of colitis at the time points examined. The administration of n‐3 PUFAs P.R. alone and treatments including Mesna prevent shortening of colon at statistically significant levels. Numbers on the y‐axis of bar graphs correspond to the mean ± SEM of the parameter assessed; asterisks denote statistical significance between each experimental group with the control colitic rats (black bar); *P < 0.05, **P < 0.01.
Mesna and n‐3 PUFAs promote healing of DSS‐induced mucosal injury and ameliorate colonic inflammation
Upon histopathological examination, the colon of control rats was normal and that of DSS‐treated animals had the typical features of DSS‐induced ulcerative colitis (Mähler et al. 1998; Karamanavi et al. 2014). Briefly, the colon of DSS‐exposed rats had multiple mucosal ulcers at various stages of re‐epithelialization and healing, with underlying pyogranulomatous tissue beds of variable maturity regarding fibrosis and neovascularization. The remaining colonic mucosa and submucosa were infiltrated multifocally by mononuclear cells and neutrophils. Crypt distortion, regenerative epithelial hyperplasia and reactive atypia with goblet cell loss were often seen. By comparing the histopathological scores of mucosal erosion and ulceration between groups, we found that both Mesna and n‐3 PUFAs contributed significantly to the restoration of mucosal architecture at 7 and 14 days after the DSS time points (Figure 2a). Likewise, the analysis of histopathological scores of inflammation in non‐ulcerated areas of colonic mucosa suggested that the regimens used significantly reduced colonic inflammation. However, the anti‐inflammatory effect primarily correlated with Mesna and not with n‐3 PUFAs treatment (Figure 2b).
Figure 2.
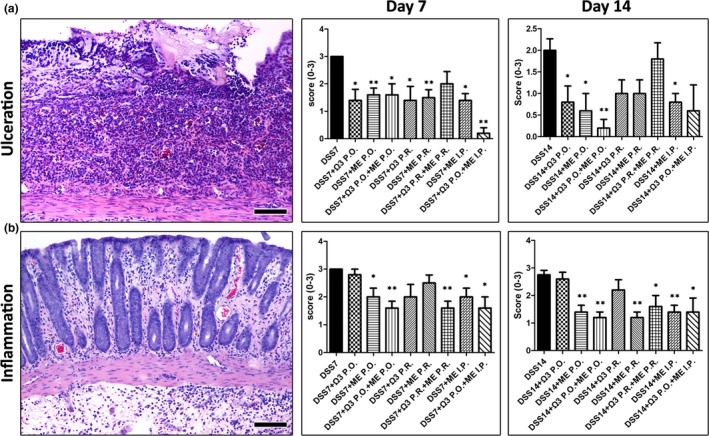
Mesna and n‐3 PUFAs ameliorate the histopathological lesions of DSS colitis. (a) Typical DSS‐induced ulcer from control colitic rat at 7 days after the removal of DSS. There is a granular tissue ulcer bed covered by necrotic debris and absent surface epithelium. Histopathological scores for the loss of epithelial integrity at 7 and 14 days after DSS highlight the beneficial effects of Mesna and n‐3 PUFAs treatments. (b) Non‐ulcerated colonic mucosa from control colitic rat showing colitis with mucosal and submucosal oedema, inflammatory cell infiltration and hyperaemia. Histopathological scores of inflammation suggest that treatments containing Mesna suppress colonic inflammation. Haematoxylin and eosin. Scale bars: 100 μm. Numbers on the y‐axis of bar graphs correspond to the mean ± SEM of histopathological scores. The asterisks indicate statistical significance between each experimental group with the control colitic rat group (black bar); *P < 0.05, **P < 0.01.
Mesna modulates critical inflammatory cells and factors and suppresses colonic epithelial cell apoptosis
Having determined that Mesna was the primary factor which downregulated colonic inflammation, we next analysed colonic mucosal ulcers for known Mesna‐associated anti‐inflammatory effects (Shusterman et al. 2003; Kabasakal et al. 2004; Sener et al. 2004, 2005; Ypsilantis et al. 2006, 2008). For that, we probed the presence of neutrophils and the expression of iNOS and NF‐κΒ p65 by immunohistochemistry. Quantitative histomorphometry of MPO‐positive cells (neutrophils) in the colonic ulcers of DSS‐treated experimental groups of rats revealed that treatments involving Mesna significantly downregulated neutrophils. This effect was not observed in rats treated with n‐3 PUFAs alone (Figure 3a). However, n‐3 PUFAs acted synergistically with Mesna to further reduce neutrophil numbers (DSS+ME P.O. vs. DSS+Ω3 + ME P.O., P < 0.001 and DSS+ME I.P. vs. DSS+Ω3 P.O.+ME I.P., P < 0.001 at 7 days post‐DSS). Likewise, treatments containing Mesna, but not n‐3 PUFAs alone, were able to significantly upregulate iNOS‐positive cells (Figure 3b) and reduce nuclear localization of NF‐κΒ p65 (Figure 4a) in the wound beds of colonic ulcers.
Figure 3.
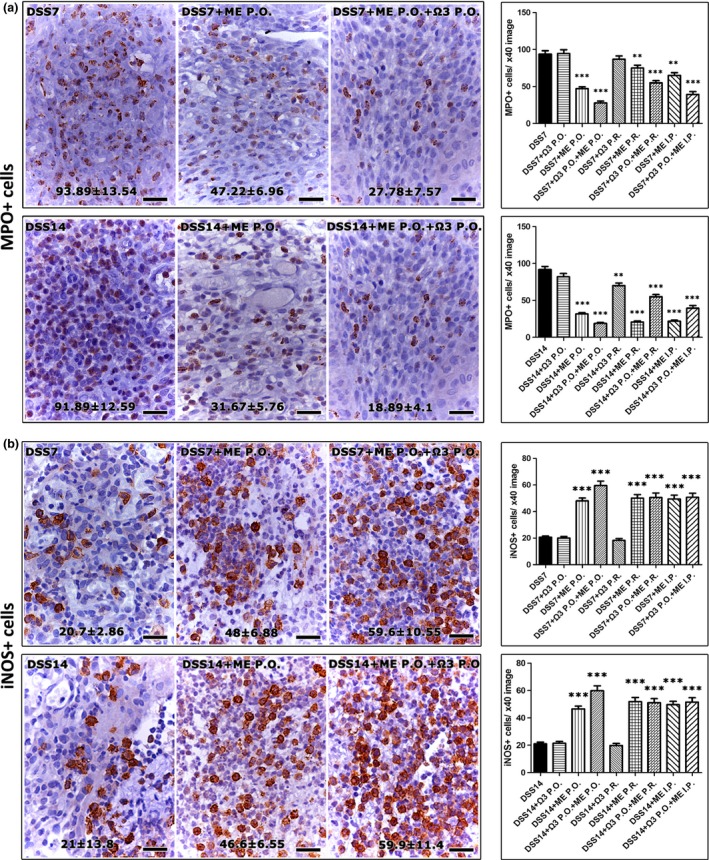
Mesna downregulates neutrophils and upregulates iNOS in the DSS‐induced colonic ulcers. (a) Immunohistochemically labelled MPO+ cells (neutrophils) at the pyogranulomatous tissue of colonic mucosa ulcer beds. Treatment schemes containing Mesna decrease neutrophils at statistically significant levels. (b) iNOs‐specific immunohistochemistry of DSS‐induced ulcers. Mesna administration correlates with a significant increase of i‐NOS positive cells. IHC, diaminobenzidine chromogen, haematoxylin counterstain. Scale bars: 25 μm. Numbers in the bottom of figures and the y‐axis of bar graphs depict the mean ± SEM of IHC‐labelled cells in high‐power magnification images. The asterisks indicate statistical significance between each experimental group with the control colitic rat group (black bar); **P < 0.01, ***P < 0.001.
Figure 4.
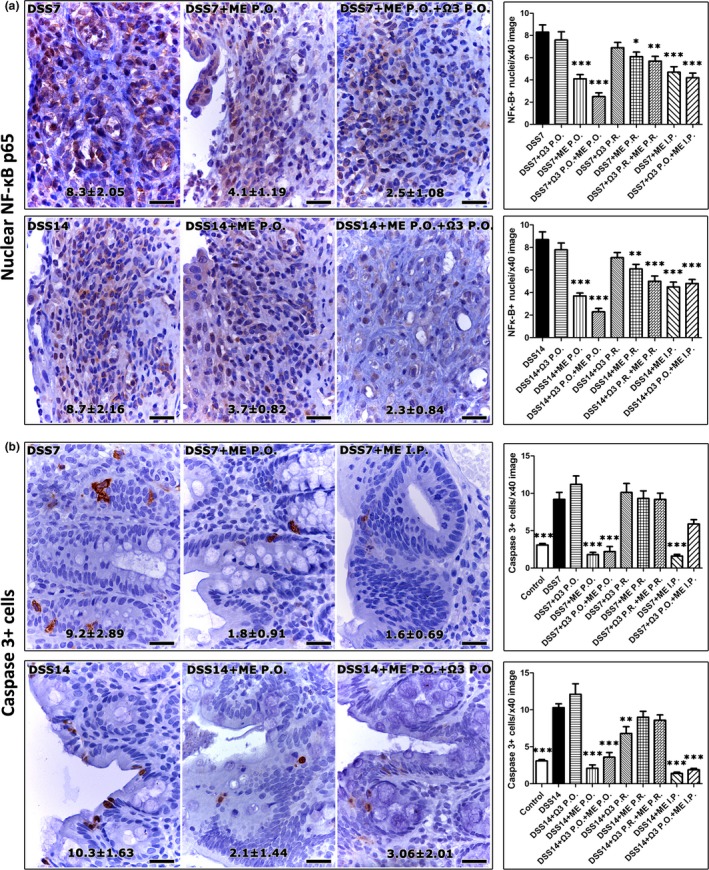
Mesna decreases the nuclear translocation of NF‐κΒ p65 in the DSS‐induced colonic ulcers and suppresses epithelial cell apoptosis in non‐ulcerated areas. (a) A fraction of the inflammatory cells populating the granulomatous tissue of ulcer beds show positive nuclear immunoreactivity for NF‐κΒ p65. The statistical comparison of histomorphometric counts suggests that Mesna reduces the number of cells with nuclear NF‐κΒ p65 at statistically significant levels. (b) Apoptotic epithelial cells have cytoplasmic cleaved caspase‐3. The analysis of morphometric counts shows that the effect of Mesna in reducing colonic epithelial cell apoptosis is significant. IHC, diaminobenzidine chromogen, haematoxylin counterstain. Scale bars: 25 μm. Numbers in the bottom of figures and the y‐axis of bar graphs depict the mean ± SEM of IHC‐labelled cells in high‐power magnification images. The asterisks indicate statistical significance between each experimental group with the control colitic rat group (black bar); *P < 0.05, **P < 0.01, ***P < 0.001.
Mesna has been previously reported to affect epithelial cell apoptosis (Ypsilantis et al. 2004, 2006, 2008). Therefore, we next examined apoptotic cells in the non‐ulcerated areas of the colonic mucosa of rats, by immunohistochemistry. As expected, the DSS‐treated colitic controls had significantly increased (P < 0.001) colonic epithelial cell apoptosis compared to the untreated controls at both time points examined. The colonic mucosa of colitic rats treated with Mesna, however, had significantly less apoptotic cells compared to colitic apoptotic controls, matching apoptotic cell levels of non‐DSS‐treated control rats. The administration of n‐3 PUFAs alone did not reduce the DSS‐associated colonic epithelial cell apoptosis (Figure 4b).
Discussion
In the present study, we demonstrate that a combined treatment using Mesna and n‐3 PUFAs improved the outcome of DSS‐induced ulcerative colitis in rats. By testing different routes of administration and schemes, we found that p.o. and i.p. dosing of Mesna was more efficient compared to topical p.r. treatment. Also, although n‐3 PUFAs are not sufficient to improve colitis when given alone, they work synergistically with Mesna to enhance its beneficial effects.
ROS have a pivotal role in the pathogenesis of human IBD, including UC (Pavlick et al. 2002; Rezaie et al. 2007; Zhu & Li 2012). Excessive levels of ROS have been found in the circulating leucocytes and colorectal specimens of patients with UC (Grisham & Granger 1988; Williams et al. 1990; D'Odorico et al. 2001). The overproduction of ROS also characterizes experimental colitis, including that induced by DSS in rodents (Keshavarzian et al. 1990, 1992; Nieto et al. 2000). As Mesna primarily acts as a scavenger of ROS (Kabasakal et al. 2004; Sener et al. 2005), our results showing its beneficial effects in ulcerative colitis were not surprising. Our finding matches Shusterman et al.'s previous study (2003) which showed that Mesna attenuates colitis induced by the colitogenic substance trinitrobenzene sulphonic acid (TNBS) in rats. In that report, Mesna was given intrarectally immediately after the i.r. administration of TNBS. The results presented here strengthen the proposed therapeutic role of Mesna in colitis by showing its beneficial effects in a different model system, where colitis is induced by DSS. Further, they provide additional important clues for treatment of colitis with Mesna. First, they suggest that although the topical colonic mucosa treatment with Mesna given p.r. attenuates colitis as previously reported (Shusterman et al. 2003), p.o. and i.p. administration routes are significantly superior in that regard. Second, they show that Mesna is not only efficient in blocking the initiation of colitis (Shusterman et al. 2003), but also may attenuate established colonic lesions as well. Consistently with these findings in rodent models of chemically induced colitis, Mesna has been reported to protect the intestinal epithelium against cis‐platinum‐ (Allan et al. 1986) or ifosfamide‐ (Ypsilantis et al. 2004) related toxicity and ischaemia–reperfusion mucosal injury (Ypsilantis et al. 2006, 2008).
In our study, we find that Mesna treatment significantly decreased MPO+ neutrophils in the bed of colonic ulcers, which is in line with previous reports showing that Mesna decreases MPO activity in TNBS colitis of rats (Shusterman et al. 2003). A timely clearance of acute phase neutrophils is necessary for normal wound healing (Savill 1997, 2001; Lerman & Kim 2015). Therefore, the effect of Mesna on reducing neutrophils in mucosal ulcers may have contributed to the overall improved outcome of DSS colitis. In addition to ROS reduction, Mesna has been linked with the downregulation of NF‐κB activity in the intestine and other tissues (Bubici et al. 2006; Gloire et al. 2006; Ypsilantis et al. 2008). NF‐κB is one of the most powerful and pleiotropic regulators of inflammatory processes (Schreiber et al. 1998; Atreya et al. 2008; Wullaert 2010). In our study, the Mesna‐treated rats showed reduction of the nuclear translocation of NF‐κΒ p65 in the inflammatory component of colonic ulcers. As the overproduction of ROS interrelates with NF‐κΒ activation (Bubici et al. 2006; Gloire et al. 2006), it is not possible to tell which of these two Mesna‐related effects came first in the mechanism of suppression of colonic inflammation. Answering this question warrants further studies that may provide important clues with broader relevance in the field of mucosal inflammation.
An interesting finding of our study is that the beneficial effects of Mesna co‐exist with increased numbers of iNOS‐positive cells in ulcerative colitis lesions. Although this observation matches similar findings in the TNBS‐induced colitis of rats (Shusterman et al. 2003), the role of iNOS in colitis is controversial (Boughton‐Smith et al. 1993; Miller et al. 1993; Rachmilewitz et al. 1995; Beckman & Koppenol 1996; Ferreti et al. 1997; McCafferty et al. 1997; Kwiecien et al. 2002). A great body of literature in the field suggests that increased iNOS activity leading to overproduction of NO has deleterious effects on colonic mucosa and contributes to colonic injury (Miller et al. 1993; Pfeiffer & Qiu 1995; Rachmilewitz et al. 1995; Videla et al. 2007; Farghaly & Thabit 2014). Indeed, iNOS inhibitors suppressed colitis in various different animal models of IBD including the acetic acid and TNBS models (Pfeiffer & Qiu 1995; McCafferty et al. 1997; Farghaly & Thabit 2014). The detrimental effects of iNOS‐derived NO on colonic tissue are thought to be mediated by the activation of NF‐κΒ (Peng et al. 1995; Kang et al. 2000; Andresen et al. 2005). However, this is in contradiction with our findings showing that increased iNOS coincides with reduced NF‐κB activation to confer attenuation of colitis after Mesna treatment. Our findings, however, agree with other studies proposing that iNOS and NO ameliorate the acute phase of intestinal inflammation (McCafferty et al. 1997, 1999; Shusterman et al. 2003; Ohtake et al. 2010; Jädert et al. 2013). One possible explanation between discrepant results on the role of iNOS in ulcerative colitis lies in the cellular source of iNOS examined in each case. In our study, we probe iNOS expression in the ulcer‐associated inflammatory cells. iNOS‐positive leucocytes, such as neutrophils, produce NO that combines with superoxide to form peroxynitrite (Rachmilewitz et al. 1993). In situ detection of this particular toxic molecule localized it primarily in close proximity to oxidant producing inflammatory cells (Dijkstra et al. 1998). In the case of our study, however, Mesna‐treated rats may be protected from the production of peroxynitrite. Despite the high levels of iNOS‐positive cells in ulcers, Mesna‐induced exhaustion of superoxide (Gressier et al. 1994; Shusterman et al. 2003; Kabasakal et al. 2004; El‐Medany et al. 2005) may preclude the formation of peroxynitrite. Thus, Mesna‐treated rats may benefit from NO‐regulated mucosal healing, without the concomitant negative effects of peroxynitrite cell toxicity.
One protective effect of NO on colonic epithelial cells may relate to apoptosis. Indeed, NO has been previously shown to protect several different cell types from apoptosis induced by oxidative stress (Kim et al. 1999; Fiorucci et al. 2001). This may explain the Mesna‐associated reduced colonic epithelial cell apoptosis observed in our study. However, the overall suppression of mucosal inflammation and reduction of ROS at mucosal surface (Ypsilantis et al. 2006, 2008) due to Mesna treatment may also explain this finding.
There is a growing body of evidence supporting the antioxidant and anti‐inflammatory effect of n‐3 PUFAs, such as the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Serhan & Savill 2005; Calder 2006a,b, 2008; Fetterman & Zdanowicz 2009; Tjonneland et al. 2009; Hou et al. 2011). The anti‐inflammatory effect of n‐3 PUFAs was investigated in both human clinical trials and animal models of colitis with conflicting results (Yuceyar et al. 1999; Nieto et al. 2002; Andoh et al. 2003; Serhan & Savill 2005; Calder 2006a,b; Chapkin et al. 2007; Tjonneland et al. 2009; Hou et al. 2011; Marion‐Letellier et al. 2013) In DSS‐induced colitis, n‐3 PUFAs have been reported to have both ameliorating and aggravating effects (Hokari et al. 2013; Tyagi et al. 2014). This discrepancy is most probably due to the amount of n‐3 PUFAs administered, as too much dietary fat could be harmful in colitis even if the type of fat is n‐3 PUFAs (Hokari et al. 2013). The dosing scheme containing EPA and DHA we administered in our study, when given alone, had a positive effect in the restoration of colonic epithelial integrity. However, it did not alter indices of inflammation and epithelial cell apoptosis. Nonetheless, n‐3 PUFAs when combined with Mesna acted synergistically and enhanced its overall beneficial effect.
In a ROS‐rich inflammatory environment as in DSS colitis, the lipid peroxidation of n‐3 PUFAs may result in the production of toxic radicals that could contribute further to colonic mucosa damage (Nieto et al. 1998; Shimizu et al. 2001). In the therapeutic regimen used here, Mesna, a known anti‐oxidant, may have blocked lipid peroxidation, thus preventing potential negative side effects of n‐3 PUFAs.
The present study taken together with previous reports suggests that the co‐administration of Mesna and n‐3 PUFAs could be considered as a novel complement to current UC management.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Michael Doulberis for technical assistance with histology and immunohistochemistry. We are also grateful to Professors Zaphiris Abas (†1966–2013) and Paschalis Fortomaris and Dr. George Papadopulos for help with statistical analyses.
References
- Allan S.G., Smyth J.F., Hay F.G., Leonard R.C. & Wolf C.R. (1986) Protective effect of sodium‐2‐mercaptoethanesulfonate on the gastrointestinal toxicity and lethality of cis‐ diamminedichloroplatinum. Cancer Res. 46, 3569–3573. [PubMed] [Google Scholar]
- Andoh A., Tsujikawa T., Ishizuka I. et al (2003) N‐3 fatty acid‐rich diet prevents early response of interleukin‐6 elevation in trinitrobenzene sulfonic acid‐induced enteritis. Int. J. Mol. Med. 12, 721–725. [PubMed] [Google Scholar]
- Andresen L., Jørgensen V.L., Perner A., Hansen A., Eugen‐Olsen J., Rask‐Madsen J. (2005) Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 54, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya I., Atreya R. & Neurath M.F. (2008) NF‐kappaB in inflammatory bowel disease. J. Intern. Med. 263, 591–596. [DOI] [PubMed] [Google Scholar]
- Beckman J.S. & Koppenol W.H. (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271(5 Pt 1), C1424–C1437. [DOI] [PubMed] [Google Scholar]
- Boughton‐Smith N.K., Evans S.M., Hawkey C.J. et al (1993) Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet 342, 338–340. [DOI] [PubMed] [Google Scholar]
- Bubici C., Papa S., Dean K. & Franzoso G. (2006) Mutual cross‐talk between reactive oxygen species and nuclear factor‐kappa B: molecular basis and biological significance. Oncogene 25, 6731–6748. [DOI] [PubMed] [Google Scholar]
- Calder P.C. (2006a) Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 75, 197–202. [DOI] [PubMed] [Google Scholar]
- Calder P.C. (2006b) n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83, 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- Calder P.C. (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 52, 885–897. [DOI] [PubMed] [Google Scholar]
- Chapkin R.S., Davidson L.A., Ly L., Weeks B.R., Lupton J.R., McMurray D.N. (2007) Immunomodulatory effects of (n‐3) fatty acids: putative link to inflammation and colon cancer. J. Nutr. 137, 200S–204S. [DOI] [PubMed] [Google Scholar]
- Cooper H.S., Murthy S.N.S., Shah R.S. & Sedergran D.J. (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249. [PubMed] [Google Scholar]
- Dijkstra G., Moshage H., van Dullemen H.M. et al (1998) Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J. Pathol. 186, 416–421. [DOI] [PubMed] [Google Scholar]
- D'Odorico A., Bortolan S., Cardin R. et al (2001) Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand. J. Gastroenterol. 36, 1289–1294. [DOI] [PubMed] [Google Scholar]
- El‐Medany A., Hagar H.H., Moursi M., At Muhammed R., El‐Rakhawy F.I., El‐Medany G. (2005) Attenuation of bleomycin‐induced lung fibrosis in rats by Mesna. Eur. J. Pharmacol. 509, 61–70. [DOI] [PubMed] [Google Scholar]
- Farghaly H.S. & Thabit R.H. (2014) L‐arginine and aminoguanidine reduce colonic damage of acetic acid‐induced colitis in rats: potential modulation of nuclear factor‐κB/p65. Clin. Exp. Pharmacol. Physiol. 41, 769–779. [DOI] [PubMed] [Google Scholar]
- Ferreti M., Gionchette P., Rizzello F. et al (1997) Intracolonic release of nitric oxide during trinitrobenzenesulfonic acid rat colitis. Dig. Dis. Sci. 42, 2606–2611. [DOI] [PubMed] [Google Scholar]
- Fetterman J.W. Jr, Zdanowicz M.M. (2009) Therapeutic potential of n‐3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 66, 1169–1179. [DOI] [PubMed] [Google Scholar]
- Fiorucci S., Distrutti E., Ajuebor M.N. et al (2001) NO–mesalamine protects colonic epithelial cells against apoptotic damage induced by proinflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G654–G665. [DOI] [PubMed] [Google Scholar]
- Gloire G., Legrand‐Poels S. & Piette J. (2006) NF‐kappa B activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72, 1493–1505. [DOI] [PubMed] [Google Scholar]
- Gressier B., Cabanis A., Lebegue S. et al (1994) Decrease of hypochlorous acid and hydroxyl radical generated by stimulated human neutrophils: comparison in vitro of some thiol‐containing drugs. Methods Find. Exp. Clin. Pharmacol. 16, 9–13. [PubMed] [Google Scholar]
- Grisham M.B. & Granger D.N. (1988) Neutrophil‐mediated mucosal injury. Role of reactive oxygen metabolites. Dig. Dis. Sci. 33, 6S–15S. [DOI] [PubMed] [Google Scholar]
- Hokari R., Matsunaga H. & Miura S. (2013) Effect of dietary fat on intestinal inflammatory diseases. J. Gastroenterol. Hepatol. 28, 33–36. [DOI] [PubMed] [Google Scholar]
- Hou J.K., Abraham B. & El‐Serag H. (2011) Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am. J. Gastroenterol. 106, 563–573. [DOI] [PubMed] [Google Scholar]
- Jädert C., Phillipson M., Holm L., Lundberg J.O., Borniquel S. (2013) Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox. Biol. 2, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantchou P., Morois S., Clavel‐Chapelon F., Boutron‐Ruault M.C. & Carbonnel F. (2010) Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 105, 2195–2201. [DOI] [PubMed] [Google Scholar]
- Kabasakal L., Sehirli A.O., Cetinel S., Cikler E., Gedik N. & Sener G. (2004) Mesna (2‐mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 75, 2329–2340. [DOI] [PubMed] [Google Scholar]
- Kang J.L., Lee K., Castranova V. (2000) Nitric oxide up‐regulates DNA‐binding activity of nuclear factor‐kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol. Cell. Biochem. 215, 1–9. [DOI] [PubMed] [Google Scholar]
- Karamanavi E., Angelopoulou K., Lavrentiadou S. et al (2014) Urokinase‐type plasminogen activator deficiency promotes neoplasmatogenesis in the colon of mice. Transl. Oncol. 7, 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A., Morgan N.G., Sedghi S., Gordon J.H. & Doria M. (1990) Role of reactive oxygen metabolites in experimental colitis. Gut 31, 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A., Doria M.I., Sedghi S. et al (1992) Mitomycin‐C‐induced colitis in rats: a new animal model of acute colonic inflammation implicating reactive oxygen species. J. Lab. Clin. Med. 120, 778–791. [PubMed] [Google Scholar]
- Kim Y.M., Chung H.T., Kim S.S. et al (1999) Nitric oxide protects PC12 cells from serum deprivation‐induced apoptosis by cGMP‐dependent inhibition of caspase signaling. J. Neurosci. 19, 6740–6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien S., Brzozowski T., Konturek P. & Konturek S.J. (2002) The role of reactive oxygen species in action of nitric oxide‐donors on stress‐induced gastric mucosal lesions. J. Physiol. Pharmacol. 53, 761–773. [PubMed] [Google Scholar]
- Lerman Y.V. & Kim M. (2015) Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Drug Targets. 15, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C.H., Mojica W.A., Newberry S.J. et al (2005) Systematic review of the effects of n‐3 fatty acids in inflammatory bowel disease. Am. J. Clin. Nutr. 82, 611–619. [DOI] [PubMed] [Google Scholar]
- Mähler M., Bristol I.J., Leiter E.H. et al (1998) Differential susceptibility of inbred mouse strains to dextran sulfate sodium‐induced colitis. Am. J. Physiol. 274, G544–G551. [DOI] [PubMed] [Google Scholar]
- Marion‐Letellier R., Savoye G., Beck P.L., Panaccione R. & Ghosh S. (2013) Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm. Bowel Dis. 19, 650–661. [DOI] [PubMed] [Google Scholar]
- McCafferty D., Mudgett J.S., Swain M.G. & Kubes P. (1997) Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 112, 1022–1027. [DOI] [PubMed] [Google Scholar]
- McCafferty D.M., Miampamba M., Sihota E., Sharkey K.A., Kubes P. (1999) Role on inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut 45, 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Chotinaruemol S., Sadowska‐Krowicka H. et al (1993) Nitric oxide: the Jekyll and Hyde of gut inflammation. Agents Actions Suppl. 39, C180–C182. [DOI] [PubMed] [Google Scholar]
- Morris G.P., Beck P.L., Herridge M.S., Depew W.T., Szewczuk M.R. & Wallace J.L. (1989) Hapten‐induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96, 795–803. [PubMed] [Google Scholar]
- Nieto N., Fernandez M.I., Torres M.I., Rios A., Suarez M.D. & Gil A. (1998) Dietary monounsaturated n‐3 and n‐6 long‐chain polyunsaturated fatty acids affect cellular antioxidant defense system in rats with experimental ulcerative colitis induced by trinitrobenzene sulfonic acid. Dig. Dis. Sci. 43, 2676–2687. [DOI] [PubMed] [Google Scholar]
- Nieto N., Torres M.I., Fernández M.I. et al (2000) Experimental ulcerative colitis impairs antioxidant defense system in intestine. Dig. Dis. Sci. 45, 1820–1827. [DOI] [PubMed] [Google Scholar]
- Nieto N., Torres M., Rios A., Gil A. (2002) Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J. Nutr. 132, 11–19. [DOI] [PubMed] [Google Scholar]
- Ohtake K., Koga M., Uchida H. et al (2010) Oral nitrite ameliorates dextran sulfate sodium‐induced acute experimental colitis in mice. Nitric Oxide 23, 65–73. [DOI] [PubMed] [Google Scholar]
- Pavlick K.P., Laroux F.S., Fuseler J. et al (2002) Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 33, 311–322. [DOI] [PubMed] [Google Scholar]
- Peng H.B., Libby P. & Liao J. (1995) Induction and stabilization of I‐κΒ by nitric oxide mediates inhibition of NF‐κΒ. J. Biol. Chem. 270, 214–219. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C.J. & Qiu B.S. (1995) Effects of chronic nitric oxide synthase inhibition on TNB‐induced colitis in rats. J. Pharm. Pharmacol. 47, 827–832. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J.S., Karmeli .F. et al (1993) Peroxynitrite‐induced rat colitis—a new model of colonic inflammation. Gastroenterology 105, 1681–1688. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J.S., Bachwich D., Karmeli F., Ackerman Z. & Podolsky D.K. (1995) Enhanced colonic nitric oxide generation and stimulated nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut 36, 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie A., Parker R.D. & Abdollahi M. (2007) Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 52, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Ruggiero C., Lattanzio F., Lauretani F., Gasperini B., Andres‐Lacueva C. & Cherubini A. (2009) Omega‐3 polyunsaturated fatty acids and immune‐mediated diseases: inflammatory bowel disease and rheumatoid arthritis. Curr. Pharm. Des. 15, 4135–4148. [DOI] [PubMed] [Google Scholar]
- Savill J. (1997) Apoptosis in resolution of inflammation. J. Leukoc. Biol. 61, 375–380. [DOI] [PubMed] [Google Scholar]
- Savill J. (2001) Apoptosis in post‐streptococcal glomerulonephritis. Kidney Int. 60, 1203–1214. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S. & Hampe J. (1998) Activation of nuclear factor kappa B in inflammatory bowel disease. Gut 42, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener G., Sehirli O., Erkanli G., Cetinel S., Gedik N. & Yeğen B. (2004) 2‐Mercaptoethane sulfonate (MESNA) protects against burn‐induced renal injury in rats. Burns 30(6), 557–564. [DOI] [PubMed] [Google Scholar]
- Sener G., Sehirli O., Cetinel S., Yegen B.G., Gedik N. & Ayanoglu‐Dulger G. (2005) Protective effects of MESNA (2‐mercaptoethane sulphonate) against acetaminophen‐induced hepatorenal oxidative damage in mice. J. Appl. Toxicol. 25, 20–29. [DOI] [PubMed] [Google Scholar]
- Serhan C.N. & Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Igarashi J., Ohtuka Y., Oguchi S., Kaneko K. & Yamashiro Y. (2001) Effects of n‐3 polyunsaturated fatty acids and vitamin E on colonic mucosal leukotriene generation, lipid peroxidation, and microcirculation in rats with experimental colitis. Digestion 63, 49–54. [DOI] [PubMed] [Google Scholar]
- Shusterman T., Sela S., Cohen H., Kristal B., Sbeit W. & Reshef R. (2003) Effect of the antioxidant Mesna (2–mercaptoethane sulfonate) on experimental colitis. Dig. Dis. Sci. 48, 1177–1185. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Hanai H., Tozawa K. et al (2002) Curcumin prevents and ameliorates trinitrobenzene sulfonic acid–induced colitis in mice. Gastroenterology 123, 1912–1922. [DOI] [PubMed] [Google Scholar]
- Tjonneland A., Overvad K., Bergmann M.M. et al (2009) Linoleic acid, a dietary n‐6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case‐control study within a European prospective cohort study. Gut 58, 1606–1611. [DOI] [PubMed] [Google Scholar]
- Tyagi A., Kumar U., Santosh V.S., Reddy S., Mohammed S.B. & Ibrahim A. (2014) Partial replacement of dietary linoleic acid with long chain n‐3 polyunsaturated fatty acids protects against dextran sulfate sodium‐induced colitis in rats. Prostaglandins Leukot. Essent. Fatty Acids 91, 289–297. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M. & Telser J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84. [DOI] [PubMed] [Google Scholar]
- Videla S., Vilaseca J., Medina C. et al (2007) Modulatory effect of nitric oxide on mast cells during induction of dextran sulfate sodium colitis. Dig. Dis. Sci. 52, 45–51. [DOI] [PubMed] [Google Scholar]
- Williams J.G., Hughes L.E. & Hallett M.B. (1990) Toxic oxygen metabolite production by circulating phagocytic cells in inflammatory bowel disease. Gut 31, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A. (2010) Role of NF‐kappaB activation in intestinal immune homeostasis. Int. J. Med. Microbiol. 300, 49–56. [DOI] [PubMed] [Google Scholar]
- Ypsilantis P., Tentes I., Assimakopoulos S.F., Kortsaris A., Scopa C.D. & Simopoulos C. (2004) Mesna ameliorates intestinal mucosa damage after ifosfamide administration in the rabbit at a dose related manner. J. Surg. Res. 121, 84–91. [DOI] [PubMed] [Google Scholar]
- Ypsilantis P., Lambropoulou M., Tentes I., Kortsaris A., Papadopoulos N. & Simopoulos C. (2006) Mesna protects intestinal mucosa from ischemia‐reperfusion injury. J. Surg. Res. 134, 278–284. [DOI] [PubMed] [Google Scholar]
- Ypsilantis P., Tentes I., Lambropoulou M. et al (2008) Prophylaxis with Mesna prevents oxidative stress induced by ischemia‐reperfusion in the intestine via inhibition of nuclear factor‐kB activation. J. Gastroenterol. Hepatol. 23, 328–335. [DOI] [PubMed] [Google Scholar]
- Yuceyar H., Ozutemiz O., Huseyinov A. et al (1999) Is administration of n‐3 fatty acids by mucosal enema protective against trinitrobenzene‐induced colitis in rats? Prostaglandins Leukot. Essent. Fatty Acids 61, 339–345. [DOI] [PubMed] [Google Scholar]
- Zhu H. & Li Y.R. (2012) Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp. Biol. Med. (Maywood). 237, 474–480. [DOI] [PubMed] [Google Scholar]


