Summary
Acute lung injury (ALI) is one of the most serious complications in traumatic patients and is an important part of multiple organ dysfunction syndrome (MODS). Recombinant human brain natriuretic peptide (rhBNP) is a peptide with a wide range of biological activity. In this study, we investigated local changes in oxidative stress and the NF‐κB‐dependent matrix metalloproteinase‐9 (MMP‐9) pathway in rats with trauma/haemorrhagic shock (TH/S)‐induced ALI and evaluated the effects of pretreatment with rhBNP. Forty‐eight rats were randomly divided into four groups: sham operation group, model group, low‐dosage rhBNP group and high‐dosage rhBNP group (n = 12 for each group). Oxidative stress and MPO activity were measured by ELISA kits. MMP‐9 activity was detected by zymography analysis. NF‐κB activity was determined using Western blot assay. With rhBNP pretreatment, TH/S‐induced protein leakage, increased MPO activity, lipid peroxidation and metalloproteinase (MMP)‐9 activity were inhibited. Activation of antioxidative enzymes was reversed. The phosphorylation of NF‐κB and the degradation of its inhibitor IκB were suppressed. The results suggested that the protection mechanism of rhBNP is possibly mediated through upregulation of anti‐oxidative enzymes and inhibition of NF‐κB activation. More studies are needed to further evaluate whether rhBNP is a suitable candidate as an effective inhaling drug to reduce the incidence of TH/S‐induced ALI.
Keywords: acute lung injury, antioxidative enzymes, brain natriuretic peptide, NF‐κB, trauma/haemorrhagic shock
Haemorrhagic shock, ischaemia reperfusion and sepsis are still the major causes of death in the injured host (Murphy et al. 2004; Kher et al. 2005). Despite significant advances in resuscitation and critical care, haemorrhage still contributes to mortality after trauma accounting for over 30% of deaths resulted from trauma worldwide (Santry & Alam 2010). Haemorrhagic shock triggers inflammatory responses characterized by increasing proinflammatory cytokines and adhesion molecules and induces lung inflammation including acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). ALI/ARDS after trauma often leads to multiple organ dysfunction failure (MODF) and increases mortality (Jarrar et al. 1999). Therefore, lung injury after haemorrhagic shock remains the leading cause of death after trauma despite advances in intensive care.
Neutrophils are activated and migrate into the lung during the development of ALI, degranulate and generate superoxide anion (Grommes & Soehnlein 2011). Myeloperoxidase (MPO) produces hypochlorous acid from hydrogen peroxide and chloride anion and serves as a marker for neutrophil activation (Kuo et al. 2011). Reactive oxygen species (ROS) produced by neutrophils are important for bactericidal function. The antioxidative enzymes (AOE), including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), are able to prevent lung injury induced by ROS (Kuo et al. 2011). In addition, matrix metalloproteinase‐9 (MMP‐9) has been known to participate in acute inflammation (Borregaard et al. 2007). As an essential transcriptional factor, NF‐κB has been shown to regulate MMP‐9 expression in LPS‐induced ALI (Corbel et al. 2000).
Brain natriuretic peptide (BNP) is part of the atria natriuretic peptide (ANP) family and first isolated from porcine brain (Maekawa et al. 1988). As a cardiac hormone mainly secreted by ventricular myocytes, BNP was used as a biomarker to evaluate heart failure (Lehr et al. 1991; Mueller et al. 2004). It has vasodilatory and natriuretic functions that counteract the vasoconstricting and fluid‐retaining effect of the rennin–angiotensin system. Recombinant human brain natriuretic peptide (rhBNP), a manmade peptide made by gene engineering, is widely used clinically for the treatment of uncompensated heart failure (Burger & Burger 2001). Recently, BNP has also been used in critical care units for purposes such as guiding fluid therapy, predicting clinical outcomes of critical illness (Yamanouchi et al. 2010; Wang et al. 2012; Li et al. 2013). In our previous studies, we found that rhBNP had protective effects on lipopolysaccharide (LPS)‐induced organ injuries (Song et al. 2013; Li et al. 2014; Yang et al. 2014). However, the effect of rhBNP on trauma‐ and haemorrhage‐induced ALI and the underlying mechanism is still unclear. Thus, in the present study, we aimed to determine how rhBNP exerts its protective effect in trauma/haemorrhagic shock (T/HS)‐induced ALI in an animal model and to find out the mechanism involved.
Materials and methods
Materials
RhBNP was taken from Nuodikang Biological Pharmaceutical Company Ltd. (Chengdu, China). Other reagents, unless specifically started elsewhere, were purchased from Sigma (St. Louis, MO, USA). Antibodies against IκB, phospho‐p65, p65, β‐actin and second antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA). CAT, SOD and GPx activity assay kits were obtained from Cayman (Ann Arbor, MI, USA). Malondialdehyde (MDA) assay kit was purchased from TAKARA Biotechnology (Dalian, China).
Animals
Adult male Sprague‐Dawley rats (310–385 g) were obtained from the Experimental Center of General Hospital of Shenyang Military District (Shenyang, China). The rats were housed in air‐filtered, temperature‐controlled units with free access to food and water. All protocols were in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Ethical approval
This study was approved by the Management Committee of Experimental Animal Center of Shenyang Military District, Shenyang, China (A2012056). All animal experiments were performed according to the guidelines of the Animal Welfare Act and the Guide for Care and Use of Laboratory Animals from the National Institutes of Health.
Trauma–haemorrhagic shock Model in Rats
The TH/S rat model was performed as described previously (Xia et al. 2013). Rats were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) prior to the induction of tissue trauma by a midline laparotomy incision (5 cm). The abdomen was closed in layers, and polyethylene catheters were cannulated in both femoral arteries and the right femoral vein. 1% lidocaine was used to reduce postoperative pain. Rats were placed on a board in a prone position and allowed to awaken. Then, they were bled to a target mean arterial blood pressure (BP) of 30–35 mmHg in 15 min and maintained for 45 min. A continuous blood pressure monitoring system (Powerlab 8/30, AD instruments, Colorado Springs, CO, USA) was used to record in during the experiment. After that, the animals were resuscitated with their shed blood and lactated Ringer's solution in 1:2 ratio. After resuscitation was finished, the catheters were removed, the skin incisions were closed with sutures, and the vessels were ligated. The animals were sacrificed at 2 h after the end of resuscitation or sham operation. The lung tissues were then harvested and stored in a −70°C freezer.
Forty‐eight rats were randomly divided into four groups: sham operation group, model group, low‐dosage rhBNP group (L‐rhBNP) and high‐dosage rhBNP group (H‐rhBNP) (n = 12 for all groups). Sham‐operated animals underwent the surgery, but neither haemorrhage nor resuscitation was performed. Rats of L‐rhBNP group were injected with 30 μg/kg of rhBNP for 30 min before surgery. Similarly, rats of H‐rhBNP group were injected with 60 μg/kg of rhBNP. The right lungs of six rats in each group were collected for Western blot assay and the left lungs for CAT, SOD and GPx activity assays. For the other six rats in each group, bronchoalveolar lavage fluids (BALFs) were collected for protein concentration assay, leucocyte infiltration assessment and MMP activity assay.
Histological analysis
The right lungs were removed quickly, and 10% formalin was infused into the trachea. The lung tissues were sliced, embedded in paraffin and sectioned (4‐μm thickness). The sections were stained with haematoxylin and eosin. Two pathologists evaluated the extent of lung injury based on the lung injury scoring system (Deree et al. 2007). Categories of inflammatory cell infiltration, pulmonary oedema and intra‐alveolar haemorrhage were used to grade on a scale of normal (0), mild (1), moderate (2) or severe (3) injury, with a maximum possible score of 12.
Bronchoalveolar lavage fluid collection
Bronchoalveolar lavage fluid (BALF) was obtained as described previously (Ci et al. 2012). The left lung was lavaged with 2 ml of cold phosphate‐buffered saline (PBS) for three times. The BALF was collected immediately and centrifuged at 500 g for 10 min at 4°C, and the cell‐free supernatants were stored at −80°C for protein concentration and MMP activity assays. Protein concentrations in the cell‐free BALF were detected using Bio‐Rad protein assay reagents. A standard curve was generated in the same fashion using bovine serum albumin. Total leucocyte content was determined by counting the cells in the pellet using a standard Giemsa stain.
Measurement of oxidative stress
Oxidative stress parameters were measured as described previously (Trocha et al. 2014). Lung homogenate was prepared and centrifuged, and the supernatant was used for assays with commercially available kits (TAKARA Biotechnology). MDA levels were measured with the thiobarbituric acid reaction. SOD, CAT and GPx activities were determined using commercially available kits (Cayman) following the manufacturer's instructions.
Measurement of MPO
As a common indicator of neutrophil sequestration, MPO activity was measured as described previously (Li et al. 2007). The snap‐frozen lung tissues were homogenized in PBS solution (pH 6.0) which contained 0.5% hexadecyltrimethylammonium bromide, sonicated twice for 30 s on ice and centrifuged at 12,000 g for 15 min at 4°C. Then, the supernatant was collected and mixed 1: 30 (v/v) with assay buffer containing the substrate o‐dianisidine hydrochloride (0.2 mg/ml) and 0.2 mM hydrogen peroxide. Standard MPO (Sigma) was used in parallel to determine MPO activity in the sample. Absorbance change was determined at 460 nm for 5 min, and MPO activity was evaluated as unit per gram of wet lung tissue per min.
Zymography analysis of matrix metalloproteinase‐9
A gelatin zymography protease assay was used to measure the activity of MMP‐9 in BALF (Yang et al. 2008). BALF was prepared with SDS sample buffer which contained 63 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol and 0.0025% bromophenol blue without boiling or reduction. Then, the BALF was subjected to 0.1% gelatin–8% SDS‐PAGE electrophoresis. After electrophoresis was finished, the gels were washed twice in 2.5% Triton X‐100 for 1 h. The gels were then incubated at 37°C for 16 h in reaction buffer which contained 40 mM Tris–HCl (pH 8.0), 10 mM CaCl2 and 0.01% NaN3. The gels were stained with Coomassie Brilliant R‐250 and destained in a solution of 7.5% acetic acid and 5% methanol.
Western blot analysis of lung tissue
The tissues were taken from the right lung for Western blot analysis. They were homogenized in lysis buffer (pH 7.4) containing 50 mmol/l Tris–HCl, 0.25 mol/l sucrose, 1 mmol/l EDTA, 20 mmol/l CHAPS and 20 mmol/l PMSF. The lung tissue was then centrifuged at 13,000 g for 20 min at 4°C. Coomassie brilliant blue was mixed with the supernatant for Western blot analysis. The concentration of proteins in the supernatant was detected by enzyme‐labelled instrument; 100 μg of proteins was added per lane and separated by 10% SDS‐PAGE and electrophoretically transferred to polyvinylidene difluoride membrane; 5% (w/v) non‐fat dried milk was used to block the membranes for 1 h at room temperature to reduce non‐specific binding. The membranes were then washed with PBS containing 0.1% Tween‐20 (PBST) and probed with antibodies including anti‐β‐actin, anti‐IκB and phosphorylated and non‐phosphorylated forms of anti‐p65. A 1:10,000 (v/v) dilution of horseradish peroxidase‐labelled IgG was then added at room temperature for 1 h, and the blots were developed using ECL Western blotting reagents.
Statistical analysis
Statistical analyses were performed using anova followed by the Bonferroni's t‐test for multigroup comparisons. Significance was accepted at P < 0.05 for all tests. Data are expressed as mean ± standard deviation.
Results
Effects of rhBNP on TH/S‐induced histological changes in lung
Lung tissue specimens were taken 12 h after surgery in the presence or absence of rhBNP. The sham operation group samples indicated normal lung morphology (Figure 1a). In the model group (Figure 1b), marked interstitial oedema and inflammatory cell infiltration were found. However, these histological alterations were markedly reduced in the specimens taken from the L‐rhBNP group (Figure 1c) and the H‐rhBNP group (Figure 1d). All pulmonary histological scores were obviously higher in the specimens from the model group compared to the sham operation group, whereas the scores were intermediate in the specimens from the L‐rhBNP group and the H‐ rhBNP group (P < 0.05) (Figure 2a).
Figure 1.
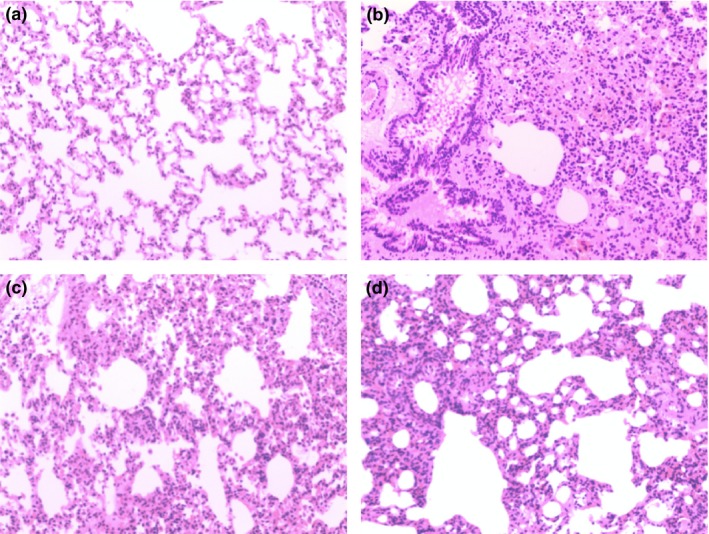
Effects of rhBNP on TH/S‐induced histological changes in lung. The lungs from the sham operation group (a), the model group (b), the L‐rhBNP group (c) and the H‐rhBNP group (d) with rhBNP pretreatment 30 min before surgery were subjected to HE staining. Representative images of HE‐stained lung sections from each group are shown (magnification: 100 × ). b‐ Marked interstitial oedema and inflammatory cell infiltration were found. c, d‐ Such pathological changes were attenuated by rhBNP pretreatment.
Figure 2.
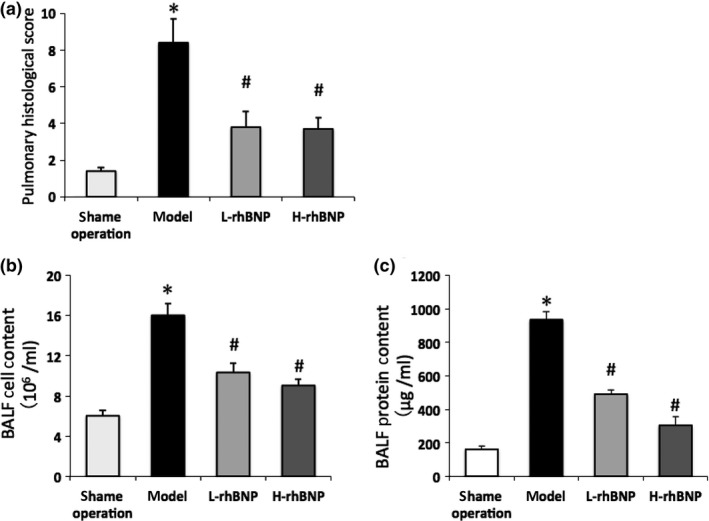
Effects of rhBNP on pulmonary histological scores, TH/S‐induced protein accumulation and leucocytes infiltration in BALF. (a) Pulmonary histological scores. (b) leucocytes count in BALF. (c) Pulmonary permeabilities determined by protein contents quantification in cell‐free BALF. Values are expressed as mean ± SD (n = 12 in each group). *Represents a significant difference between the indicated and the operation group; #between the indicated and model groups, P < 0.05.
Beneficial effects of rhBNP on TH/S‐induced ALI in rats
Trauma and haemorrhage can lead to ischaemia reperfusion injury and increase lung vascular permeability and result in protein leakage and leucocytes infiltration (Deitch et al. 2004). To evaluate the effect of rhBNP on ALI, we examined the alteration in the permeability of the pulmonary barrier and the leucocytic infiltration which had occurred in the lungs of the rats with or without rhBNP pretreatment. Total protein concentration in BALF determined the permeability of pulmonary barrier. The results indicated that protein concentration in BALF was significantly increased after trauma–haemorrhagic shock when compared to sham operation rats (P < 0.05). However, the protein leakage was significantly reduced in the rats pretreated with rhBNP at 30 and 60 μg/kg for 30 min (P < 0.05) (Figure 2b). The number of infiltrating leucocytes was counted on BALF smear preparations stained with Giemsa. In Figure 2c, trauma–haemorrhagic shock without rhBNP pretreatment caused an extensive leucocytes infiltration in lung tissues. With the pretreatment of rhBNP, trauma–haemorrhage‐induced leucocytes infiltration was significantly inhibited (P < 0.05) (Figure 2c). These results indicated that rhBNP exerted a protective effect in trauma–haemorrhage‐induced ALI rats.
Effects of rhBNP on TH/S‐induced oxidative stress in lung
As shown in Table 1, the level of MDA was significantly increased in model group animals relative to sham operation group animals (P < 0.05). Low‐dosage and high‐dosage rhBNP pretreatment diminished the MDA level by 50%, when compared to the model group. The activities of the three kinds of antioxidant enzymes (SOD, GPx and CAT) were significantly lower in model group animals than in sham operation group animals (P < 0.05, Table 1). Notably, such a decline in the activities of these enzymatic antioxidants was significantly prevented by rhBNP pretreatment (P < 0.05). These results suggested that rhBNP was able to reduce trauma–haemorrhage‐induced oxidative stress.
Table 1.
Measurement of oxidative stress and MPO activity in the lung
| Variables | Sham operation group | Model group | L‐rhBNP group | H‐rhBNP group |
|---|---|---|---|---|
| MDA (nmol/mg) | 6.18 ± 1.34 | 22.37 ± 1.94* | 12.22 ± 2.06*, # | 11.72 ± 1.83*, # |
| SOD (U/mg) | 272.27 ± 16.98 | 161.32 ± 16.06* | 198.36 ± 10.47*, # | 202.56 ± 12.32*, # |
| CAT (U/mg) | 22.31 ± 2.30 | 12.76 ± 2.17* | 19.28 ± 2.74*, # | 18.17 ± 2.43*, # |
| GPx (U/mg) | 205.32 ± 52.31 | 140.31 ± 62.30* | 182.23 ± 41.71*, # | 185.33 ± 40.46*, # |
| MPO (U/g) | 0.38 ± 0.05 | 1.41 ± 0.11* | 0.51 ± 0.09*, # | 0.60 ± 0.12*, # |
Data were presented as mean ± standard.
*P < 0.05 vs. the sham operation group; # P < 0.05 vs. model group. MDA, malondialdehyde; SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; MPO, myeloperoxidase.
Effects of rhBNP on TH/S‐induced MPO activity in lung
MPO activity is an index of neutrophil infiltration and plays an important role in the progression of ALI (Borregaard et al. 2007). MPO activity was significantly upregulated in the lung in model group (P < 0.05). Differently, pretreatment with rhBNP reduced MPO activity, and there was no significant difference between low‐dosage and high‐dosage rhBNP (Table 1). These results indicated that rhBNP inhibited the trauma–haemorrhage‐induced neutrophil recruitment.
Effects of rhBNP on TH/S‐induced MMP‐9 activity in BALF
MMP‐9 plays a key role in neutrophil infiltration. The effect of rhBNP on MMP‐9 activity in BALF was analysed using a gelatin zymograph. The activity of MMP‐9 was significantly increased in model group rats as compared with the sham operation group (P < 0.05). Pretreatment with 30 and 60 μg/kg of rhBNP significantly reduced the activation of MMP‐9 (P < 0.05) (Figure 3).
Figure 3.
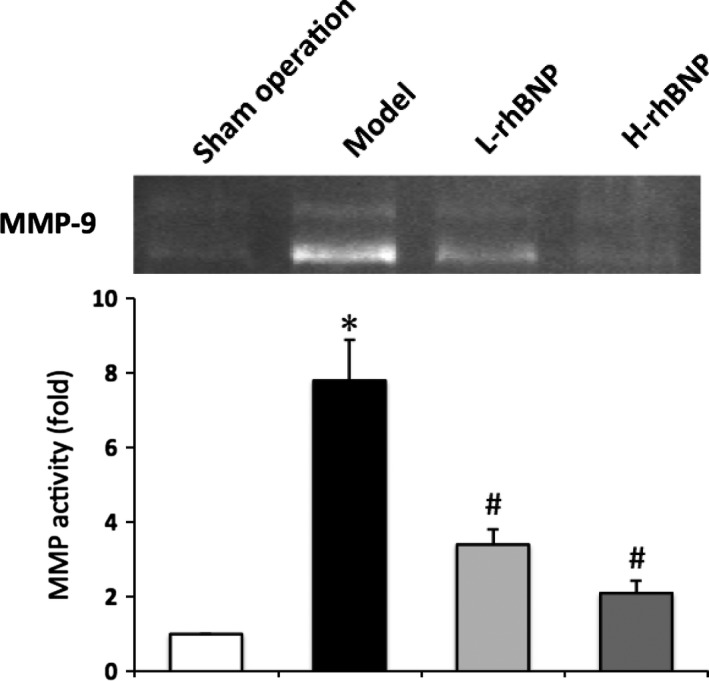
Effects of rhBNP on TH/S‐induced MMP‐9 activation in lung tissue. The activity of MMP‐9 in BALF was analysed by zymography assay. The fold of MMP‐9 activation between the treated and sham operation groups was calculated. Values are expressed as mean ± SD (n = 12 in each group). *Represents a significant difference between the indicated and the operation group; #between the indicated and model groups, P < 0.05.
Effects of rhBNP on TH/S‐induced NF‐κB activation
Activation and tyrosine phosphorylation of NF‐κB appear to be an important mechanism mediating MMP‐9 activation associated with acute lung injury (Kang et al. 2001). After the surgery of trauma and haemorrhage, tyrosine phosphorylation of NF‐κB p65 increased, which was suppressed by rhBNP pretreatment, and significant inhibitory effects began at 30 μg/kg (P < 0.05) (Figure 4a). Similar to tyrosine phosphorylation of NF‐κB p65, trauma‐ and haemorrhage‐induced injury promoted IκB degradation, and this increased degradation was attenuated by 30 and 60 μg/kg of rhBNP (P < 0.05) (Figure 4b).
Figure 4.
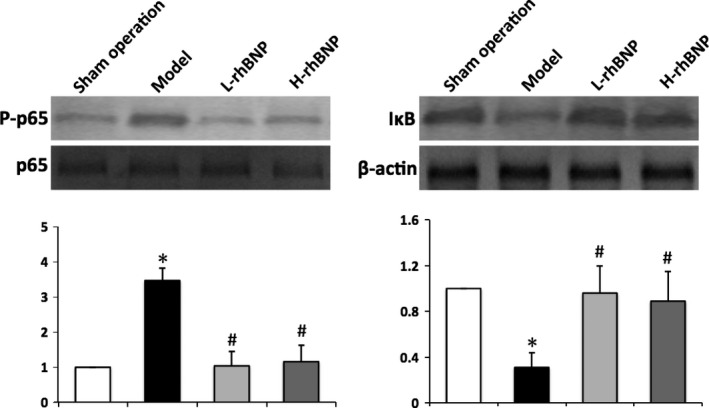
Effects of rhBNP on TH/S‐induced NF‐κB p65 phosphorylation and IκB degradation in lung tissue. Lung tissues harvested from post‐treated rats were analysed by Western blotting. The fold of NF‐κB p65 phosphorylation and IκB degradation between the treated and sham operation groups was calculated. Values are expressed as mean ± SD (n = 12 in each group). *Represents a significant difference between the indicated and the operation group; #between the indicated and model groups, P < 0.05.
Discussion
Trauma/haemorrhagic shock triggers a systemic inflammatory response characterized by an increase in proinflammatory cytokines and induces pulmonary inflammation that can lead to ALI. Although the characteristics of ALI cannot be fully duplicated in animal models, T/HS models in rats are considered to mimic the clinical development of T/HS‐induced ALI (Roy et al. 2013). It has been known that the leakage of protein‐rich fluid into the alveolar space results from increased permeability of the alveolar–capillary barrier which is caused by widespread damage and leucocyte activation in ALI (Grommes & Soehnlein 2011). Despite some recent advances, there is no effective treatment for ALI. Thus, it is extremely important to find an effective drug and to investigate the mechanisms which can be deployed against T/HS‐induced ALI. In the present study, we demonstrated that rhBNP has potent anti‐inflammatory properties which act against the protein leakage and leucocyte infiltration that is seen in the T/HS‐induced ALI model.
As the most abundant form of leucocyte in humans, neutrophils are an important component of the inflammatory response that characterizes ALI (Abraham 2003). Neutrophils are essential contributors to lung inflammation as shown by the deterioration in pulmonary function in patients with lung injury as neutropenia resolves (Azoulay et al. 2002). Then, neutrophils rapidly enter the pulmonary parenchyma and intestine. MPO is stored within the primary granules and known to be a marker of neutrophil presence and activity in circulating blood, generating hydrogen peroxide, a potent bactericide and tissue injury mediator (Borregaard et al. 2007). Events in lung tissue involving inflammatory cells may be mirrored by the MPO level of these cells. Experimental data in this study demonstrated that the pretreatment of rhBNP downregulated the activation of MPO in the lung tissues of rats. This result implied that rhBNP confers a protection against T/HS‐induced ALI through reducing the activation of MPO in rats.
BNP is mainly produced by ventricular myocytes and used as a biomarker for heart failure (Lehr et al. 1991; Mueller et al. 2004). There are four different groups of natriuretic peptides identified to date: ANP, BNP, C‐type natriuretic peptide (CNP) and dendroaspis natriuretic peptide, a D‐type natriuretic peptide (DNP) (Pandit et al. 2011). So far, three kinds of natriuretic peptide receptors (NPRs) have been found and widely distributed in the human body such as the heart, lung, brain, kidney. RhBNP is artificially synthesized by recombinant DNA technology, and its amino acid sequence, space structure and biological activity are the same as that of the endogenous BNP. Therefore, the target organs of natriuretic peptides are the heart, lung, brain and kidney, but the specific target cell is not very clear in each organ. Our previous study found that inductions of IL‐6 and TNF‐α could be attenuated in the L‐rhBNP and the H‐rhBNP groups. Furthermore, MPO and MDA activities were significantly lower in the H‐rhBNP group compared to those in the ALI group. RhBNP pretreatment exerts a protective effect and may be associated with its significant biological properties in a LPS‐induced lung injury animal model (Song et al. 2013). Our data indicate that rhBNP treatment may exert protective effects and may be associated with changes in endogenous antioxidant enzymes. Thus, rhBNP may be considered as a therapeutic agent for various clinical conditions involving lung injury by sepsis.
The consumption of oxygen that results in the production of superoxide anion generates ROS in activated neutrophils in ALI (El‐Benna et al. 2005). Excessive generation of ROS can induce oxidative stress and lead to lipid oxidation (Manca et al. 1991). Furthermore, ROS can cause tissue injury via protein oxidation and DNA damage (Ward 2010). Tissues may escape toxic damage induced by ROS via antioxidative enzymes including SOD, CAT and GPx. Our previous study and other studies have demonstrated that natriuretic peptide can reduce the accumulation of MDA induced by differential stimulators such as lipopolysaccharide, trauma and ischaemia/reperfusion injury (Jin et al. 2014; Yang et al. 2014). In addition, natriuretic peptide increases the activities of SOD, CAT and GPx in intestine, lung, skin and heart after the administration of LPS (Romero et al. 2013; Song et al. 2013; Subramanian & Vellaichamy 2014; Yang et al. 2014). The fact that both amounts of MDA formation and reduction in antioxidative enzymes activity can be reversed by rhBNP indicated that the reduction in MDA formation was partially due to the activation of antioxidative enzymes.
NF‐κB, a redox‐sensitive nuclear transcription factor, can be induced to be activated by ischaemia/reperfusion and ROS and plays a major role in ALI (Chiang et al. 2011; Lv et al. 2011). NF‐κB is usually maintained in the cytoplasm in an inactive form bound by inhibitory protein IκB. Proteolytic degradation of phosphorylated IκB is required for NF‐κB liberation. As the predominant component of NF‐κB, phosphorylation of p65 is mediated by IκB kinase in the cytoplasm and translocates NF‐κB into the nucleus following activation (Sasaki et al. 2005). ANP is known to suppress gene expression of NF‐κB on the LPS and TNF‐α‐induced inflammatory signalling in pulmonary artery endothelial cells (Xing & Birukova 2010). ANP is also found to inhibit NF‐κB activity and TNF‐α secretion in TNF‐α‐stimulated pulmonary microvascular and macrovascular endothelial monolayers (Irwin et al. 2005). In human alveolar epithelial cells, natriuretic peptide decreases the activation of NF‐κB and has a bronchodilatory and anti‐inflammatory activity (Hellermann et al. 2004). In the previous study, we found that rhBNP could reduce intestinal tissue damage in response to LPS injection in the dog sepsis models through downregulating pro‐inflammatory cytokines by a mechanism of suppressing IκB phosphorylation and NF‐κB expression (Yang et al. 2014). The present study demonstrated that trauma and haemorrhagic shock resulted in NF‐κB activation and IκB degradation in lung tissue, and rhBNP pretreatment prevented these manifestations. These results suggested that the prevention of T/HS‐induced ALI by rhBNP might be due to the inhibition of NF‐κB activation pathway.
MMP‐9 is one of the families of MMPs which degrades ECM. MMP‐9 is found in the tertiary granules of neutrophils and is rapidly released following inflammatory factors. MMP‐9 has been implicated to be involved in the pathogenesis of ALI and regulated by NF‐κB (Kang et al. 2001). The activity level of MMP‐9 in trauma‐ and infection‐induced organ injury has been reported to be increased (Teng et al. 2012). Natriuretic peptide prevents the increase in MMP‐9 in adult fibroblasts (Parthasarathy et al. 2013). Zymograph analysis showed that rhBNP inhibited MMP‐9 activation in the BALF of TH/S‐induced ALI in rats. Thus, we concluded that TH/S‐induced ALI was protected by rhBNP via suppressing MMP‐9 activation.
In conclusion, our findings suggest the possibility of using rhBNP as an effective drug for the prevention of ALI associated with T/HS. The protection mechanism of rhBNP is by upregulating antioxidative enzymes and inhibiting NF‐κB activation. More studies are needed to further evaluate whether rhBNP is a suitable candidate as an effective inhaled drug to reduce the incidence of TH/S‐induced ALI.
Author contributions
ZS, XZ, YG, MH and ML carried out the experimental work and analysis of data. HJ and LW conceived the study and carried out data analysis. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Conflict of interest
There are no conflict of interest issues regarding any of the authors.
Acknowledgements
This work was supported by Chinese Postdoctoral Science Foundation (2014M552693, to Z. S.) and Science and Technology Project of Liaoning, China (2013225089, to Z. S.).
Zhi Song and Xiu Zhao contributed equally to this work.
References
- Abraham E. (2003) Neutrophils and acute lung injury. Crit. Care Med. 31, S195–S199. [DOI] [PubMed] [Google Scholar]
- Azoulay E., Darmon M., Delclaux C. et al (2002) Deterioration of previous acute lung injury during neutropenia recovery. Crit. Care Med. 30, 781–786. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Sorensen O.E. & Theilgaard‐Monch K. (2007) Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28, 340–345. [DOI] [PubMed] [Google Scholar]
- Burger M.R. & Burger A.J. (2001) BNP in decompensated heart failure: diagnostic, prognostic and therapeutic potential. Curr. Opin. Investig. Drugs 2, 929–935. [PubMed] [Google Scholar]
- Chiang C.H., Chuang C.H. & Liu S.L. (2011) Apocynin attenuates ischemia‐reperfusion lung injury in an isolated and perfused rat lung model. Transl. Res. 158, 17–29. [DOI] [PubMed] [Google Scholar]
- Ci X., Chu X., Wei M., Yang X., Cai Q. & Deng X. (2012) Different effects of farrerol on an OVA‐induced allergic asthma and LPS‐induced acute lung injury. PLoS ONE 7, e34634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel M., Boichot E., Lagente V. (2000) Role of gelatinases MMP‐2 and MMP‐9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 33, 749–754. [DOI] [PubMed] [Google Scholar]
- Deitch E.A., Forsythe R., Anjaria D. et al (2004) The role of lymph factors in lung injury, bone marrow suppression, and endothelial cell dysfunction in a primate model of trauma‐hemorrhagic shock. Shock 22, 221–228. [DOI] [PubMed] [Google Scholar]
- Deree J., Martins J., de Campos T. et al (2007) Pentoxifylline attenuates lung injury and modulates transcription factor activity in hemorrhagic shock. J. Surg. Res. 143, 99–108. [DOI] [PubMed] [Google Scholar]
- El‐Benna J., Dang P.M., Gougerot‐Pocidalo M.A. & Elbim C. (2005) Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. 53, 199–206. [PubMed] [Google Scholar]
- Grommes J. & Soehnlein O. (2011) Contribution of neutrophils to acute lung injury. Mol. Med. 17, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellermann G., Kong X., Gunnarsdottir J. et al (2004) Mechanism of bronchoprotective effects of a novel natriuretic hormone peptide. J. Allergy Clin. Immunol. 113, 79–85. [DOI] [PubMed] [Google Scholar]
- Irwin D.C., Tissot van Patot M.C., Tucker A. & Bowen R. (2005) Direct ANP inhibition of hypoxia‐induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L849–L859. [DOI] [PubMed] [Google Scholar]
- Jarrar D., Chaudry I.H. & Wang P. (1999) Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review). Int. J. Mol. Med. 4, 575–583. [DOI] [PubMed] [Google Scholar]
- Jin X., Zhang Y., Li X., Zhang J. & Xu D. (2014) C‐type natriuretic peptide ameliorates ischemia/reperfusion‐induced acute kidney injury by inhibiting apoptosis and oxidative stress in rats. Life Sci. 117, 40–45. [DOI] [PubMed] [Google Scholar]
- Kang J.L., Lee H.W., Lee H.S. et al (2001) Genistein prevents nuclear factor‐kappa B activation and acute lung injury induced by lipopolysaccharide. Am. J. Respir. Crit. Care Med. 164, 2206–2212. [DOI] [PubMed] [Google Scholar]
- Kher A., Wang M., Tsai B.M. et al (2005) Sex differences in the myocardial inflammatory response to acute injury. Shock 23, 1–10. [DOI] [PubMed] [Google Scholar]
- Kuo M.Y., Liao M.F., Chen F.L. et al (2011) Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFkappaB pathways in mice with endotoxin‐induced acute lung injury. Food Chem. Toxicol. 49, 2660–2666. [DOI] [PubMed] [Google Scholar]
- Lehr H.A., Guhlmann A., Nolte D., Keppler D. & Messmer K. (1991) Leukotrienes as mediators in ischemia‐reperfusion injury in a microcirculation model in the hamster. J. Clin. Investig. 87, 2036–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kovacs E.J., Schwacha M.G., Chaudry I.H. & Choudhry M.A. (2007) Acute alcohol intoxication increases interleukin‐18‐mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L1193–L1201. [DOI] [PubMed] [Google Scholar]
- Li N., Zhang Y., Fan S., Xing J. & Liu H. (2013) BNP and NT‐proBNP levels in patients with sepsis. Front Biosci. 18, 1237–1243. [DOI] [PubMed] [Google Scholar]
- Li N., Jin H.X., Song Z., Bai C.Z., Cui Y. & Gao Y. (2014) Protective effect of recombinant human brain natriuretic peptide on acute renal injury induced by endotoxin in canines. Cell Biochem. Biophys. 70, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Lv X., Wang Z.M., Huang S.D., Song S.H., Wu F.X. & Yu W.F. (2011) Emulsified isoflurane preconditioning reduces lung injury induced by hepatic ischemia/reperfusion in rats. Int. J. Med. Sci. 8, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa K., Sudoh T., Furusawa M. et al (1988) Cloning and sequence analysis of cDNA encoding a precursor for porcine brain natriuretic peptide. Biochem. Biophys. Res. Commun. 157, 410–416. [DOI] [PubMed] [Google Scholar]
- Manca D., Ricard A.C., Trottier B. & Chevalier G. (1991) Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology 67, 303–323. [DOI] [PubMed] [Google Scholar]
- Mueller C., Scholer A., Laule‐Kilian K. et al (2004) Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N. Engl. J. Med. 350, 647–654. [DOI] [PubMed] [Google Scholar]
- Murphy T.J., Paterson H.M., Mannick J.A. & Lederer J.A. (2004) Injury, sepsis, and the regulation of Toll‐like receptor responses. J. Leukoc. Biol. 75, 400–407. [DOI] [PubMed] [Google Scholar]
- Pandit K., Mukhopadhyay P., Ghosh S. & Chowdhury S. (2011) Natriuretic peptides: diagnostic and therapeutic use. Indian J. Endocrinol. Metab. 15(Suppl 4), S345–S353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A., Gopi V., Umadevi S. et al (2013) Suppression of atrial natriuretic peptide/natriuretic peptide receptor‐A‐mediated signaling upregulates angiotensin‐II‐induced collagen synthesis in adult cardiac fibroblasts. Mol. Cell. Biochem. 378, 217–228. [DOI] [PubMed] [Google Scholar]
- Romero M., Caniffi C., Bouchet G. et al (2013) Sex differences in the beneficial cardiac effects of chronic treatment with atrial natriuretic Peptide in spontaneously hypertensive rats. PLoS ONE 8, e71992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S.K., Emr B., Sadowitz B. et al (2013) Preemptive application of airway pressure release ventilation prevents development of acute respiratory distress syndrome in a rat traumatic hemorrhagic shock model. Shock 40, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santry H.P. & Alam H.B. (2010) Fluid resuscitation: past, present, and the future. Shock 33, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C.Y., Barberi T.J., Ghosh P. & Longo D.L. (2005) Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}‐independent NF‐{kappa}B pathway. J. Biol. Chem. 280, 34538–34547. [DOI] [PubMed] [Google Scholar]
- Song Z., Cui Y., Ding M.Z., Jin H.X. & Gao Y. (2013) Protective effects of recombinant human brain natriuretic peptide against LPS‐Induced acute lung injury in dogs. Int. Immunopharmacol. 17, 508–512. [DOI] [PubMed] [Google Scholar]
- Subramanian V. & Vellaichamy E. (2014) Atrial natriuretic peptide (ANP) inhibits DMBA/croton oil induced skin tumor growth by modulating NF‐kappaB, MMPs, and infiltrating mast cells in Swiss albino mice. Eur. J. Pharmacol. 740, 388–397. [DOI] [PubMed] [Google Scholar]
- Teng L., Yu M., Li J.M. et al (2012) Matrix metalloproteinase‐9 as new biomarkers of severity in multiple organ dysfunction syndrome caused by trauma and infection. Mol. Cell. Biochem. 360, 271–277. [DOI] [PubMed] [Google Scholar]
- Trocha M., Merwid‐Lad A., Chlebda E. et al (2014) Influence of ezetimibe on selected parameters of oxidative stress in rat liver subjected to ischemia/reperfusion. Arch. Med. Sci. 10, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wu Y., Tang L. et al (2012) Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta‐analysis. Crit. Care 16, R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P.A. (2010) Oxidative stress: acute and progressive lung injury. Ann. N. Y. Acad. Sci. 1203, 53–59. [DOI] [PubMed] [Google Scholar]
- Xia X., Wang X., Li Q., Li N. & Li J. (2013) Essential amino acid enriched high‐protein enteral nutrition modulates insulin‐like growth factor‐1 system function in a rat model of trauma‐hemorrhagic shock. PLoS ONE 8, e77823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J. & Birukova A.A. (2010) ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc. Res. 79, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi S., Kudo D., Endo T., Kitano Y. & Shinozawa Y. (2010) Blood N‐terminal proBNP as a potential indicator of cardiac preload in patients with high volume load. Tohoku J. Exp. Med. 221, 175–180. [DOI] [PubMed] [Google Scholar]
- Yang S.F., Yang W.E., Kuo W.H., Chang H.R., Chu S.C. & Hsieh Y.S. (2008) Antimetastatic potentials of flavones on oral cancer cell via an inhibition of matrix‐degrading proteases. Arch. Oral Biol. 53, 287–294. [DOI] [PubMed] [Google Scholar]
- Yang H., Song Z., Jin H., Cui Y., Hou M. & Gao Y. (2014) Protective effect of rhBNP on intestinal injury in the canine models of sepsis. Int. Immunopharmacol. 19, 262–266. [DOI] [PubMed] [Google Scholar]


