Summary
A key role of bacterial biofilm in the pathogenesis of chronic rhinosinusitis (CRS) with (CRSwNP) and without nasal polyps (CRSsNP) is commonly accepted. However, the impact of some bacterial species isolated from inflamed sinus mucosa on biofilm formation is unclear. In particular, the role of Staphylococcus epidermidis as aetiological agents of CRS is controversial. Moreover, the effect of biofilm formation on neutrophil infiltration and activity in CRSwNP calls for explanation. In this study, biofilms were found in three of 10 patients (mean age = 46 ± 14) with CRS undergoing endoscopic sinus surgery by means of scanning electron microscopy. Unexpectedly, S. epidermidis was the primary isolated bacteria and was also found to be present in all biofilm‐positive mucosa specimens, indicating its pivotal role in the pathogenesis of severe chronic infections associated with biofilm formation. We have also measured the activity of myeloperoxidase (MPO), the most abundant neutrophil enzyme, to demonstrate the presence of neutrophils in the samples tested. Our present results show that the level of MPO in CRS associated with biofilm is lower than that without biofilm. It may suggest either a low number of neutrophils or the presence of a type of neutrophils with compromised antimicrobial activity, described as biofilm‐associated neutrophils (BAN). Finally, we conclude that further studies with a large number of CRS cases should be performed to establish the association between S. epidermidis and other frequently isolated bacterial species from paranasal sinuses, with the severity of CRS, biofilm formation and the infiltration of BAN.
Keywords: biofilm, biofilm‐associated neutrophils, chronic rhinosinusitis, endoscopic sinus surgery, inflammation, myeloperoxidase, Staphylococcus epidermidis, scanning electron microscopy
Chronic rhinosinusitis (CRS) is one of the most common inflammatory diseases of the upper respiratory tract. Chronic rhinosinusitis represents a persistent inflammation of nasal and sinus mucosa with and without the presence of nasal polyps. Some investigators suggest that CRS is associated with different systemic diseases such as bronchial asthma, Non‐steroidal anti‐inflammatory drugs (NSAIDs) allergy, cystic fibrosis, sarcoidosis and aspirin‐induced asthma (Fokkens et al. 2012). However, in the majority of CRS, the aetiology, the pathogenesis and the role of bacterial infections in the development of the disease are unclear (Pandak et al. 2011). In addition, the association of neutrophil infiltration in CRS with and without nasal polyps is not well documented (Hirotsu et al. 2011).
Since 2004, when the relationship between CRS and biofilms was first described, a number of studies have been published to demonstrate the role of bacterial biofilms in the recalcitrant nature of the disease (Perloff & Palmer 2004; Marcinkiewicz et al. 2013). However, due to the distinct detection methodology, reported rates of biofilms in the CRS population vary from 30% to 100% (Głowacki et al. 2008; Wang et al. 2014). Moreover, various bacterial species have been shown to be associated with CRS biofilms including Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Haemophilus influenza, Streptococcus pneumonia and Moraxella catarrhalis, out of which the role of S. epidermidis is the most controversial (O'Gara & Humphreys 2001; Ferguson & Stolz 2005). It is commonly accepted that the presence of S. aureus biofilm is associated with a poor prognosis of illness and with an unfavourable clinical outcome such as persistent sinusitis symptoms with ongoing mucosal inflammation and high risk of recurrent infections (Głowacki et al. 2008; Günther et al. 2009; Thurlow et al. 2011). In contrast, the pathologic role of S. epidermidis in CRS has been documented by some investigators in spite of the emergence of the coagulase‐negative staphylococci (S. epidermidis) as major nosocomial pathogens associated with infections of implanted medical devices (Sachse et al. 2008; Pandak et al. 2011). Moreover, coagulase‐negative staphylococci (S. epidermidis) were the most common bacteria found within ethmoid and maxillary sinuses (31–51% of isolates) (Fokkens et al. 2012). Nonetheless, the impact of some nasal microbiota in the pathogenesis of CRS, as well as the role of neutrophil infiltration in CRS with (CRSwNP) and without polyps (CRSsNP), is still unclear. The presence of neutrophils, which are the major cells of inflammation, have been demonstrated in both CRSwNP and CRSsNP. However, some reports demonstrated comparable neutrophil infiltration in both CRSwNP and CRSsNP, whereas the eosinophylic infiltrate was significantly smaller in CRSsNP (Fokkens et al. 2012; Ikeda et al. 2013). Furthermore, it has been suggested that neutrophils may be the most important pathologic agent of CRS (Fokkens et al. 2012).
Neutrophils, which are the major phagocytes of acute inflammatory response, are the key effector cells of innate immunity (Nathan 2006). These cells effectively kill the planktonic form of various bacterial strains playing beneficial role in host defence against microbial infections (Nauseef & Borregaard 2014). By contrast, the detrimental role of neutrophils was reported in the pathogenesis of a number of chronic inflammatory diseases such as arthritis, acne and atherosclerosis (Klebanoff 2005). Importantly, the role of neutrophils in chronic infections and their interactions with biofilm are still not clear (Marcinkiewicz et al. 2013; Hirschfeld 2014). We hypothesize that the biofilm environment, similar to the tumour environment (Fridlender et al. 2009; Galdiero et al. 2013), is able to drive neutrophils towards distinct phenotypes, either N1 or N2 cells. Thus, depending on the specificity of a bacterial strain, biofilm‐associated neutrophils (BAN) will be polarized towards either anti‐biofilm N1‐type or pro‐biofilm N2‐type cells. For example, it has been reported that not only P. aeruginosa biofilm was resistant to the immune attack but also the formation of the biofilm was accelerated in the presence of neutrophils (Jesaitis et al. 2003). The phenotype of these cells was not estimated, but they behave as N2 pro‐biofilm neutrophils.
In this study, we have examined the presence of biofilm in mucosal specimens of patients undergoing endoscopic sinus surgery (ESS) for idiopathic CRS (see exclusions below). Moreover, we have identified biofilm‐forming bacterial species isolated from the examined mucosal specimens. In addition, the enzymatic activity of myeloperoxidase (MPO), a marker of tissue neutrophil content and an indicator of the oxidative stress in inflammatory diseases, was compared between mucosal samples with and without biofilm.
Materials and methods
Patients and study design
This was a prospective, double‐blind study. Chronic rhinosinusitis patients undergoing ESS in the Department of Otolaryngology, Jagiellonian University Medical College were recruited for the study. Chronic rhinosinusitis was diagnosed based on the definition from the EPOS 2012 position paper on rhinosinusitis and nasal polyps (Fokkens et al. 2012). Study exclusion criteria were unwillingness to take part in the study, asthma, hypersensitivity to NSAIDs, cystic fibrosis, immunosuppressive disorders (e.g. AIDS, diabetes), hypothyroidism, gastro‐oesophageal reflux disease and ESS reoperation. Intra‐operative complications such as cerebrospinal fluid leakage and postoperative, histopathologic diagnosis of a malignant process or a foreign body in the sinus also excluded the patient from the study. About 10 of 179 CRS patients who underwent ESS in our clinic in 2013 were qualified for the study based on the above‐mentioned criteria. For each of those 10 patients (from 1 to 10 – see Table 1, Figure 3), demographic, clinical and laboratory characteristics were recorded.
Table 1.
Patient's characteristics
| Patient number | Gender | Age (years) | Clinical diagnosis | VASa [M/S] | Biofilm presence | Bacterial identification | Origin of mucosa specimen |
|---|---|---|---|---|---|---|---|
| 1 | Female | 58 | CRSwNP | 9 [S] | No |
Staphylococcus aureus
Pseudomonas aeruginosa |
Maxilliary sinus |
| 2 | Female | 60 | A‐CRSwNP | 10 [S] | No | Staphylococcus epidermidis | Ethmoid sinus |
| 3 | Male | 58 | CRSwNP | 7 [M] | No |
Staphylococcus epidermidis
Clostridium perfringens Veilonella parvula Streptococcus agalactiae Streptococcus mitis |
Sphenoid sinus |
| 4 | Female | 54 | CRSwNP | 9 [S] | Yes | Staphylococcus epidermidis | Sphenoid sinus |
| 5 | Male | 40 | A‐CRSsNP | 9 [S] | No |
Staphylococcus epidermidis
Propionibacterium acnes |
Sphenoid sinus |
| 6 | Male | 28 | CRSwNP | 9 [S] | Yes |
Staphylococcus epidermidis
Propionibacterium acnes Prevotella intermedia |
Sphenoid sinus |
| 7 | Male | 61 | A‐CRSwNP | 10 [S] | No |
Staphylococcus epidermidis
Streptococcus mitis |
Ethmoid sinus |
| 8 | Male | 32 | CRSwNP | 10 [S] | Yes |
Staphylococcus epidermidis
Klebsiella pneumonia |
Ethmoid sinus |
| 9 | Female | 29 | CRSwNP | 7 [M] | No |
Moraxella catarrhalis
Peptostreptococcus Lactococcus lactis subsp. cremoris |
Maxilliary sinus |
| 10 | Female | 39 | CRSsNP | 8 [M] | No | Aerococcus uranie | Maxilliary sinus |
The intensity of subjective CRS symptoms was assessed using a 0–10 point visual analogue scale. M, moderate; S, severe; CRSwNP, chronic rhinosinusitis with nasal polyps; A‐CRS, allergic chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps.
Figure 3.
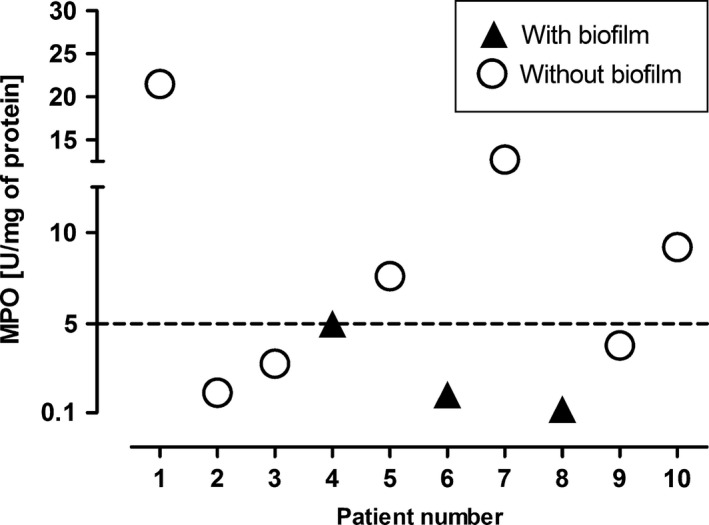
Comparison of MPO activity in mucosa specimens with and without biofilm. MPO activity: ▲ (n = 3) − 2.1 ± 2.51 vs. ○ (n = 7) – 8.4 ± 7.02 U/mg, P = 0.077 (unpaired t‐test with Welch's correction).
Ethics
All patients gave their written and informed consent prior to inclusion into the study. The research protocol was approved by the Jagiellonian University Ethics Committee (registry number KBET/229/B/2011). The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Pre‐operative and follow‐up assessment
Each patient, pre‐operatively and during both follow‐up visits, filled out a questionnaire assessing the presence of concomitant diseases (asthma, hypersensitivity to NSAIDs, allergic rhinitis) and the intensity of subjective CRS symptoms. The intensity of subjective CRS symptoms was assessed using a 0–10 point visual analogue scale (VAS) – the patients assessed the amount of discharge on the pharynx posterior wall, the degree of smell impairment, facial pain and progressive nasal congestion. Pre‐operative radiologic assessment included computed tomography (CT) of paranasal sinuses (bone window, a minimum of two projections). Each CT was assessed by the operator and an experienced radiologist for any anatomic or pathologic changes. Sinuses assessed included the maxillary, anterior and posterior ethmoid, and sphenoidal and frontal sinuses, as well as the ostiomeatal complex. Post‐ESS, each patient was treated according to the EPOS 2012 guidelines (Fokkens et al. 2012).
Intra‐operative procedures
Each patient underwent ESS in general anaesthesia. During surgery, at least five mucosa specimens were taken from each patient qualified for the study. These specimens (approximately 10 × 10 mm) were acquired from the frontal, maxillary, sphenoid, anterior and posterior ethmoid, sinuses, frontal recess, middle nasal concha and the middle nasal meatus. Only ciliated epithelium was sampled. Directly after excision, the mucosa fragments were rinsed in saline and submerged in a fixative solution. The specimens were taken for analysis through scanning electron microscopy (SEM). Some specimens were transported to the immuno‐microbiological laboratory for bacteriological analyses and MPO assessment.
Material preparation for SEM – biofilm identification
Acquired tissue samples were prepared for SEM assessment within 30 min after excision. Mucosa fragments were fixed using a solution of 2.5% glutaraldehyde in 0.2 M cacodylate buffer (pH 7.4). Each fragment was immersed in this solution for 24 h in 4°C. Next, after thorough rinsing in 0.2 M cacodylate buffer, which was exchanged several times, the samples were fixed again, this time in 2% osmium tetroxide, for 3 h. Next, the tissues were dehydrated in rising concentrations of ethyl alcohol and absolute acetone and dried in CO2. The dried specimens were mounted on plates using silver glue and sputter‐coated with gold using a JFC‐1100 sputter (Jeol, Tokyo, Japan). The casts were examined in a JSM 35‐CF scanning electron microscope (Jeol) at 25 kV. Each biofilm was assessed independently by three examiners, who had no contact with the patients and who were blind to the result of the clinical assessment. The basic criterion for diagnosing a biofilm was the presence of spherical and rod‐shaped structures, surrounded by a matrix and situated on the epithelium.
Bacterial identification
The collected mucosa samples were suspended in Schaedler broth (SAB; Difco, Detroit, MI, USA) with 10% glycerol and stored at −20°C for up to 1 week. The samples were then transported to the microbiology laboratory in dry ice. In the laboratory, the samples were thawed, weighed and homogenized in 1 ml of SAB. The procedure for crushing samples was carried out within the anaerobic chamber (N2 – 85%, H2 – 10% and CO2 – 5%, MACS‐MG 500 Work Station, DW Scientific, Shipley, West Yorkshire, UK) using a glass homogenizer. Homogenized samples were then serially diluted with SAB, and 100 μl aliquots of serial dilutions were placed on the following media: Columbia blood agar (Difco) with 5% sheep blood for streptococci and staphylococci (incubation for 24 h at 37°C in aerobic conditions), chocolate agar (BD, San Diego, CA, USA) for Haemophilus spp., Neisseria spp. and Moraxella spp. (incubation for 48 h at 37°C in microaerophilic conditions) and Schaedler agar (Difco) for anaerobic bacteria (incubation for 48 h at 37°C in anaerobic conditions). Strict anaerobic and microaerophilic atmosphere was maintained using GENbox anaerobic and microaer system (bioMerieux, Marcy l'Etoile, France). Moreover, to detect yeast‐like fungi, Sabouraud agar (Difco) was used at 37°C for 24 h in aerobic conditions. Cultured bacteria were counted to determine the bacterial cell count (CFU/g of tissue), and then, all morphologically different colonies were further identified to genus and species using the following API sets (bioMerieux): API Staph (staphylococci), API Strep (streptococci and enterococci), API 20A (anaerobic bacteria) and API NH (Haemophilus spp., Neisseria spp. and Moraxella spp.).
Myeloperoxidase activity measurement
Myeloperoxidase activity was measured in mucosa specimens as described before (Marcinkiewicz et al. 2004). Briefly, mucosa specimens were homogenized in ice‐cold 0.5% hexadecyltrimethylammonium (Sigma–Aldrich, Steinheim, Germany) in 50 mM potassium phosphate buffer, pH 6.0. The tissue was freeze‐thawed three times and dispersed by vortex. Suspensions were centrifuged at 4000 g for 15 min at 4°C. Aliquots (0.01 ml) of the supernatant were mixed with 0.29 ml of 50 mM phosphate buffer, pH 6.0, containing 0.167 mg/ml o‐dianisidine dihydrochloride (Sigma‐Aldrich, Germany) and 0.0005% hydrogen peroxide. About 200 μl sample of the mixture was placed in a 96‐well flat‐bottom plate and incubated for 20 min at room temperature. The absorbance was measured at 460 nm using a Sumal‐PE2 spectrophotometer. The activity of MPO was calculated from MPO (Sigma‐Aldrich, St. Louis, MO, USA) standard curve and expressed in units (U). One unit of MPO activity was defined as the degradation of 1 μmol of hydrogen peroxide per minute at room temperature. Each sample was tested in duplicate. The total protein concentration in the mucosa specimens was measured by the use of the bicinchoninic acid protein test (BCA; Sigma–Aldrich).
Statistical analysis
Statistical significance of differences between groups was analysed using unpaired t‐test with Welch's correction. Results are presented as mean ± SD values. A P‐value <0.05 was considered statistically significant. Analysis was performed using GraphPad Prism v. 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) .
Results
Patient's basic characteristics
Of 10 selected patients, five were males and five were females. Their ages ranged from 29 to 61. Seven patients rated their symptoms as severe (VAS score 9–10) and three as moderate (VAS score 7–8) (Table 1). All allergic patients (A‐CRSwNP) reported a very high VAS score (9–10). Interestingly, a higher mean VAS score of 9.33 ± 0.33 was shown in CRS patients with a biofilm than in non‐biofilm CRS patients (the mean VAS score = 7.75 ± 0.36).
Demonstration of bacterial biofilms in the mucosa specimens – SEM analysis
To detect biofilms in the collected mucosa fragments, we have used SEM imaging. Biofilms were found in three of 10 patients (30%). In these SEM images, the presence of spherical structures, surrounded by a matrix and situated on the epithelium, and water channels were visualized (Figure 1). We did not find any rod‐shaped structures. Importantly, S. epidermidis, the spherical‐shaped bacteria, was isolated in all mucosa fragments covered with biofilms (Table 1). On the other hand, undamaged cilia were demonstrated in two non‐biofilm samples, both with moderate CRS (VAS score‐7) (Figure 2a,b). The epithelium of the remaining CRS patients without biofilm was at least partially devoid of cilia (Figure 2c). This observation has important clinical relevance, as healthy cilia are vital for the proper mucous passages in paranasal sinuses. Mucus that remains in sinuses for extended periods of time becomes infected with bacteria present in the nasal cavity (Fokkens et al. 2012).
Figure 1.
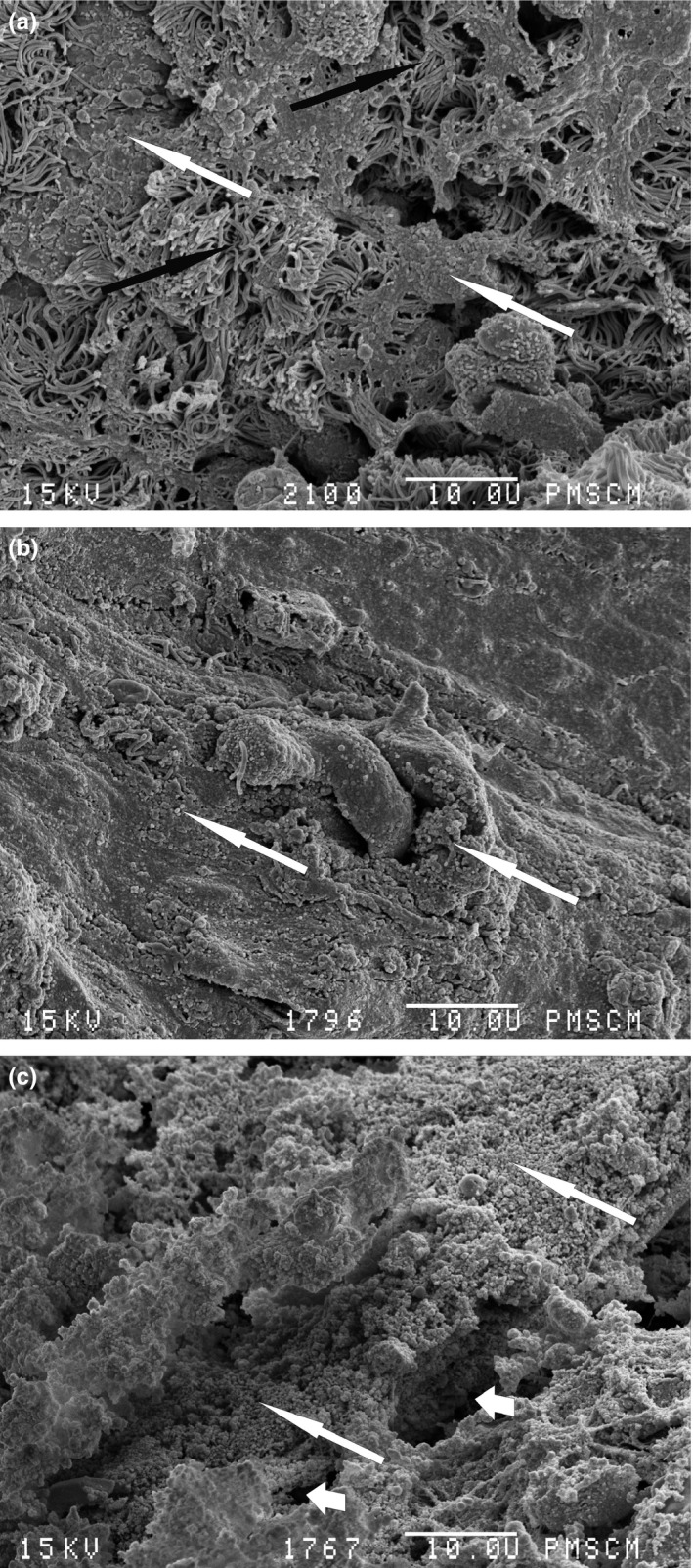
Scanning electron microscopy image. Biofilm structure. (a) Patient no. 8, black arrow – cilia, white arrow – biofilm matrix with multiple bacterial cells; (b) patient no. 4, white arrow – biofilm matrix with multiple bacterial cells; (c) patient no. 6, long white arrow – biofilm matrix with multiple bacterial cells, short white arrows – water channels. Magnification 2000×.
Figure 2.
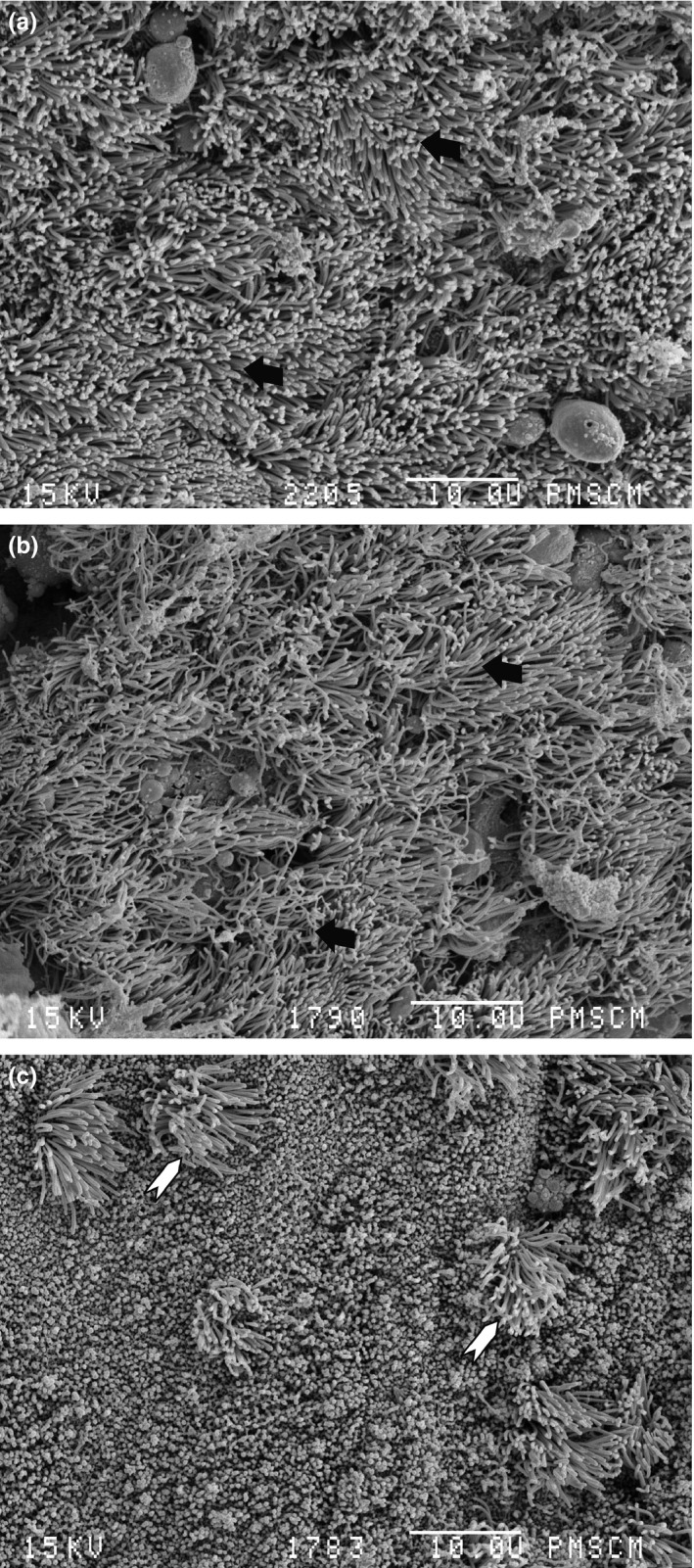
Scanning electron microscopy image. No biofilm structure can be seen. (a) Patient no. 9 with normal epithelium and cilia, black arrow – cilia; (b) patient no. 3 with normal epithelium and cilia, black arrow – cilia; (c) patient no. 2, non‐ciliated cylindrical epithelium with few spots of cilia, white arrow head – cilia spots. Magnification 2000×.
Identification of bacteria strains isolated from mucosa specimens
Microbiological analysis of mucosal specimens taken from 10 patients with CRS showed a high diversity of bacterial flora (Table 2). The most commonly isolated species of bacteria was S. epidermidis. Its presence was found in seven patients, in two of which S. epidermidis appeared in a monoculture, while in five patients, it was isolated with other bacteria. S. aureus and other species of bacteria were isolated from individual patients (incidence 10%). The density of bacteria in mucosa fragments varied from 102 to 105 CFU per gram tissue tested. There were no significant differences between bacteria species in numbers of isolated bacterial cells.
Table 2.
Bacterial species isolated from mucosa specimens of 10 patients participating in the study
| Groups of bacteria | Species | The number of isolates among 10 patients | CFU/g |
|---|---|---|---|
| Staphylococci | Staphylococcus aureus | 1 | 3.7 × 104 |
| Staphylococcus epidermidis | 7 | 1.8 × 102–2.3 × 104 | |
| Streptococci | Streptococcus agalactiae | 1 | 6.5 × 102 |
| Streptococcus mitis | 2 | 6.4 × 102–2.0 × 104 | |
| Anaerobic bacteria | Clostridium perfringens | 1 | 1.3 × 103 |
| Veilonella parvula | 1 | 1.0 × 104 | |
| Propionibacterium acnes | 2 | 8.1 × 102–5.3 × 105 | |
| Prevotella intermedia | 1 | 1.7 × 102 | |
| Peptostreptococcus | 1 | 2.3 × 103 | |
| Other | Pseudomonas aeruginosa | 1 | 1.5 × 103 |
| Klebsiella pneumoniae | 1 | 4.1 × 103 | |
| Moraxella catarrhalis | 1 | 2.6 × 103 | |
| Aerococcus uranie | 1 | 3.0 × 102 | |
| Lactococcus lactis cremoris | 1 | 4.9 × 103 |
Demonstration of neutrophil infiltration in mucosa fragments based on the measurement of MPO activity
To detect the tissue content of neutrophils in mucosa samples, the activity of MPO was measured. As shown in Figure 3, MPO activity ranged from 0.1 to 26 units per milligram of total protein extracted from the tissue, in all samples tested. The low level of MPO (<3 U/mg), similar to the background level of MPO within un‐inflamed mucosa or non‐neutrophil inflammation (Bradley et al. 1982; Nowak et al. 2010), was demonstrated in six of 10 (60%) patients. The high, inflammatory‐like level of MPO (>9 U/mg), suggesting neutrophil infiltration, was shown in 30% samples. Importantly, the mucosa specimens with bacterial biofilms contained MPO of low activity (0.1–0.5 U/mg) (No. 4, 6, 8 – see patient's characteristic in Table 1).
Discussion
For years, bacterial infections have been considered to have an established primary role in the pathogenesis of acute rhinosinusitis (ARS), while their role in CRS remains unclear. Such opinion was supported by a non‐effective antibiotic therapy in a majority of cases of CRS in contrast to ARS (Fokkens et al. 2012). Moreover, it has been postulated that bacteria found in chronically inflamed sinuses are associated with colonization but not infection of a sinus mucosa (Pandak et al. 2011). Our understanding of the role of bacteria in CRS has changed over the last decade when a number of papers demonstrated the presence of bacterial biofilm in mucosa specimens taken from patients with both CRSsNP and CRSwNP. Presently, a key role of biofilms in the pathogenesis of CRS with high resistance to antibiotics and to immune attack has been commonly accepted (Prince et al. 2008; Psaltis et al. 2008; Danielsen et al.2014).
The aim of this study was to identify biofilm‐forming bacterial species in mucosa specimens taken from patients with idiopathic CRS and to answer the question of whether neutrophils are present in the tested inflamed tissue samples. Moreover, we compared the activity of neutrophil MPO in the mucosal samples with and without biofilm. For this preliminary pilot study, we have qualified 10 patients who underwent ESS and fulfilled all the above‐mentioned criteria of exclusion.
All the selected patients showed clinical symptoms of moderate and severe chronic rhinosinusitis (CRS with the VAS score ranged from 7 to 10) confirmed by CT. Three of them have been diagnosed as allergic CRS (A‐CRS). Analysis of SEM images showed the presence of biofilm in three of 10 patients (incidence 30%). All of them were diagnosed as having severe non‐allergic CRS with nasal polyps (CRSwNP). Recently, we have reported the presence of biofilm in 33 of 80 patients with CRS, which demonstrated the incidence of 41%, corresponding to other data (Ferguson & Stolz 2005; Głowacki et al. 2013).
Unexpectedly, in the present study, S. epidermidis has been identified in all biofilm‐positive samples. On the other hand, in non‐biofilm mucosa samples, various pathogenic (S. aureus, P. aeruginosa) and habitant bacteria of the upper respiratory tract (S. epidermidis, S. mitis) have been found. Interestingly, S. epidermidis was isolated from 7 of 10 cases (incidence 70%), while S. aureus, a major cause of infectious diseases in humans, was found only in one patient (incidence 10%). The low frequency of S. aureus in our study, isolated only in one case, may be explained by the observed inverse correlation between S. epidermidis and S. aureus (Iwase et al. 2010). Recently, it has been demonstrated that serine protease especially secreted by S. epidermidis inhibits S. aureus colonization and the formation of S. aureus biofilm (Sugimoto et al. 2013; Vandecandelaere et al. 2014). Moreover, it was shown that S. aureus biofilms were more sensitive towards the neutrophil attack than S. epidermidis biofilms (Guenther et al. 2009). Interestingly, in our study, S. aureus was isolated from maxillary sinus, while S. epidermidis, in all positive cases, was isolated from ethmoidal or sphenoidal sinuses. This observation may be explained by the fact that sphenoethmoid recess serves as a common drainage pathway for ethmoid and sphenoid sinuses, but also may serve as a gateway to infections. It also confirms the idea that the ethmoid sinus area is a major focus for the initiation of CRS (Kennedy 2004). Therefore, our results confirmed data from other studies showing that S. epidermidis, the most prevalent bacteria of the skin and mucosa microflora, is not only a major nosocomial pathogen associated with infections of implanted medical devices but is also the most frequently isolated bacteria in CRS patients (O'Gara & Humphreys 2001). The high prevalence of S. epidermidis in CRS with biofilm formation may be explained by its permanent colonization of nasal cavity (Ramakrishnan et al. 2013) and massive production of slime, an exopolysaccharide essential for the irreversible adherence of S. epidermidis to mucosa in chronic infections (Nayak et al. 2011). However, in our study, S. epidermidis was mainly isolated along with other bacterial species. It suggests that multispecies biofilms exist in these patients. The occurrence of mixed biofilms has an important clinical implication as interactions among species within a biofilm lead to increased resistance to antibiotics and host defence compared to the mono‐species biofilms (Elias & Banin 2012; Burmølle et al. 2014). Therefore, it may correspond with the severity of CRS and therapeutic difficulties.
The next task of this study was to demonstrate the presence of neutrophils in inflamed mucosa of CRS patients with and without biofilm in mucosa specimens taken during ESS. Neutrophils, as first‐line defence cells in acute inflammation, kill planktonic bacteria either during phagocytosis or through a formation of neutrophil extracellular trap (Nauseef & Borregaard 2014). The effectiveness of a bactericidal activity of neutrophils depends on a cooperation of a number of proteolytic enzymes and reactive oxygen species (Klebanoff 2005). However, biofilm environment may polarize infiltrating neutrophils into functionally new subtypes of cells, namely into BAN. These cells have limited bactericidal capacity and, under some circumstances, can be even killed by biofilm‐derived toxins (Drenkard 2003; Jensen et al. 2007; Alhede et al. 2009). Some reports have demonstrated neutrophilic infiltration in CRS (Rowe‐Jones et al. 1997; Hirotsu et al. 2011). However, the presence and the role of BAN subtypes (N1 vs. N2 neutrophils) in chronic inflammations associated with biofilm, including CRS, need to be explained.
In this study, we have tried to estimate the presence of neutrophils in inflamed mucosa in CRS patients by measuring the enzymatic activity of MPO, a marker for tissue neutrophil content (Bradley et al. 1982). Myeloperoxidase, the most abundant neutrophil enzyme, is a key enzyme catalysing the production of bactericidal and highly reactive agents such as hypochlorous acid and tyrosyl radicals (Klebanoff et al. 2013). Therefore, an activity of MPO at a site of inflammation should be closely related to the microbicidal capability of neutrophils and to their contribution to the inflamed tissue damage (Schneider & Issekutz 1996). We have found such an association in the experimental model of collagen‐induced arthritis in mice. The level of MPO activity in joint tissue correlated with the neutrophil infiltration and with the severity of arthritis (Kwaśny‐Krochin et al. 2002; Marcinkiewicz et al. 2007). Herein, in our mucosa samples, the levels of MPO activity ranged from the low background level to the high level of MPO related to the inflamed tissue associated with neutrophil infiltration. Analysis of these preliminary results showed the lower level of MPO in the mucosa specimens with biofilm than that observed in the samples without biofilm (Figure 3). On the other hand, the level of MPO was not correlated with the type of identified bacterial species. However, the highest activity of MPO, indicating the presence of neutrophil infiltration, was demonstrated in mucosa specimens infected with S. aureus. Nevertheless, we detected the low level of MPO (<5 U/mg protein) in a majority of our patients (60%), all with CRSwNP. These preliminary results are in agreement with the recent data of Wang et al. (2014). They noticed that neutrophils were expressed poorly in CRSwNP. However, we did not demonstrate statistically significant differences in the degree of MPO activity due to high variations between individual specimens tested. It may be explained by the fact that the enzymatic activity of MPO, the assay widely used by researches, can vary considerably between individuals even if the amount of MPO protein is similar (Prokopowicz et al. 2012; Pulli et al. 2013). Such situation has to be taken into account especially in chronic inflammatory diseases associated with biofilm, as a number of antioxidants and agents neutralizing bactericidal molecules associated with biofilm environment have been described (Jesaitis et al. 2003; Hirschfeld 2014). Therefore, the number of biofilms associated neutrophils may not correspond to the MPO activity measured. Instead, it is closely correlated with the amount of intracellular MPO protein (Pulli et al. 2013). Importantly, in the case of measurement of neutrophil infiltration in biological samples using the enzymatic activity of MPO assay, the results should be complemented with MPO expression or protein‐level measurements which would provide more precise information regarding the abundance of the MPO molecule and the content of neutrophils (Pulli et al. 2013).
In conclusion, further studies with a larger number of CRS patients should be performed to estimate the association between the severity of CRS and the presence of different bacterial species, including S. epidermidis, and their ability to form biofilm. Additional experiments may also identify distinct subsets of neutrophils and/or macrophages polarized by the biofilm environment, as have been described in the tumour microenvironment (Fridlender et al. 2009; Galdiero et al. 2013). Finally, in the light of the important roles of different neutrophil subsets in chronic and recurrent infections, the identification of mechanisms and conditions required for polarization of BAN in CRS may provide a basis for new diagnostic and therapeutic strategies.
Acknowledgements
The authors would like to thank Prof. Jacek Składzień for giving helpful advice and Agnieszka Bator for the complement of patient data. This study was supported by Jagiellonian University College of Medicine grant no. K/ZDS/002964 and K/ZDS/003851 and the Ministry of Science and Higher Education (Poland) grant no. NN401547040.
References
- Alhede M., Bjarnsholt T., Jensen P.Ø. et al (2009) Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155, 3500–3508. [DOI] [PubMed] [Google Scholar]
- Bradley P.P., Priebat D.A., Christensen R.D. et al (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78, 206–209. [DOI] [PubMed] [Google Scholar]
- Burmølle M., Ren D., Bjarnsholt T. et al (2014) Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 22, 84–91. [DOI] [PubMed] [Google Scholar]
- Danielsen K.A., Eskeland O., Fridrich‐Aas K. et al (2014) Bacterial biofilms in patients with chronic rhinosinusitis: a confocal scanning laser microscopy study. Rhinology 52, 150–155. [DOI] [PubMed] [Google Scholar]
- Drenkard E. (2003) Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 5, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Elias S. & Banin E. (2012) Multi‐species biofilms: living with friendly neighbors. FEMS Microbiol. Rev. 36, 990–1004. [DOI] [PubMed] [Google Scholar]
- Ferguson B.J. & Stolz D.B. (2005) Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am. J. Rhinol. 19, 452–457. [PubMed] [Google Scholar]
- Fokkens W.J., Lund V.J., Mullol J. et al (2012) European position paper on Rhinosinusitis and nasal polyps 2012. Rhinol. Suppl. 23, 1–298. [PubMed] [Google Scholar]
- Fridlender Z.G., Sun J., Kim S. et al (2009) Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: “N1” versus “N2” TAN. Cancer Cell 16, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero M.R., Garlanda C., Jaillon S. et al (2013) Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 228, 1404–1412. [DOI] [PubMed] [Google Scholar]
- Głowacki R., Strek P., Zagórska‐Świeży K. et al (2008) Biofilm from patients with chronic rhinosinusitis. Morphological SEM studies. Otolaryngol. Pol. 62, 305–310. [DOI] [PubMed] [Google Scholar]
- Głowacki R., Tomaszewski K.A., Stręk P. et al (2013) The influence of bacterial biofilm on the clinical outcome of chronic rhinosinusitis: a prospective, double‐blind, scanning electron microscopy study. Eur. Arch. Otorhinolaryngol. 271, 1015–1021. [DOI] [PubMed] [Google Scholar]
- Guenther F., Stroh P., Wagner C. et al (2009) Phagocytosis of staphylococci biofilms by polymorphonuclear neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int. J. Artif. Organs 32, 565–573. [DOI] [PubMed] [Google Scholar]
- Günther F., Wabnitz G.H., Stroh P. et al (2009) Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol. Immunol. 46, 1805–1813. [DOI] [PubMed] [Google Scholar]
- Hirotsu M., Kikuchi K., Kusunoki T. et al (2011) Comparison of bacterial examinations between eosinophilic and neutrophilic chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 131, 997–1001. [DOI] [PubMed] [Google Scholar]
- Hirschfeld J. (2014) Dynamic interactions of neutrophils and biofilms. J. Oral. Microbiol. 6, 26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Shiozawa A., Ono N. et al (2013) Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 123, E1–E9. [DOI] [PubMed] [Google Scholar]
- Iwase T., Uehara Y., Shinji H. et al (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349. [DOI] [PubMed] [Google Scholar]
- Jensen P.Ø., Bjarnsholt T., Phipps R. et al (2007) Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum‐sensing‐controlled production of rhamnolipid by Pseudomonas aeruginosa . Microbiology 153, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Jesaitis A.J., Franklin M.J., Berglund D. et al (2003) Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171, 4329–4339. [DOI] [PubMed] [Google Scholar]
- Kennedy D.W. (2004) Pathogenesis of chronic rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 193, 6–9. [DOI] [PubMed] [Google Scholar]
- Klebanoff S.J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625. [DOI] [PubMed] [Google Scholar]
- Klebanoff S.J., Kettle A.J., Rosen H. et al (2013) Myeloperoxidase: a front‐line defender against phagocytosed microorganisms. J. Leukoc. Biol. 93, 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaśny‐Krochin B., Bobek M., Kontny E. et al (2002) Effect of taurine chloramine, the product of activated neutrophils, on the development of collagen‐induced arthritis in DBA 1/J mice. Amino Acids 23, 419–426. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J., Biedroń R., Maresz K. et al (2004) Oxidative modification of type II collagen differentially affects its arthritogenic and tolerogenic capacity in experimental arthritis. Arch. Immunol. Ther. Exp. 52, 284–291. [PubMed] [Google Scholar]
- Marcinkiewicz J., Głuszko P., Kontny E. et al (2007) Is Taurolidine a candidate for treatment of rheumatoid arthritis? Clin. Exp. Rheumatol. 25, 211–218. [PubMed] [Google Scholar]
- Marcinkiewicz J., Strus M. & Pasich E. (2013) Antibiotic resistance: a “dark side” of biofilm‐associated chronic infections. Pol. Arch. Med. Wewn. 123, 309–313. [PubMed] [Google Scholar]
- Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- Nauseef W.M. & Borregaard N. (2014) Neutrophils at work. Nat. Immunol. 15, 602–611. [DOI] [PubMed] [Google Scholar]
- Nayak N., Satpathy G., Nag H.L. et al (2011) Slime production is essential for the adherence of Staphylococcus epidermidis in implant‐related infections. J. Hosp. Infect. 77, 53–56. [DOI] [PubMed] [Google Scholar]
- Nowak B., Głuszko P., Ciszek‐Lenda M. et al (2010) High‐dose methotrexate ameliorates collagen‐induced arthritis but does not inhibit the release of proinflammatory cytokines by peritoneal macrophages in mice. Cent. Eur. J. Immunol. 35, 128–137. [Google Scholar]
- O'Gara J.P. & Humphreys H. (2001) Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 50, 582–587. [DOI] [PubMed] [Google Scholar]
- Pandak N., Pajić‐Penavić I., Sekelj A. et al (2011) Bacterial colonization or infection in chronic sinusitis. Wien. Klin. Wochenschr. 123, 710–713. [DOI] [PubMed] [Google Scholar]
- Perloff J.R. & Palmer J.N. (2004) Evidence of bacterial biofilms on frontal recess stents in patients with chronic rhinosinusitis. Am. J. Rhinol. 18, 377–380. [PubMed] [Google Scholar]
- Prince A.A., Steiger J.D., Khalid A.N. et al (2008) Prevalence of biofilm‐forming bacteria in chronic rhinosinusitis. Am. J. Rhinol. 22, 239–245. [DOI] [PubMed] [Google Scholar]
- Prokopowicz Z., Marcinkiewicz J., Katz D.R. et al (2012) Neutrophil myeloperoxidase: soldier and statesman. Arch. Immunol. Ther. Exp. (Warsz) 60, 43–54. [DOI] [PubMed] [Google Scholar]
- Psaltis A.J., Weitzel E.K., Ha K.R. et al (2008) The effect of bacterial biofilms on post‐sinus surgical outcomes. Am. J. Rhinol. 22, 1–6. [DOI] [PubMed] [Google Scholar]
- Pulli B., Ali M., Forghani R. et al (2013) Measuring myeloperoxidase activity in biological samples. PLoS ONE 8, e67976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V.R., Feazel L.M., Gitomer S.A. et al (2013) The microbiome of the middle meatus in healthy adults. PLoS ONE 8, e85507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe‐Jones J.M., Trendell‐Smith N., Shembekar M. et al (1997) Polypoid rhinosinusitis in patients with host defence deficiencies: cellular infiltration and disease severity. Rhinology 35, 113–117. [PubMed] [Google Scholar]
- Sachse F., von Eiff C., Becker K. et al (2008) Proinflammatory impact of Staphylococcus epidermidis on the nasal epithelium quantified by IL‐8 and GRO‐alpha responses in primary human nasal epithelial cells. Int. Arch. Allergy Immunol. 145, 24–32. [DOI] [PubMed] [Google Scholar]
- Schneider T. & Issekutz A.C. (1996) Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J. Immunol. Methods 198, 1–14. [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Iwamoto T., Takada K. et al (2013) Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host‐pathogen interaction. J. Bacteriol. 195, 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow L.R., Hanke M.L., Fritz T. et al (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186, 6585–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecandelaere I., Depuydt P., Nelis H.J. et al (2014) Protease production by Staphylococcus epidermidis and its effect on Staphylococcus aureus biofilms. Pathog. Dis. 70, 321–331. [DOI] [PubMed] [Google Scholar]
- Wang X., Du J. & Zhao C. (2014) Bacterial biofilms are associated with inflammatory cells infiltration and the innate immunity in chronic rhinosinusitis with or without nasal polyps. Inflammation 37, 871–879. [DOI] [PubMed] [Google Scholar]


