Summary
To delay age‐related bone loss, physical activity is recommended during growth. However, it is unknown whether interval training is more efficient than continuous training to increase bone mass both quickly and to a greater extent. The aim of this study was to compare the effects of a 10‐week interval training regime with a 14‐week continuous training regime on bone mineral density (BMD). Forty‐four male Wistar rats (8 weeks old) were separated into four groups: control for 10 weeks (C10), control for 14 weeks (C14), moderate interval training for 10 weeks (IT) and moderate continuous training for 14 weeks (CT). Rats were exercised 1 h/day, 5 day/week. Body composition and BMD of the whole body and femur respectively were assessed by dual‐energy X‐ray absorptiometry at baseline and after training to determine raw gain and weight‐normalized BMD gain. Both trained groups had lower weight and fat mass gain when compared to controls. Both trained groups gained more BMD compared to controls when normalized to body weight. Using a 30% shorter training period, the IT group showed more than 20% higher whole body and femur BMD gains compared to the CT. Our data suggest that moderate IT was able to produce faster bone adaptations than moderate CT.
Keywords: body composition, bone mineral density, exercise, rat, running
Osteoporosis is characterized by a dramatic decrease in bone mineral density (BMD) increasing the risk of fracture. Exercise is known to induce bone benefits and to prevent osteoporosis (Bonaiuti et al. 2002). It has been demonstrated that impact exercises such as artistic or rhythmic gymnastics improved BMD (Maimoun et al. 2013). Exercise stimulates bone formation (calcitonin, osteocalcin, alkaline phosphatase) and slightly limits bone resorption (PTH, NTx, CTx, TRAP, pyridinoline) as demonstrated by observations regarding circulating hormones and bone biomarkers (Tartibian et al. 2011; Maurel et al. 2013). Consequently, the bone remodelling leads to an enhancement of the microarchitecture. Generally rats have been used to study and understand the effects of exercise on bone, mainly by means of treadmill (Iwamoto et al. 2005). To date, there has been considerable heterogeneity of exercise duration and intensity, as well as heterogeneity of its effects, and this does not allow researchers to establish a consensus about the most appropriate form of training. Some authors reported a negative effect (Bourrin et al. 1994) or no gain (Cavalie et al. 2002) as a result of treadmill training on bone. In 2005, a review focused on the effect of treadmill exercise in female rats clearly indicated that running did not modify BMD as measured by DXA in growing rats (Iwamoto et al. 2005). There is a lack of data concerning in vivo BMD gains. Moreover, to our knowledge, no study has shown in vivo whole body BMD gains using running. Only two studies reported extremely localized in vivo BMD gains in the tibia and the vertebrae using continuous training (Yeh et al. 1993; Yao et al. 2004). The first of these employed 14‐month‐old female Sprague–Dawley rats, running (17 m/min) 1 h/day, 5 days/week, and reported tibia/fibula BMD gain after 16 weeks of training (non‐significant after 9 weeks). The vertebral BMD was significantly higher in trained rats after 9 weeks and remained higher after 16 weeks compared to controls (Yeh et al. 1993). The second study was performed on 10‐month‐old male Wistar rats, running (20–30 m/min) 1 h 30 m/day, 5 days/week. Tibial metaphysis BMD gain was significantly higher after 4 and 5 weeks of training compared to controls (Yao et al. 2004). The heterogeneity of exercise effects in the literature might be explained by differences in age, sex, rat species and bone analysed, but also by the heterogeneity of the exercise protocols.
Many animal models and various training modes, including treadmill running (Kemi et al. 2005), jumping (Ducomps et al. 2004) and resistance training (Sanches et al. 2014), have been used to investigate the effects and mechanisms of exercise. In addition, the time commitment for most interval training programmes is considerably lower than that traditionally prescribed in low‐intensity continuous exercise training programmes (Gibala & McGee 2008). The different regimes that have been prescribed have been highlighted in the current debates in the exercise literature in the rat. Different investigators are attempting to determine the impacts of the different loads and the time spent to practise and the optimum regime has not as yet emerged.
It is perhaps expected that moderate interval training (IT) produces faster bone adaptations than does a moderate continuous training (CT). Actually, in a preliminary study, Chen et al. have shown that rats exercised by IT had higher ex vivo femur BMD compared with untrained controls in 4 weeks (Chen et al. 2004). To our knowledge, studies aiming to compare CT and IT exercises on BMD gain, particularly regarding the time efficacy have not been described in the literature.
Consequently, we hypothesized that an IT programme would be able to increase BMD in a shorter period than CT. Thus, the aim of this study was to compare a 10‐week moderate IT programme with a 14‐week moderate CT programme on whole body and femur BMD gains in male growing rats.
Materials and methods
Animals
Forty‐four male Wistar rats, 8 weeks old at baseline, were received from Janvier Breeding (Le Genest St Isle, France) and acclimatized for 2 weeks, until 10 weeks old, at constant temperature (21°C ± 2°C), under a 12 h/12 h light/dark cycle. Rats were divided randomly into four groups: control followed for 10 weeks (C10), control followed for 14 weeks (C14), IT programme followed for 10 weeks (IT) and moderate CT programme followed for 14 weeks (CT). All rats had an access to commercial standard diet (M20; SDS, France) and water ad libitum. All animals were allowed to move freely in standardized cages. The procedure for the care and euthanasia of the animals was in accordance with the European Community Standards on the Care and Use of Laboratory Animals. The study was approved by a board institution and an ethics committee (agreement no C45‐234‐9 and 2011‐11‐2) from the French Institute INSERM (Institut National de la Santé et de la Recherche Médicale) and from the agriculture council (Ministère de l'agriculture, France, approval ID: INSERM45‐001). This study has been performed in accordance with the ethical standards for animals.
Ethical Approval statement
This study has been performed in accordance with the ethical standards for animals.
Maximal aerobic speed test
Maximal aerobic speed (MAS), evaluated in m/min, was measured in the two trained groups by an incremental exercise test as previously used in our laboratory (Maurel et al. 2011). This test, achieved on a calibrated treadmill, gives the level of aerobic aptitude which corresponds to the maximal oxygen uptake (Hoydal et al. 2007). The protocol used was first described by Boissiere et al. (2008). The test started by 5 min of warm‐up to a 7.5 m/min running speed, and then, the running speed was increased by 1.5 m/min every 2 min. MAS was determined when the rat was not able to sustain the speed. To validate our training programme, MAS was evaluated at the end of each training programme (IT, week 10; CT, week 14).
Treadmill training protocols
Duration
The same treadmill was used in both exercise protocols with no incline as described by Ip et al. (2009). It has been shown that moderate 10‐week IT was efficient to improve cardiovascular function and performance in rats (Kemi et al. 2005). In contrast, two studies reported no BMD gain using a CT of this duration (Bonnet et al. 2007a,b). However, a study revealed positive effects on weight‐normalized femur BMD with 14 weeks of CT (Pajamaki et al. 2003). Consequently, we decided to compare 10 weeks for IT with a longer CT of 14 weeks.
Intensity
During the first week, the treadmill speed was progressively increased (from 7 to 15 m/min). The speeds were progressively increased during the study (Table 1). Both training protocols lasted 60 min/session, one session/day, 5 days/week during 10 (IT) or 14 (CT) weeks (Boudenot et al. 2012; Maurel et al. 2013).
Table 1.
Training programme, intensity scores and speed progression
| Weeks | Session duration (min/day) | Continuous Training (CT) | Interval Training (IT) | ||
|---|---|---|---|---|---|
| Speeds (m/min) | Intensity scores | Speeds (m/min) | Intensity scores | ||
| 1 | 20–40 | 7 | 1.0 | 8/14 | 2.0 |
| 2 | 60 | 10 | 1.0 | 10/15.3 | 1.3 |
| 3 | 60 | 14 | 1.0 | 10/15.3 | 1.3 |
| 4 | 60 | 15 | 1.0 | 11/19 | 1.3 |
| 5 | 60 | 16 | 2.0 | 11/19 | 1.3 |
| 6 | 60 | 16 | 2.0 | 12/20 | 1.3 |
| 7 | 60 | 17 | 2.0 | 13/23 | 1.5 |
| 8 | 60 | 17 | 2.0 | 13/23 | 1.5 |
| 9 | 60 | 17 | 2.0 | 14/23 | 1.5 |
| 10 | 60 | 17 | 2.0 | 15/24 | 1.5 |
| 11 | 60 | 20 | 2.0 | ||
| 12 | 60 | 20 | 2.0 | ||
| 13 | 60 | 20 | 2.0 | ||
| 14 | 60 | 20 | 2.0 | ||
Interval training lasted 10 weeks, and continuous training lasted 14 weeks. Interval training alternated between moderate and high intensity; thus, two values are presented (low/high). Intensity scores were calculated as described in the Materials and Methods section (arbitrary unit).
It is considered that rats are walking under 10 m/min (Jarvinen et al. 1998), are running at slight speed until 15 m/min (Patch & Brooks 1980), are running at moderate speed until 20 m/min (Chae & Kim 2009; Ip et al. 2009) and are running at intense speed above 20 m/min (Bourrin et al. 1994). Intensity scores were adapted to obtain three intensity levels (I#1, I#2 and I#3) corresponding to three coefficients (I#1: low speed ≤15 m/min, coefficient 1; I#2 moderate speed: 15–20 m/min, coefficient 2; I#3 intensive speed: >20 m/min, coefficient 3). Then, scores were calculated based on the following equation (Mujika et al. 1995; Atlaoui et al. 2007):
The intensity score was calculated each week for both training groups. Then, the averaged intensity score was calculated as the mean value of each week's score. This score gives information about the difficulty of the exercise. The higher the score is, the more difficult the exercise is. The intensity, volumes and workload of both trainings are presented in Table 2.
Table 2.
Intensity, volumes and workload of both trainings
| CT | IT | |
|---|---|---|
| Maximal aerobic speed (m/min) | 29.2 ± 4.5 | 33.9 ± 3.5* |
| Averaged running speeds (m/min) | 16 | 12/20 |
| Averaged Intensity scores | 1.71 | 1.44 |
| Effective volume of session (min) | 60 | 53 |
| Total effective volume (h) | 70 | 46 |
| Workload | 120 | 66 |
CT, continuous training; IT, interval training. Intensity scores were calculated as described in the Materials and Methods section (arbitrary unit). Effective volume of training excludes passive recovery period (i.e. treadmill stopped). Workload was calculated by multiplying intensity score and total effective volume (arbitrary unit). IT alternated between moderate and high intensity; thus, two values are presented for averaged running speeds (low/high). Only maximal aerobic speed parameter was statistically evaluated as we obtained only one value per training, not per rat, for remaining parameters.
*P = 0.01.
Moderate interval training
The speeds used for this training are presented in Table 1.
The IT programme consisted of 5 min of moderate speed (Chae & Kim 2009; Ip et al. 2009), then 2 min of intensive running (Bourrin et al. 1994) and 1 min of passive recovery (i.e. treadmill stopped). These three periods were repeated sevenfold, and the training session finished by 4 min of moderate speed. At the end of this 1‐h training session, the rats were placed back in their cages. The IT programme lasted 10 weeks.
Moderate continuous training
The CT programme was considered as moderate exercise training (Chae & Kim 2009; Ip et al. 2009). In this protocol, rats had to run for 1 h without recovery during the training session. At the end of this training session, the rats were placed back in their cages. The CT programme lasted 14 weeks. The speeds used for this training are presented in Table 1.
Dual‐energy X‐ray absorptiometry assessment
Body composition was evaluated by DXA (Discovery; Hologic, Bedford, MA, USA). Rats were previously anesthetized with a dose of 0.1 ml of pentobarbital per 100 g body weight diluted in saline solution 50% v/v. Using the small animal‐specific software, we measured total weight, lean mass, fat mass and BMD on the whole body. Moreover, we used the high‐resolution software to analyse BMD on the femur. The root‐mean‐square coefficient of variation of in vivo whole body BMD was 0.87%, and for femur BMD, it was 0.41%. All these parameters were assessed before and after each training programme. Thus, body composition gain (g), BMD gain (g/cm²) and weight‐normalized BMD gain (mg/cm²/g) were calculated. Gains were calculated by the formula: (end value) – (baseline value). As trained rats had a lower body weight and as body weight is a major determinant of bone mineral density, whole body BMD gains were normalized using the formula: whole body BMD gain/whole body weight gain (Kannus et al. 1996; Pajamaki et al. 2003).
Data analysis
The normality of the distributions was assessed with the Shapiro–Wilk test and the homogeneity of the variances with the Levene test. Time effect was controlled using one‐way anova. Between‐groups comparisons were performed by a one‐way anova. The relationships between either final body weight or final lean mass and final BMD were study using Pearson correlation. The Tukey post hoc test was used to determine differences between the groups following the anova. Student's t‐test was used to compare MAS of trained rats. These statistical analyses were performed with the software SAS 9.3. Data are presented as mean ± standard deviation (SD). A significance level of P < 0.05 was used for all statistical tests.
Results
Comparison of interval and continuous training performances
All training parameters and performances are presented in both Figure 1 and Table 2.
Figure 1.
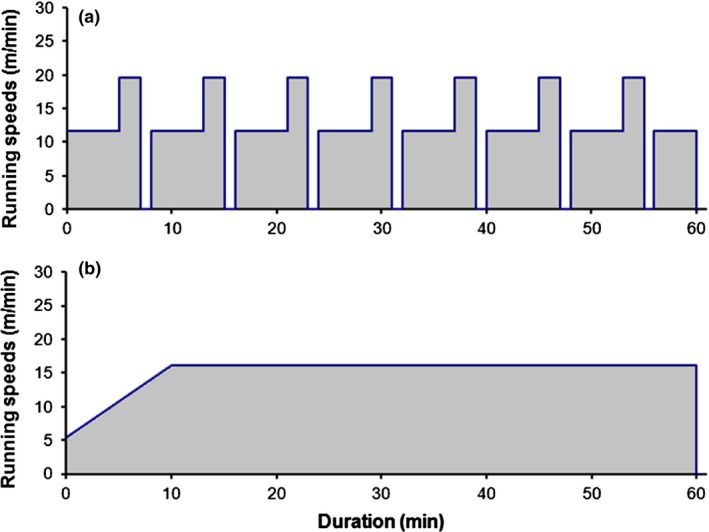
Training protocols: interval training (a) and moderate continuous training (b). The interval training programme consisted of seven cycles of 5 min of moderate speed (i.e. 12 m/min), then 2 min of intensive running (i.e. 20 m/min) and 1 min of passive recovery (i.e. treadmill stopped), and the training session finished by 4 min of moderate speed. Both training sessions lasted 1 h per day, 5 days a week. Running speeds correspond to the averaged speeds during all the protocols, expressed in metres per minute (m/min). Corresponding speeds for each week and training are reported in Table 1.
The averaged intensity score was 19% higher in CT than in IT. Workload was 82% higher in CT compared to IT. Higher workload implies higher quantity of training as it is composed of both intensity and time spent at exercise.
At the end of the two protocols, MAS was 4.7 m/min higher in IT compared to CT (P < 0.01). It reflects the efficiency of the moderate intermittent protocol compared to the moderate continuous protocol, although both workload and intensity scores were lower in IT compared to CT.
Body composition gains
Data are presented in Table 3. Concerning body weight and fat mass gains, no difference was observed between the control groups C10 and C14, and between the trained groups IT and CT. However, these latter parameters were significantly lower in IT (body weight: −25%; fat mass: −58%) and CT (body weight: −37%; fat mass: −48%) compared to their respective controls. Concerning lean mass gains, CT had significant lower values than its control C14 (−32%), when no difference was observed between IT and its control C10.
Table 3.
Body composition, at baseline and at the end of each protocol, and body composition gains in the four groups
| C10 (a) n = 11 | IT (b) n = 11 | C14 (c) n = 12 | CT (d) n = 10 | |
|---|---|---|---|---|
| Baseline | ||||
| Body weight (g) | 386.2 ± 14.4‡ | 384.6 ± 15.4§ | 414.7 ± 25.4 | 416.9 ± 24.9 |
| Lean Mass (g) | 335.4 ± 12.9‡ | 338.7 ± 12.0 | 361.2 ± 21.9 | 346.2 ± 23.0 |
| Fat Mass (g) | 40.5 ± 6.3 | 36.2 ± 6.9§ | 42.5 ± 10.0§ | 59.4 ± 10.2 |
| Fat Mass (%) | 10.5 ± 1.6§ | 9.4 ± 1.5§ | 10.2 ± 2.2§ | 14.3 ± 2.2 |
| End | ||||
| Body weight (g) | 615.2 ± 45.0* | 556.6 ± 32.8* , † | 641.6 ± 56.3* | 560.5 ± 38.6* , ‡ |
| Lean Mass (g) | 479.4 ± 32.5* , ‡ | 473.7 ± 31.9* | 515.0 ± 30.8* | 450.2 ± 28.4* , ‡ |
| Fat Mass (g) | 111.4 ± 32.0* | 66.1 ± 19.2* , † | 107.0 ± 35.7* | 92.7 ± 18.0* |
| Fat Mass (%) | 18.0 ± 4.4* | 11.8 ± 3.3* , † , ‡ , § | 16.4 ± 4.3* | 16.5 ± 2.4* |
| Gains | ||||
| Body weight (g) | 229.0 ± 33.5 | 171.9 ± 30.6† | 226.9 ± 45.5 | 143.7 ± 24.9‡ |
| Lean Mass (g) | 144.0 ± 23.8 | 135.0 ± 30.6 | 153.8 ± 35.3 | 103.9 ± 15.3‡ |
| Fat Mass (g) | 70.9 ± 27.6 | 29.9 ± 15.8† | 64.5 ± 27.1 | 33.3 ± 12.1‡ |
Data are presented as mean ± SD.
*End values were significantly higher than the baseline values (P < 0.0001), in all groups and parameters. Gains were calculated as end values – baseline values. Control 10 (C10) and interval training (IT) were followed for 10 weeks, and control 14 (C14) and moderate continuous training (CT) were followed for 14 weeks. Differences between groups were considered significant at P < 0.05.
†Significantly lower than C10; ‡significantly lower than C14; §significantly lower than CT.
Bone mineral density
IT group had significant higher whole body and femur BMD gains compared to CT group (24% and 23% respectively). Moreover, the whole body and femur BMD gains were lower in CT than in C14 (−25% and −30% respectively), but no significant difference was seen between IT and its control C10. Data are reported in Table 4.
Table 4.
Bone mineral density (BMD) at baseline and at the end of each protocol, BMD gains and weight‐normalized BMD gains in the four groups
| C10 (a) n = 11 | IT (b) n = 11 | C14 (c) n = 12 | CT (d) n = 10 | |
|---|---|---|---|---|
| Baseline | ||||
| Whole body BMD (g/cm²) | 0.147 ± 0.004‡ | 0.144 ± 0.006§ | 0.154 ± 0.005 | 0.155 ± 0.005 |
| Femur BMD (g/cm²) | 0.253 ± 0.011‡ | 0.253 ± 0.013§ | 0.272 ± 0.013 | 0.281 ± 0.013 |
| End | ||||
| Whole body BMD (g/cm²) | 0.194 ± 0.008* , ‡ | 0.191 ± 0.006* | 0.205 ± 0.006* | 0.194 ± 0.006* , ‡ |
| Femur BMD (g/cm²) | 0.349 ± 0.014* , ‡ | 0.353 ± 0.012* | 0.389 ± 0.020* | 0.364 ± 0.013* , ‡ |
| Gains | ||||
| Whole body BMD (g/cm²) | 0.047 ± 0.006 | 0.047 ± 0.005 | 0.051 ± 0.005 | 0.038 ± 0.004† , ‡ |
| Femur BMD (g/cm²) | 0.096 ± 0.014‡ | 0.101 ± 0.009 | 0.117 ± 0.017 | 0.082 ± 0.011† , ‡ |
| Normalized gains | ||||
| Normalized whole body BMD (mg/cm²/g) | 0.206 ± 0.036† , § | 0.283 ± 0.060 | 0.231 ± 0.038† , § | 0.274 ± 0.051 |
Data are presented as mean ± SD.
*End values were significantly higher than the baseline values (P < 0.0001), in all groups and parameters. Gains were calculated as end values – baseline values. Normalized BMD gains were calculated by whole body BMD gain divided by body weight gain. Control 10 (C10) and interval training (IT) were followed for 10 weeks, and control 14 (C14) and moderate continuous training (CT) were followed for 14 weeks.
Differences between groups were considered significant at P < 0.05. †significantly lower than IT; ‡significantly lower than C14; §significantly lower than CT.
We observed a significant moderate correlation between final body weight and final BMD (r = 0.5, P = 0.0004). Moreover, as trained rats had a lower body weight and as body weight is a major determinant of bone mineral density, whole body BMD gains were normalized to body weight. As a result, when whole body BMD gains were normalized, we observed a significant positive effect of exercise. Therefore, values were +37% higher for IT compared to C10, and +19% for CT compared to C14 (Table 4).
Lean mass was positively correlated with femur BMD (r = 0.57, P < 0.0001) and whole body BMD (r = 0.66, P < 0.0001).
Discussion
Few animal studies have compared training modalities on bone status. Our study is the first to compare moderate IT and moderate CT on BMD in rats. According to our hypothesis, moderate IT was able to induce faster and higher BMD gains than moderate CT despite shorter training time.
The major finding of this study was the positive effect of exercise on normalized BMD gains. Interestingly, our data suggest that training quality is more important than training quantity, as our IT produced better effects than CT despite lower global workload. Indeed, IT rats showed higher whole body and femur BMD gains compared to CT. No BMD gain has been found using 12 weeks of moderate CT (1 h/day, 6 days/week) in female Wistar rats (Cavalie et al. 2002). In contrast, higher BMD values have been shown only after 4 weeks with high‐speed IT (25 m/min, 1 h/day, 5 days/week) compared to untrained rats (Chen et al. 2004). This intensity effect has been reported on BMD normalized to body weight (Kannus et al. 1996). In this study, BMD values were normalized to make the groups comparable in terms of loading environment. Indeed, the moderate CT (running speed: 18 m/min) produced better effects on the rat femur than the slight CT running speed: 12 m/min) reported previously (Kannus et al. 1996).
So, the exercise intensity seems to be a major determinant for bone benefits. It has been shown that benefits increase linearly with running speed in both human (Mercer et al. 2010) and animals (Rubin & Lanyon 1982), meaning that higher running speed leads to higher mechanical loadings. Impact induce bone matrix deformation, leading to interstitial higher bone fluid flow (Qin & Hu 2014). It is possible that high impact produced by higher speeds leads to an increase of fluid exchange into the lacuno‐canalicular network. Osteocytes communicate with each other via molecules transported by fluid flow such as Ca2+, nitric oxide (NO), prostaglandin E2 (PGE2) and Wnt (Bonewald & Johnson 2008), which are released in higher amounts with fluid flow (Bonewald & Johnson 2008; Kamel et al. 2010). These molecules are known to favour bone formation and bone remodelling. Heavy mechanical loadings provided by exercise not only stimulate bone formation but also decrease bone resorption. Indeed, mechanical loadings have led to a lower expression of sclerostin, which is an antagonist of the Wnt/β‐catenin pathway (Papanicolaou et al. 2009; Moustafa et al. 2012). These mechanisms could explain why IT, composed of 7 sequences of high running speeds (2 min), has increased the gain of BMD in less time (10 weeks versus 14 weeks) than CT. The high speeds in IT were 10–40% higher than the speeds used in CT.
Thus, it appears that maintaining a satisfactory intensity is a major determinant of training quality. Moreover, the high impacts perceived by the skeleton during high intensity were followed by passive recovery in IT protocol. We have hypothesized that such a passive recovery was needed to recover aerobic and bone functions. Turner and Robling suggested that recovery limits the bone ‘desensitization’ phenomenon observed after the first few minutes of exercise (Turner & Robling 2004). Umemura et al. also suggested that 30 s of recovery was necessary between jumps to recover the mechanosensor sensitivity in order to enhance the effects of loading (Umemura et al. 2002). Our IT protocol with cycles of 5 min of moderate speed, 2 min of intensive running and 1 min of recovery was able to increase BMD gain in less time than our moderate continuous training. Besides, the whole body and femur BMD gains were respectively 24% and 23% higher in IT vs. CT. Adding rest periods is another determinant of training quality. So, both intensity and recovery are interesting parameters allowing researchers to reduce the time spent for animal experiment in order to obtain exercise benefits quickly.
It is important to dissect the effect of growth from the effect of exercise on BMD. All rats were growing during the protocol (Table 3 and Table 4). At sacrifice, although BMD values were higher for C14 than for C10, our analysis revealed no differences in femur length (P = 0.58) and surface (P = 0.22) between groups (data not shown) suggesting a slowdown of growth. Beyond this growing effect, the major effect on BMD may be attributable to exercise, mainly for IT. We observed significantly lower body weight and fat mass gains in both trained groups compared to controls, as previously observed with exercise (Cavalie et al. 2003; Frisbee et al. 2006). Due to this lower body weight in trained vs. controls, weight‐normalized BMD gains were used to isolate the exercise effect as previously employed in order to make the groups comparable in terms of loadings effects (Kannus et al. 1996; Pajamaki et al. 2003). IT and CT had respectively 37% and 19% higher normalized BMD gains compared to their respective controls. Moreover, lean mass gains were higher for IT compared to CT, while fat mass gains did not differ. Recently, it has been suggested that muscle exerts endocrine regulation of bone mass; a deletion of myostatin increases both muscle mass and bone strength and density (Brotto & Johnson 2014). Moreover, BMD and lean mass were correlated in our study. The positive effect of IT on muscle could possibly also affect bone through myokine influence. Thus, the higher BMD gain in IT compared to CT might be explained in part by this specific crosstalk between muscle and bone (Brotto & Johnson 2014).
Conclusion
Moderate IT produces quicker BMD adaptations than moderate CT. IT could be efficient to improve peak bone mass in human and is also a lesser time‐consuming strategy. This kind of exercise has to be tested to counteract the osteopenia‐induced deleterious effects on bone.
Disclosures
The authors have no conflict of interest.
Acknowledgements
Arnaud BOUDENOT obtained a young investigator award at the 2011 annual congress of Association des Chercheurs en Activités Physiques et Sportives (ACAPS), in Rennes (France), for the preliminary results.
The authors thank Eric DOLLEANS and Zahra ACHIOU from I3MTO Laboratory in Orléans, for animal experiment and technical assistance. We thank Gérard NOYER from University of Orléans for technical help on treadmill for the training of the rats and Philippe MOREAU from the Laboratory of Neurobiology in Orléans for the housing of the rats.
We confirm that all listed authors meet ICMJE authorship criteria and that nobody who qualifies for authorship has been excluded.
References
- Atlaoui D., Pichot V., Lacoste L., Barale F., Lacour J.R. & Chatard J.C. (2007) Heart rate variability, training variation and performance in elite swimmers. Int. J. Sports Med. 28, 394–400. [DOI] [PubMed] [Google Scholar]
- Boissiere J., Eder V., Machet M.C., Courteix D. & Bonnet P. (2008) Moderate exercise training does not worsen left ventricle remodeling and function in untreated severe hypertensive rats. J. Appl. Physiol. 104, 321–327. [DOI] [PubMed] [Google Scholar]
- Bonaiuti D., Shea B., Iovine R. et al (2002) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. CD000333. [DOI] [PubMed] [Google Scholar]
- Bonewald L.F. & Johnson M.L. (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N., Beaupied H., Vico L. et al (2007a) Combined effects of exercise and propranolol on bone tissue in ovariectomized rats. J. Bone Miner. Res. 22, 578–588. [DOI] [PubMed] [Google Scholar]
- Bonnet N., Benhamou C.L., Beaupied H. et al (2007b) Doping dose of salbutamol and exercise: deleterious effect on cancellous and cortical bones in adult rats. J. Appl. Physiol. 102, 1502–1509. [DOI] [PubMed] [Google Scholar]
- Boudenot A., Pallu S., Lespessailles E. & Jaffré C. (2012) Effets de l'entraînement intermittent sur la composition corporelle, la masse et la densité minérale osseuses chez le rat. Sci. Sports 23, 188–191. [Google Scholar]
- Bourrin S., Genty C., Palle S., Gharib C. & Alexandre C. (1994) Adverse effects of strenuous exercise: a densitometric and histomorphometric study in the rat. J. Appl. Physiol. 76, 1999–2005. [DOI] [PubMed] [Google Scholar]
- Brotto M. & Johnson M.L. (2014) Endocrine crosstalk between muscle and bone. Curr. Osteoporos. Rep. 12, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalie H., Malochet S., Lebecque P., Horcajada‐Molteni M.N., Picherit C. & Barlet J.P. (2002) Effects of endurance running and/or isoflavones on bone mineral density in ovariectomized adult female rat. Sci. Sports 17, 312–314. [Google Scholar]
- Cavalie H., Horcajada‐Molteni M.N., Lebecque P. et al (2003) Progressive isometric force training and bone mass in rats. J. Musculoskelet. Neuronal Interact. 3, 47–52. [PubMed] [Google Scholar]
- Chae C.H. & Kim H.T. (2009) Forced, moderate‐intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3‐kinase signaling in the hippocampus of induced aging rats. Neurochem. Int. 55, 208–213. [DOI] [PubMed] [Google Scholar]
- Chen X., Aoki H. & Fukui Y. (2004) Effect of exercise on the bone strength, bone mineral density, and metal content in rat femurs. Biomed. Mater. Eng. 14, 53–59. [PubMed] [Google Scholar]
- Ducomps C., Larrouy D., Mairal A., Doutreloux J.P., Lebas F., Mauriege P. (2004) Effects of jump training on procollagen alpha(1)(i) mRNA expression and its relationship with muscle collagen concentration. Can. J. Appl. Physiol. 29, 157–171. [DOI] [PubMed] [Google Scholar]
- Frisbee J.C., Samora J.B., Peterson J. & Bryner R. (2006) Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 291, H2483–H2492. [DOI] [PubMed] [Google Scholar]
- Gibala M.J. & McGee S.L. (2008) Metabolic adaptations to short‐term high‐intensity interval training: a little pain for a lot of gain? Exerc. Sport Sci. Rev. 36, 58–63. [DOI] [PubMed] [Google Scholar]
- Hoydal M.A., Wisloff U., Kemi O.J. & Ellingsen O. (2007) Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 14, 753–760. [DOI] [PubMed] [Google Scholar]
- Ip T.Y., Peterson J., Byrner R. & Tou J.C. (2009) Bone responses to body weight and moderate treadmill exercising in growing male obese (fa/fa) and lean Zucker rats. J. Musculoskelet. Neuronal Interact. 9, 155–166. [PubMed] [Google Scholar]
- Iwamoto J., Takeda T. & Sato Y. (2005) Effect of treadmill exercise on bone mass in female rats. Exp. Anim. 54, 1–6. [DOI] [PubMed] [Google Scholar]
- Jarvinen T.L., Kannus P., Sievanen H., Jolma P., Heinonen A. & Jarvinen M. (1998) Randomized controlled study of effects of sudden impact loading on rat femur. J. Bone Miner. Res. 13, 1475–1482. [DOI] [PubMed] [Google Scholar]
- Kamel M.A., Picconi J.L., Lara‐Castillo N. & Johnson M.L. (2010) Activation of beta‐catenin signaling in MLO‐Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone 47, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P., Jarvinen T.L., Sievanen H. et al (1996) Effects of immobilization, three forms of remobilization, and subsequent deconditioning on bone mineral content and density in rat femora. J. Bone Miner. Res. 11, 1339–1346. [DOI] [PubMed] [Google Scholar]
- Kemi O.J., Haram P.M., Loennechen J.P. et al (2005) Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc. Res. 67, 161–172. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Mura T. et al (2013) Specific bone mass acquisition in elite female athletes. J. Clin. Endocrinol. Metab. 98, 2844–2853. [DOI] [PubMed] [Google Scholar]
- Maurel D.B., Boisseau N., Ingrand I., Dolleans E., Benhamou C.L. & Jaffre C. (2011) Combined effects of chronic alcohol consumption and physical activity on bone health: study in a rat model. Eur. J. Appl. Physiol. 111, 2931–2940. [DOI] [PubMed] [Google Scholar]
- Maurel D.B., Boisseau N., Pallu S., Rochefort G.Y., Benhamou C.L. & Jaffre C. (2013) Regular exercise limits alcohol effects on trabecular, cortical thickness and porosity, and osteocyte apoptosis in the rat. Joint Bone Spine 80, 492–498. [DOI] [PubMed] [Google Scholar]
- Mercer J.A., Dufek J.S., Mangus B.C., Rubley M.D., Bhanot K. & Aldridge J.M. (2010) A description of shock attenuation for children running. J. Athlet. Train. 45, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa A., Sugiyama T., Prasad J. et al (2012) Mechanical loading‐related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 23, 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujika I., Chatard J.C., Busso T., Geyssant A., Barale F., Lacoste L. (1995) Effects of training on performance in competitive swimming. Can. J. Appl. Physiol. 20, 395–406. [DOI] [PubMed] [Google Scholar]
- Pajamaki I., Kannus P., Vuohelainen T. et al (2003) The bone gain induced by exercise in puberty is not preserved through a virtually life‐long deconditioning: a randomized controlled experimental study in male rats. J. Bone Miner. Res. 18, 544–552. [DOI] [PubMed] [Google Scholar]
- Papanicolaou S.E., Phipps R.J., Fyhrie D.P. & Genetos D.C. (2009) Modulation of sclerostin expression by mechanical loading and bone morphogenetic proteins in osteogenic cells. Biorheology 46, 389–399. [DOI] [PubMed] [Google Scholar]
- Patch L.D. & Brooks G.A. (1980) Effects of training on VO2 max and VO2 during two running intensities in rats. Pflugers Arch. 386, 215–219. [DOI] [PubMed] [Google Scholar]
- Qin Y.X. & Hu M. (2014) Mechanotransduction in musculoskeletal tissue regeneration: effects of fluid flow, loading, and cellular‐molecular pathways. Biomed Res. Int. 2014, 863421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C.T. & Lanyon L.E. (1982) Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J. Exp. Biol. 101, 187–211. [DOI] [PubMed] [Google Scholar]
- Sanches I.C., Conti F.F., Sartori M., Irigoyen M.C. & De Angelis K. (2014) Standardization of resistance exercise training: effects in diabetic ovariectomized rats. Int. J. Sports Med. 35, 323–329. [DOI] [PubMed] [Google Scholar]
- Tartibian B., Hajizadeh Maleki B., Kanaley J. & Sadeghi K. (2011) Long‐term aerobic exercise and omega‐3 supplementation modulate osteoporosis through inflammatory mechanisms in post‐menopausal women: a randomized, repeated measures study. Nutr. Metab. 8, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.H. & Robling A.G. (2004) Exercise as an anabolic stimulus for bone. Curr. Pharm. Des. 10, 2629–2641. [DOI] [PubMed] [Google Scholar]
- Umemura Y., Sogo N. & Honda A. (2002) Effects of intervals between jumps or bouts on osteogenic response to loading. J. Appl. Physiol. 93, 1345–1348. [DOI] [PubMed] [Google Scholar]
- Yao Z., Lafage‐Proust M.H., Plouet J., Bloomfield S., Alexandre C. & Vico L. (2004) Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J. Bone Miner. Res. 19, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Yeh J.K., Aloia J.F., Tierney J.M. & Sprintz S. (1993) Effect of treadmill exercise on vertebral and tibial bone mineral content and bone mineral density in the aged adult rat: determined by dual energy X‐ray absorptiometry. Calcif. Tissue Int. 52, 234–238. [DOI] [PubMed] [Google Scholar]


