Summary
There is evidence to show that downregulation of miR‐1 expression is closely related to cancer progression, including in nasopharyngeal carcinoma (NPC). However, the molecular mechanisms underlying miR‐1 downregulation in NPC remain largely unknown, especially its association with Epstein–Barr virus (EBV). In this study we found that restoration of miR‐1 dramatically inhibited cell invasion in vitro, together with tumour growth and metastasis in vivo. Importantly, we found that LMP1, an Epstein–Barr virus (EBV)‐associated protein, suppressed miR‐1 expression. Furthermore, we identified K‐ras as a novel direct target of miR‐1. Our results demonstrated for the first time that miR‐1 was suppressed by LMP1 and its tumour‐suppressive effects were mediated chiefly by repressing K‐ras expression. We propose that miR‐1 could serve as an independent biomarker to identify patients with different clinical characteristics.
Keywords: EBV, K‐ras, LMP1, miR‐1, nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC), which arises from the lateral walls of the nasopharynx, is highly prevalent in southern China (Wei & Sham 2005). NPC often leads to cervical lymph node metastasis, and distant metastasis has been identified as a main cause of treatment failure in patients with NPC (Chen‐Scarabelli et al. 2005). Understanding the molecular mechanisms of the metastasis steps of NPC may help to develop better strategies of treatment.
MicroRNAs (miRNAs), 19–24 nucleotides in length, regulate gene expression by imperfect base‐pairing with complementary sequences located mainly in the 3′ untranslated regions (UTRs) of target mRNAs (German et al. 2008). Emerging evidence strongly suggests crucial roles of miRNAs in tumorigenesis.
In this study, we explored the role of miR‐1 in NPC development. We found that miR‐1 was downregulated in NPC cell lines. Interestingly, miR‐1 expression was negatively regulated by LMP1. K‐ras was identified as a downstream target of miR‐1, and miR‐1 functioned as a tumour suppressor by regulating K‐ras expression.
Materials and methods
Cell line culture
All cell lines were maintained at 37°C in a humidified atmosphere with 5% CO2. The NPC cell lines (SUNE‐1, 5‐8F, HONE‐1, C666‐1) were maintained in RPMI‐1640 supplemented with 10% foetal bovine serum (FBS). NP‐69 (an immortalized nasopharyngeal epithelium cell line) was cultured in keratinocyte serum‐free medium (keratinocyte‐SFM; Gibco , BRL Co. Ltd., USA). All the cell lines were obtained from Sun Yat‐sen University. 293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS.
Lentivirus production and transduction
The pLV‐has‐miR‐1 plasmid and the negative control pLV‐miRNA‐vector were purchased from Biosettia Inc. (Biosettia, San Diego, USA). The viruses were packaged in 293T cells according to standard protocols, and the virus particles were harvested 72 h later. The packaged lentiviruses were named LV‐miR‐1. The empty lentiviral vector LV‐con was used as a control. C666‐1 cells were infected with virus particles plus 8 μg/ml Polybrene (Sigma, St Louis, MO, USA). Infected cells were selected with puromycin for up to 14 days.
Oligonucleotide transfection
siRNA duplex oligonucleotides targeting human Stat‐3 mRNA 5′‐ aacaucugccuagaucggcua‐3′ and human LMP1 mRNA 5′‐ aagagccuucucuguccacu‐3′ were synthesized by RiboBio (Guangzhou, China). Synthesized RNA duplexes of scramble miRNA and miR‐1 mimic were obtained from Genechem (Shanghai, China). Full‐length K‐ras cDNA entirely lacking the 3′‐UTR was purchased from GeneCopoeia (Rockville, MD, USA) and subcloned into the eukaryotic expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). pcDNA3.1‐LMP1 and pcDNA3.1‐Stat‐3 were kind gifts from Dr. Wei Fang (Sun Yat‐sen University, Guangzhou, China). Oligonucleotide transfection was performed with Lipofectamine 2000 reagent (Invitrogen).
Luciferase reporter assay
The 3′‐UTR untranslated region of K‐ras was amplified by PCR and cloned downstream of the firefly luciferase gene in the pGL3 vector (Promega, Madison, WI, USA). The vector was named wild‐type (WT) 3′‐UTR. Site‐directed mutagenesis of the miR‐1 binding site in K‐ras 3′‐UTR was performed using the QuickChange site‐directed mutagenesis kit (Stratagene, Cedar Creek, TX, USA) and named mutant (mt) 3′‐UTR. For reporter assays, WT or mt 3′‐UTR vector and the control vector pRL‐CMV (Promega) were cotransfected. The luciferase assay was performed using the Dual‐Luciferase Reporter Assay System (Promega) 48 h after transfection.
RNA isolation and quantitative real‐time PCR
Total RNA was extracted from cells with TRIzol reagent (Invitrogen). cDNA was synthesized with the PrimeScript RT Reagent Kit (Promega). For miR‐1, reverse transcription and qRT‐PCRs were performed by means of a qSYBR‐green‐containing PCR kit (GenePharma, Shanghai, China), and U6 snRNA was used as an endogenous control.
Western blot and Immunohistochemistry
The primary antibodies K‐ras, Stat3, phosphorylated Stat‐3, vimentin, E‐cadherin, MMP‐9 and GAPDH were purchased from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Primary antibody LMP1 was purchased from Bethyl Laboratories Inc (Montgomery, TX, USA). Western blot and immunohistochemistry assays were performed as previously described (Wei & Sham 2005).
Chip–pcr
DNA–protein complexes were immunoprecipitated from C666‐1 cells that were transfected with Stat‐3 cDNA using the Chromatin Immunoprecipitation Kit (Millipore, Billerica, MA, USA), according to the manufacturer's protocol with the following polyclonal antibodies: anti‐p‐Stat‐3 or normal mouse IgG (Millipore). The precipitated DNA was subjected to qPCR analysis using specific primers to amplify across the miR‐1 promoter region.
In vivo growth and metastasis assay
For the in vivo tumour growth studies, 1 × 106 C666‐1 cells, stably expressing miR‐1, or the control vector, were injected subcutaneously in the upper back of BALB/C‐nu/nu athymic nude mice. The length and width of the tumours were measured every 5 days using a digital calliper, and tumour volumes were calculated using the formula Volume (mm3) = L × W2/2 (length L, mm; width W, mm). After 21 days, tumour samples were carefully removed and weighed.
Statistical analysis
All data were analysed using SPSS 13.0 software (SPSS, Chicago, IL, USA). A two‐tailed Student's t‐test was used for the comparison between two independent groups. One‐way anova was used to determine the differences between groups. P values of <0.05 were considered as statistically significant.
Ethical approval statement
This study was conducted with the approval of the Ethical and Scientific Committees of Guangzhou Medical University with its permitted number: JF‐008. All animal procedures were performed in accordance with institutional guidelines. The metastasis assay was performed as previously described (Hsu et al. 2014).
Results
The LMP1‐mediated Stat‐3 pathway is involved in miR‐1 downregulation
First, the expression of miR‐1 was examined in NPC cell lines, including SUNE‐1, 5‐8F, HONE‐1, C666‐1 and nasopharyngeal epithelial (NPE) cell line NP‐69. We found that the expression level of miR‐1 was significantly lower in NPC cell lines when compared with NPE cell line NP‐69. Interestingly, the EBV‐positive cell line C666‐1 showed the lowest level of miR‐1 (P < 0.05, Figure 1a). The Western blot assay showed that C666‐1 cells expressed higher levels of LMP1, when compared with other NPC cell lines and NP‐69 (Figure 1b). Subsequently, we asked whether exogenetic overexpression of LMP1 affected miR‐1 expression. NP‐69 cells were transiently transfected with pcDNA3.1‐LMP1 or empty vector pcDNA3.1, and the overexpression efficiency of LMP1 was confirmed by Western blot analysis (Figure 1c). The qRT‐PCR assay demonstrated that miR‐1 expression in NP‐69‐pcDNA3.1‐LMP1 cells was decreased at both the primary and mature transcript levels, when compared with that in NP‐69‐pcDNA3.1 cells (P < 0.05, Figure 1d).
Figure 1.
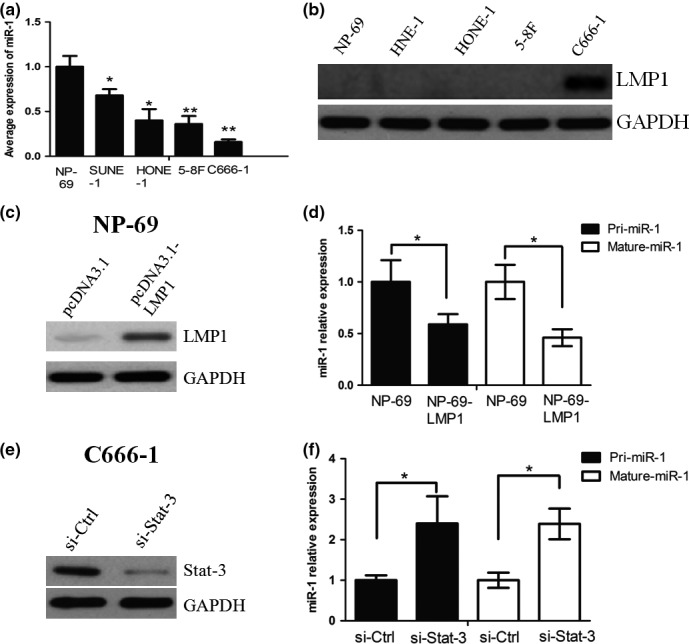
(a) miR‐1 expression in SUNE‐1, 5‐8F, HONE‐1, C666‐1 and nasopharyngeal epithelial (NPE) cell line NP‐69. (b) Western blot assay showed that C666‐1 cells expressed higher levels of LMP1, when compared with other NPC cell lines and NP‐69. (c) NP‐69 cells were transiently transfected with pcDNA3.1‐LMP1 or empty vector pcDNA3.1, and the overexpression efficiency of LMP1 was confirmed by Western blot analysis. (d) miR‐1 expression in NP‐69‐pcDNA3.1‐LMP1 cells was decreased at both the primary and mature transcript levels, when compared with that in NP‐69‐pcDNA3.1 cells. (e) siRNA was used to suppress the expression of Stat‐3 in C666‐1 cells. (f) miR‐1 expression was markedly increased when Stat‐3 was knocked down in C666‐1 cells. * and ** represent significance.
Previous studies showed that Stat‐3 could regulate microRNA expression (Rozovski et al. 2013; Ma et al. 2014), and it has been well known that LMP1 can activate the Stat‐3 signalling pathway (Kung et al. 2011). We hypothesized that LMP1 negatively regulated miR‐1 expression through Stat‐3. To test whether Stat‐3 regulated miR‐1 expression in NPC cells, we first used siRNA to suppress the expression of Stat‐3 in C666‐1 cells (Figure 1e). qPCR analysis indicated that miR‐1 expression was markedly increased when Stat‐3 was knocked down in C666‐1 cells (Figure 1f).
Furthermore, overexpression of Stat‐3 dose‐dependently inhibited miR‐1 promoter activity in NP‐69 cells as determined by luciferase assay (Fig. S1A). Using bioinformatics prediction tools, we found out the putative binding sites for Stat‐3 in the upstream regions of miR‐1 (Fig. S1B). Subsequently, we used chromatin immunoprecipitation combined with qPCR analysis to confirm that Stat‐3 could bind to the miR‐1 promoter site and suppress its expression in C666‐1 cells (Fig. S1C). Finally, it was observed that LMP1 increased the expression of phos‐Stat‐3, an active form of Stat‐3 (Fig. S1D). Taken together, these results demonstrated that LMP1 suppressed the expression of miR‐1 through activation of the Stat‐3 pathway.
Restoration of miR‐1 inhibits NPC cell invasion in vitro
We established C666‐1 cells that stably expressed high expression of miR‐1, and these cells were named LV‐miR‐1. The transwell assay showed that exogenetic overexpression of miR‐1 caused a suppression of cell invasion in the C666‐1 cells (P < 0.05, Figure 2a). Furthermore, we examined whether miR‐1 affected the expression levels of proteins involved in invasion of NPC cells. We found that overexpression of miR‐1 suppressed vimentin and MMP‐9 expression, but increased E‐cadherin expression, as determined by Western blot analysis (Figure 2b).
Figure 2.
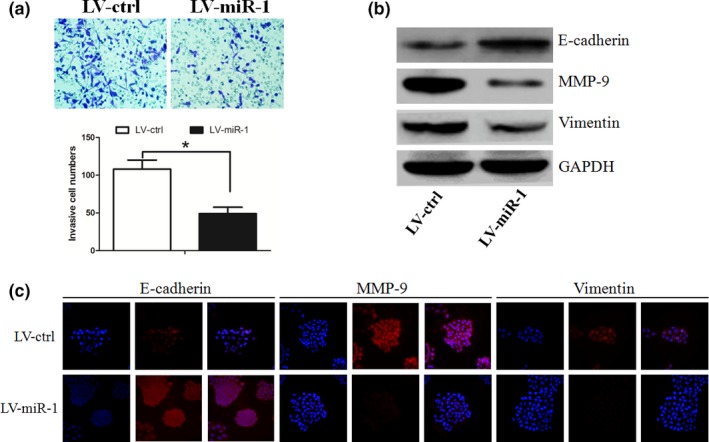
(a) Overexpression of miR‐1 caused a suppression of cell invasion in the C666‐1 cells. (b) miR‐1 suppressed vimentin and MMP‐9 expression, but increased E‐cadherin expression, as determined by Western blot analysis. * represent significance.
miR‐1 directly targets and inhibits K‐ras
TargetScan and miRanda were used to search for potential miR‐1 target genes. Among the mRNAs containing miR‐1 recognition sites in their 3′‐UTRs, we focused on K‐ras, a protein involved in invasion of NPC cells (Deng et al. 2011). To verify that K‐ras is a direct target of miR‐1, K‐ras wild‐type (WT) or mutant 3′‐UTR was subcloned into a luciferase reporter vector and cotransfected with miR‐1 or scrambled control into C666‐1 cells (Figure 3a). miR‐1 significantly inhibited the luciferase activity of the K‐ras WT 3′‐UTR but not that of the mutant in C666‐1 cells (Figure 3b). Furthermore, overexpression of miR‐1 significantly downregulated K‐ras protein levels (Figure 3c), although it did not affect K‐ras mRNA levels (data not shown). These results indicated that K‐ras was a target gene of miR‐1.
Figure 3.
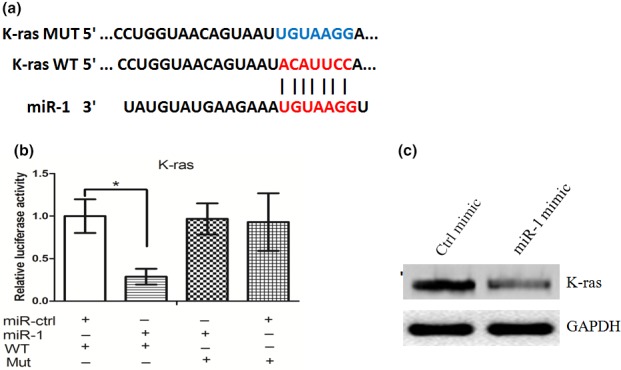
(a) K‐ras wild‐type (WT) or mutant 3′‐UTR was subcloned into a luciferase reporter vector and cotransfected with miR‐1 or scrambled control into C666‐1 cells. (b) miR‐1 significantly inhibited the luciferase activity of the K‐ras WT 3′‐UTR but not that of the mutant in C666‐1 cells. (c) Overexpression of miR‐1 significantly downregulated K‐ras protein levels. *represent significance.
Overexpression of miR‐1 attenuated the growth and metastasis of NPC cells in vivo
We next investigated the efficacy of miR‐1 against tumour growth and metastasis in vivo. Stable transfection of miR‐1 into C666‐1 cells resulted in decreased growth and tumour weight of subcutaneous xenograft tumours in nude mice, when compared with those stably transfected with empty vector (Figure 4a and b). By immunohistochemistry, we found that the expression of K‐ras, vimentin and MMP‐9 was also reduced by stable transfection of miR‐1. However, the expression of E‐cadherin was elevated in the miR‐1‐treated group (Figure 4c). In the experimental metastasis studies, C666‐1 cells stably transfected with miR‐1 established smaller lung metastatic colonies than mock group (Figure 4d). These results suggested that miR‐1 could inhibit the growth and metastasis of NPC cells in vivo.
Figure 4.
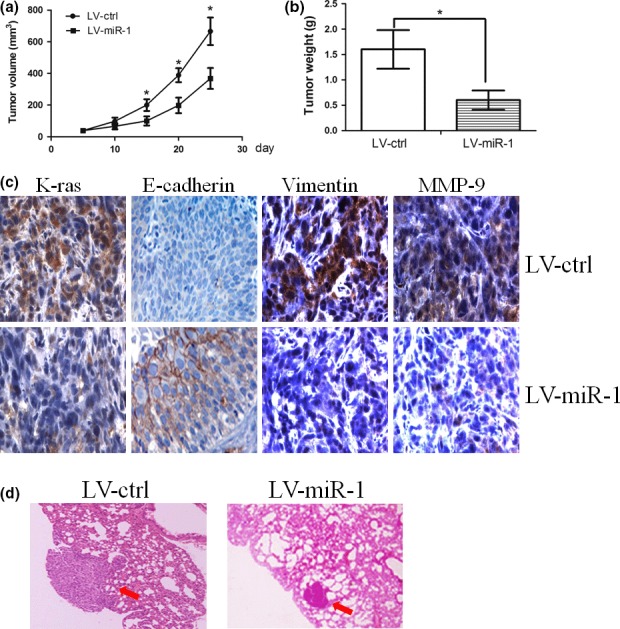
(a) and (b) Stable transfection of miR‐1 into C666‐1 cells resulted in decreased growth and tumour weight of subcutaneous xenograft tumours in nude mice, when compared with those stably transfected with empty vector. (c) Immunohistochemistry assay showed that the expression of K‐ras, vimentin and MMP‐9 was reduced by stable transfection of miR‐1. However, the expression of E‐cadherin was elevated in miR‐1‐treated group. (d) C666‐1 cells stably transfected with miR‐1 established smaller lung metastatic colonies than mock group. * represent significance.
Discussion
miR‐1 has been reported to function as a tumour suppressor, and low‐level expression of miR‐1 is associated with a poor prognostic phenotype in patients with cancer (Letelier et al. 2014; Li et al. 2015). miR‐1 has been reported to function as a tumour suppressor in a series of cancers (Chang et al. 2015). Overexpression of miR‐1 inhibited cancer cell proliferation and metastasis (Xiao et al. 2015). In a previous study, we have demonstrated that miR‐1 inhibited NPC cell angiogenesis (Lu et al. 2014). However, the role of miR‐1 in NPC cell invasion and its association with EBV is still largely unknown. In the present study, we examined the miR‐1 expression in EBV‐negative (SUNE‐1, 5‐8F, HONE‐1) and EBV‐positive NPC cell lines (C666‐1). We found that when compared with nasopharyngeal epithelial (NPE) cell line NP‐69, miR‐1 expression was downregulated in NPC cells. Interestingly, the miR‐1 expression was the lowest in EBV‐positive cells. These data suggest that miR‐1 expression was negatively associated with the EBV status.
LMP1 protein is detected in approximately 60% of tissue samples from patients with NPC, while LMP1 mRNA is detected in nasopharyngeal swabs in over 90% of patients with NPC by RT‐PCR (Ma et al. 2014). In the present study, we investigated the molecular mechanism of LMP1‐mediated miR‐1 expression. We found an inverse correlation between the expression of LMP1 and miR‐1 in NPC cells. LMP1 overexpression or silencing significantly down‐ or upregulated the miR‐1 expression in NP‐69 and C666‐1 cells respectively. Our observation revealed that LMP1 activated the Stat‐3 pathway through increasing the expression of phos‐Stat‐3. We further found that by knocking down Stat‐3, miR‐1 expression in C666‐1 cells could be increased. Finally, chromatin immunoprecipitation combined with qPCR analysis was used to confirm that Stat‐3 could bind to the miR‐1 promoter site and suppress its expression in C666‐1 cells. It is worth to note that some NPC cells, which were EBV‐negative, also presented low miR‐1 expression. This may be the reason that some other factors, such as methylation, may have contributed to miR‐1 downregulation (Datta et al. 2008).
K‐ras was identified as a direct target of miR‐1 in the present study through dual‐firefly luciferase reporter assay and Western blot analysis. K‐ras is known to regulate intracellular activities such as focal complex formation, integrin localization, adhesion, cell morphology and MMP expression (Bera et al. 2013). K‐ras is well documented in the development and progression of cancer (Jancik et al. 2010; Wang et al. 2010). Active point mutations in the K‐ras gene had been reported to stay at high rates in a various type of cancer. For instance, approximately ~50% colon cancers and thyroid cancers contain a K‐ras point mutation (Ren et al. 2012). In pancreatic cancers, the point mutation rate was as high as about 90% (Yongxiang et al. 2014). Interestingly, although the K‐ras gene mutations are rare in NPC, its upregulation has been found in 60% of the tumour tissues examined (Deng et al. 2011). Thus, targeting K‐ras is a promising strategy in the treatment of cancer, including NPC (Haghgoo et al. 2015; Kumar et al. 2015). In the present study, we demonstrated that miR‐1 functioned as a tumour suppressor by negatively regulating K‐ras expression both in vitro and in vivo. miR‐1 was commonly downregulated in NPC tissues and cell lines (Luo et al. 2012). Overexpression of miR‐1 induced apoptosis and inhibited angiogenesis in nasopharyngeal carcinoma cells (Wu et al. 2011; Lu et al. 2014). Thus, restoration of miR‐1 expression may be a novel strategy for NPC treatment.
In summary, our present work indicates that low miR‐1 levels induced by EBV via LMP1 promote invasion of EBV‐positive cells. Our findings on miR‐1 suggest that this microRNA could be employed as an effective therapeutic target for NPC in the future.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Supporting information
Figure S1. (A) Overexpression of Stat‐3 dose‐dependently inhibited miR‐1 promoter activity in NP‐69 cells as determined by luciferase assay.
References
- Bera A., Zhao S., Cao L., Chiao P.J. & Freeman J.W. (2013) Oncogenic K‐Ras and loss of Smad4 mediate invasion by activating an EGFR/NF‐kappaB Axis that induces expression of MMP9 and uPA in human pancreas progenitor cells. PLoS ONE 8, e82282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.S., Chen W.Y., Yin J.J., Sheppard‐Tillman H., Huang J. & Liu Y.N. (2015) EGF Receptor Promotes Prostate Cancer Bone Metastasis by Downregulating miR‐1 and Activating TWIST1. Cancer Res. 75, 3077–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen‐Scarabelli C., Kaza A.R. & Scarabelli T. (2005) Syncope due to nasopharyngeal carcinoma. Lancet Oncol. 6, 347–349. [DOI] [PubMed] [Google Scholar]
- Datta J., Kutay H., Nasser M.W. et al (2008) Methylation mediated silencing of MicroRNA‐1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 68, 5049–5058. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Deng M., Tang H., Zhou Y. et al (2011) miR‐216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J. Cell Sci. 124, 2997–3005. [DOI] [PubMed] [Google Scholar]
- German M.A., Pillay M., Jeong D.H. et al (2008) Global identification of microRNA‐target RNA pairs by parallel analysis of RNA ends. Nat. Biotechnol. 26, 941–946. [DOI] [PubMed] [Google Scholar]
- Haghgoo S.M., Allameh A., Mortaz E. et al (2015) Pharmacogenomics and targeted therapy of cancer: focusing on non‐small cell lung cancer. Eur. J. Pharmacol. 754, 82–91. [DOI] [PubMed] [Google Scholar]
- Hsu C.Y., Yi Y.H., Chang K.P., Chang Y.S., Chen S.J. & Chen H.C. (2014) The Epstein‐Barr virus‐encoded microRNA MiR‐BART9 promotes tumor metastasis by targeting E‐cadherin in nasopharyngeal carcinoma. PLoS Pathog. 10, e1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancik S., Drabek J., Radzioch D. & Hajduch M. (2010) Clinical relevance of KRAS in human cancers. J. Biomed. Biotechnol. 2010, 150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Mondal G., Slavik P., Rachagani S., Batra S.K., Mahato R.I. (2015) Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol. Pharm. 12, 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C.P., Meckes D.G. Jr & Raab‐Traub N. (2011) Epstein‐Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J. Virol. 85, 4399–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letelier P., Garcia P., Leal P. et al (2014) miR‐1 and miR‐145 act as tumor suppressor microRNAs in gallbladder cancer. Int. J. Clin. Exp. Pathol. 7, 1849–1867. [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu Y., Li H. et al (2015) MicroRNA‐1 promotes apoptosis of hepatocarcinoma cells by targeting apoptosis inhibitor‐5 (API‐5). FEBS Lett. 589, 68–76. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhao F.P., Peng Z. et al (2014) EZH2 promotes angiogenesis through inhibition of miR‐1/Endothelin‐1 axis in nasopharyngeal carcinoma. Oncotarget 5, 11319–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Zhang L., Li Z. et al (2012) An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med. Genomics 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Deng X., Wu M., Zhang G. & Huang J. (2014) Down‐regulation of miRNA‐204 by LMP1 enhances CDC42 activity and facilitates invasion of EBV‐associated nasopharyngeal carcinoma cells. FEBS Lett. 588, 1562–1570. [DOI] [PubMed] [Google Scholar]
- Ren J., Li G., Ge J., Li X. & Zhao Y. (2012) Is K‐ras gene mutation a prognostic factor for colorectal cancer: a systematic review and meta‐analysis. Dis. Colon Rectum 55, 913–923. [DOI] [PubMed] [Google Scholar]
- Rozovski U., Calin G.A., Setoyama T. et al (2013) Signal transducer and activator of transcription (STAT)‐3 regulates microRNA gene expression in chronic lymphocytic leukemia cells. Mol. Cancer. 12, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.L., Lopategui J., Amin M.B. & Patterson S.D. (2010) KRAS mutation testing in human cancers: The pathologist's role in the era of personalized medicine. Adv. Anat. Pathol. 17, 23–32. [DOI] [PubMed] [Google Scholar]
- Wei W.I. & Sham J.S. (2005) Nasopharyngeal carcinoma. Lancet 365, 2041–2054. [DOI] [PubMed] [Google Scholar]
- Wu C.D., Kuo Y.S., Wu H.C. & Lin C.T. (2011) MicroRNA‐1 induces apoptosis by targeting prothymosin alpha in nasopharyngeal carcinoma cells. J. Biomed. Sci. 18, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Zeng J., Li H. et al (2015) MiR‐1 downregulation correlates with poor survival in clear cell renal cell carcinoma where it interferes with cell cycle regulation and metastasis. Oncotarget 6, 13201–13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongxiang W., Liang G. & Qinshu S. (2014) Apoptosis of human pancreatic carcinoma PC‐2 cells by an antisense oligonucleotide specific to point mutated K‐ras. Pathol. Oncol. Res. 20, 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Overexpression of Stat‐3 dose‐dependently inhibited miR‐1 promoter activity in NP‐69 cells as determined by luciferase assay.


