Abstract
Background: Patients with chronic obstructive pulmonary disease (COPD) may not recognize worsening symptoms that require intensification of therapy. They may also be reluctant to contact a healthcare provider for minor worsening of symptoms. A telemedicine application for daily symptom reporting may reduce these barriers and improve patient outcomes. Materials and Methods: Patients hospitalized for a COPD exacerbation within the past year or using supplemental O2 were approached for participation. Patients received optimal COPD care and were given a telecommunication device for symptom reporting. Initial symptom scores were obtained while patients were in their usual state of health. Patients were randomly assigned to an intervention group or a control group (usual medical care). The control group patients were instructed to seek medical care if their condition worsened. The intervention group symptom scores were assessed by a computer algorithm and compared with initial values. Scores 1 or more points above the initial score generated an “alert,” and patients were reviewed by a nurse and referred to a physician who prescribed treatment. Results: Eighty-six patients were screened; 79 met entry criteria and were randomized (intervention group, n=39; control group, n=40). Twelve patients submitted five or fewer symptom reports (5 intervention; 7 control) and were excluded from the analysis. Daily peak flow and dyspnea scores improved only in the intervention group. There were no differences in hospitalization and mortality rates between groups. No serious adverse events were reported. Conclusions: A telemedicine-based symptom reporting program facilitated early treatment of symptoms and improved lung function and functional status.
Key words: : chronic obstructive pulmonary disease exacerbations, telemedicine, disease management
Introduction
Telemedicine has been advocated in managing several chronic disease states, including diabetes,1,2 hypertension,3 congestive heart failure,4 and high-risk pregnancies,5 and for improving general medical care and outcomes.3,6,7 Chronic obstructive pulmonary disease (COPD) is a chronic disease whose course is punctuated by intermittent exacerbations and has been the focus of several telemedicine or telemonitoring studies.8–10
Recently, Goldstein and O'Hoski11 concluded that the value of telemedicine in the daily management of COPD patients is unproven. They cited poor study designs, small sample sizes, inconsistent interventions, and limited follow-up as factors that must be addressed before telemedicine interventions can be considered as a standard of care for COPD patients. In this study, we hypothesized that telemedicine-based daily symptom reporting when added to optimal medical therapy will decrease hospitalizations and COPD-related mortality, as well as reduce the frequency and severity of acute COPD exacerbation symptoms, in high-risk patients.
Materials and Methods
The PennsylvaniA Study of COPD Exacerbations (PA-SCOPE) was a randomized, unblinded, parallel-group trial funded by the Pennsylvania Commonwealth Universal Research Enhancement Program. Patients were drawn from the outpatient practices of the Principal Investigators while in their usual state of health. Eligibility criteria were ages between 40 and 80 years, diagnosis of COPD, current or former smokers, COPD hospitalization within the past year or current home oxygen use, and no significant comorbidity. All patients gave institutional review board–approved written informed consent.
On the day of enrollment a complete history was obtained, a physical examination was performed, the COPD medication regimen was optimized, full pulmonary function testing was performed, and arterial blood gases were obtained.12 A 6-min walk test was performed with oxygen titration,13 demographic information was obtained, and quality of life questionnaires were completed (Modified Outcomes Study Short Form [SF-36],14 St. George's Respiratory Questionnaire [SGRQ],15 The Shortness of Breath Questionnaire,16 and the Quality of Well-being Scale17). Peak flow meters were distributed to all patients, and electronic diaries were provided for daily peak flow and symptom reporting. Patients were instructed in their use.
An initial symptom assessment was made for each patient when he or she was in the usual state of health. The components of the symptom assessment included peak expiratory flow (best of three attempts), dyspnea (modified Borg score), and sputum quantity, color, and consistency (all were considered major symptoms), as well as the presence of cough, wheeze, sore throat, nasal congestion, and temperature above 100°F (all minor symptoms). Although both groups of patients made daily symptom reports, only those made by the intervention group were evaluated using a computerized algorithm that generated a symptom score that reflected the degree to which their symptoms deviated from the symptoms obtained when in their usual state of health (Table 1). The study nurse and physician reviewed the symptom report to determine the factors that triggered the alert to direct the prescribed intervention.
Table 1.
Electronic Diary Scoring
| CATEGORY | SCORING |
|---|---|
| Breathlessness | Score 1.0 if ≥3 increments above initial value |
| Sputum quantity | Score 0.5 if change to greater amount from initial value. Choices: |
| <1 tablespoonful | |
| ≥1 tablespoonful | |
| ≥¼ cupful | |
| Sputum color | Score 0.5 if color change from initial value. Choices: |
| If initial value was none or white and the change is to yellow, green, or brown, score 0.5. | |
| If initial value was none, white, brown, or yellow and the change is to green, score 0.5. | |
| Sputum consistency | Score 0.5 if change from initial values of none, watery, or thin to thick. |
| Score is 0.0 if thick is not a change from baseline. | |
| Peak flow | Score 1.0 if ≤80% of baseline. |
| Temperature over 100°F | Score 0.5 if answer is “Yes” |
| Cough | All minor symptoms. If two or more minor symptoms are “yes” and a change from baseline, score 0.5. |
| Wheeze | |
| Sore throat | |
| Nasal congestion |
U.S. copyright TX-7-170-236, 2004, by Gerard J. Criner.
The symptom algorithm detected changes from the initial values of the patient's symptoms. However, even when in their usual state of health COPD patients are symptomatic. To quantify this burden, a symptom severity index was developed that assigned weights to each symptom. The symptoms were weighted to parallel the severity of symptoms reported in the electronic diary. These were as follows: increased sputum purulence, 0.167; thickness, 0.167; quantity, 0.084; cough, 0.125; wheeze, 0.125; sore throat, 0.125; nasal congestion, 0.125; and fever, 0.5. Peak flow rates were weighed based on National Health and Nutrition Examination Survey predicted flow rate (>0.6 to <0.8, 0.25; 0.5–0.6, 0.5; 0.37–0.49; 0.75; and <0.37, 1), and breathlessness was given a score of 0.1 up to 1 corresponding to 1–10 on the Borg dyspnea scale (Table 2). Although not necessary for the daily management of patients, the symptom index allowed us to categorize the severity of symptoms as mild (1–1.9), moderate (2–2.9), or severe (≥3) and to track symptom severity over time.
Table 2.
Symptoms Severity Index
| SYMPTOMS | MAXIMUM CONTRIBUTION TO INDEX | LEVELS |
|---|---|---|
| Breathlessness | 1 | 10 |
| Sputum | ||
| Quantitya | 0.5 | 6 |
| Color | 0.5 | 3 |
| Consistency | 0.5 | 3 |
| Peak flow (percentage of initial value) | 1 | >0.6 to <0.8 |
| 0.5–0.6 | ||
| 0.37–0.49 | ||
| <0.37 | ||
| Temperature >100° F | 0.5 | Yes/no |
| Cough | 0.125 | Yes/no |
| Wheeze | 0.125 | Yes/no |
| Sore throat | 0.125 | Yes/no |
| Nasal congestion | 0.125 | Yes/no |
| Maximum severity index | 4.5 | |
The original sputum quantity score provided for smaller sputum volumes, hence the six levels.
The Duke Activity Status Index was also reported to measure the patient's daily functional status.18 It has been shown to be a useful addition in the evaluation of functional capacity in the COPD patient population.19
Patients were randomized to either the intervention group or the control group using a computer-generated randomization scheme developed by the study statistician. All patients were instructed to report their symptoms daily using the electronic diary (Palm® model M500; TCL Corp., Sunnyvale, CA). The electronic diary had eight screens of questions that took 2–3 min to complete. Patients obtained three peak flow readings using a handheld disposable peak flow meter (AsthmaMentor®; Respironics New Jersey, Inc., Cedar Grove, NJ). The patient responded to the prompts on each of the screens regarding his or her symptoms that day (as described in Table 1) and entered the best peak flow meter reading. Once all data were entered, the electronic diary was placed into its cradle, and the daily symptom report was transmitted by telephone line to a central database. The data submitted by the patient were processed by the algorithm. If an alert was generated for an intervention group patient, the patient received a notification to call the office. Reports made by intervention patients were reviewed daily by the study nurse who contacted patients that did not call the office on their own. Two weeks after the enrollment visit, all patients received a telephone call to determine if they were having any problems with the use of the peak flow meter or the electronic diary.
Control group patients were instructed to follow their primary physician's care plan provided at the time of discharge from the clinic.
Intervention group patients were instructed to phone a 1–800 number if the symptom score generated by the algorithm reached or exceeded the predetermined threshold of 1 (“alerted”). The 1–800 number was available 24 h/day, 7 days a week and staffed by nurses and pulmonologists. Exacerbation symptoms were treated according to GOLD guidelines.20
Follow-up study visits for all patients were scheduled at 6, 12, 18, and 24 months after enrollment. At these visits an interim history, physical exam, and medication history were obtained. Also at these visits, demographic information was updated, peak flow meters were replaced, and quality of life questionnaires were obtained.
The calculation of the sample size was based on a conservative estimate assuming patients accrue to the study according to a Poisson process. Using a two-sided test at a 5% level of statistical significance, a sample size of 100 patients per group would have an 80% power to detect a 30% difference in the composite event rate (hospitalization or death) between the intervention group and the control group. Assuming a 25% dropout rate, 135 patients per group would need to be recruited.
Data Collection and Analysis
The primary end point was a composite of the number of hospitalizations and deaths, adjudicated by three pulmonologists blinded to group assignment. The secondary end points were the frequency and severity of acute exacerbation symptoms, daily peak flow, dyspnea score, and Duke Activity Status Index, as well as interval changes in the general and disease-specific quality of life questionnaires. To be included in the analysis of acute exacerbation symptoms, patients must have made five or more symptom reports between the day of enrollment and the end of their study participation.
Differences in the primary end point and the secondary end point of exacerbation symptom days greater than baseline were compared between groups using the test for differences in Poisson rates. Time to first hospitalization for each group was analyzed using Kaplan–Meier analyses. Differences between average exacerbation symptom index scores were tested for significance using a mixed-model analysis of variance for repeated measures. Quality of life data were analyzed by two-way analysis of variance for repeated measures. Yates's corrected chi-squared test was used to compare mortality between the two groups.
A Data and Safety Monitoring Board reviewed the study data for differential mortality or morbidity.
Results
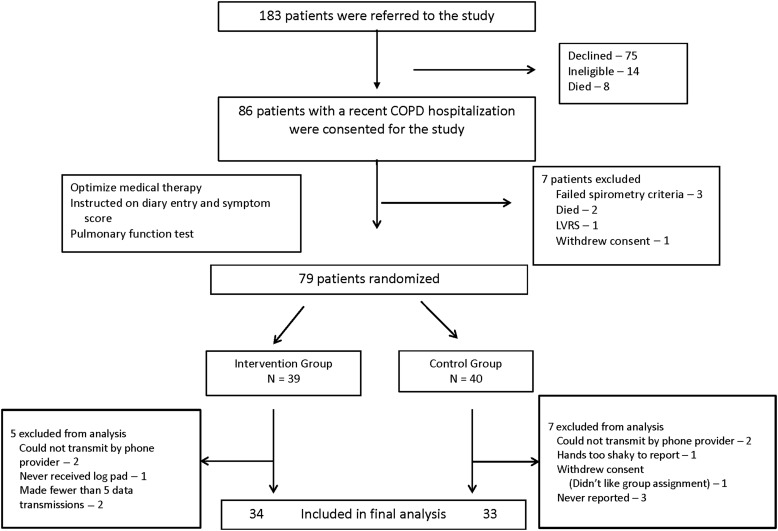
In total, 86 patients were consented. Seven patients were excluded prior to randomization (Fig. 1). Of the 79 randomized patients (39 patients to the intervention group and 40 to the control group), 12 patients were excluded from the analysis because they submitted five or fewer symptom reports (5 in the intervention group and 7 in the control group) (the CONSORT diagram appears in Fig. 1), resulting in 34 patients in the intervention group and 33 patients in the control group. There were no significant differences between the intervention group and the control group in the average number of days in the study (323±224 versus 364±210, respectively; p=0.45) and the average number of days symptom reports were submitted (264±164 versus 254±199, respectively; p=0.83). Clinical and demographic data obtained at enrollment showed that the groups were well matched (Table 3). Intervention group patients had a higher compliance rate in daily diary reporting compared with control group patients (81.4% versus 69.9%, respectively; p<0.001). There were no serious adverse events reported in either group. Two patients in the intervention group developed corticosteroid-related hyperglycemia that was treated with a temporary increase in insulin dosage.
Fig. 1.
Study flow diagram. COPD, chronic obstructive pulmonary disease; LVRS, lung volume reduction surgery.
Table 3.
Baseline Demographic and Clinical Characteristics
| INTERVENTION (N=34) | CONTROL (N=33) | P VALUE | |
|---|---|---|---|
| Age (years) | 64±6 | 63±8 | 0.58 |
| Sex (number female) | 17 | 24 | 0.08 |
| Smoking (pack-years) | 43±22 | 54±25 | 0.053 |
| BMI (kg/m2) | 29±6 | 30±7 | 0.42 |
| Number (%) of patients on oxygen | 23/34 (67%) | 23/33 (70%) | 1 |
| Oxygen (L/min) | 3.0±1.7 | 2.9±1.1 | 0.76 |
| mMRC (n) | 0.37 | ||
| 0 | 1 | 0 | |
| 1 | 5 | 3 | |
| 2 | 4 | 8 | |
| 3 | 16 | 11 | |
| 4 | 8 | 11 | |
| SGRQ total score | 59±11 | 60±12 | 0.71 |
| SF-36 | |||
| Physical component | 25±19 | 17±13 | 0.06 |
| Mental component | 66±20 | 70±17 | 0.35 |
| Long-acting beta-agonist | 28/34 (82%) | 26/33 (79%) | 0.77 |
| Inhaled corticosteroids | 29/34 (85%) | 31/33 (94%) | 0.43 |
| Long-acting anticholinergic | 22/34 (65%) | 25/33 (76%) | 0.43 |
| Short-acting beta-agonist | 34/34 (100%) | 33/33 (100%) | 1 |
| Comorbidities | |||
| DM | 3/34 (9%) | 4/33 (12%) | 0.71 |
| CAD | 7/34 (21%) | 13/33 (39%) | 0.11 |
| FVC (%) | 74±15 | 68±17 | 0.16 |
| FEV1 (%) | 31±13 | 32±15 | 0.76 |
| FEV1/FVC (%) | 34±13 | 38±13 | 0.16 |
| TLC (%) | 112±18 | 112±17 | 0.96 |
| RV (%) | 182±49 | 182±47 | 0.99 |
| DLCO (%) | 54±15 | 54±15 | 0.90 |
| pH | 7.42±0.02 | 7.42±0.02 | 0.77 |
| PaCO2 (mm Hg) | 42±7 | 45±7 | 0.29 |
| PaO2 (mm Hg) | 67±14 | 65±10 | 0.55 |
| 6MWT (min) | 262±83 | 254±96 | 0.73 |
6MWT, 6-min walk test; BMI, body mass index; CAD, coronary artery disease; DLCO, diffusing capacity for carbon monoxide; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, Modified Medical Research Council Dyspnea Scale; PaCO2, partial pressure of carbon dioxide in blood; PaO2, partial pressure of oxygen in blood; RV, residual lung volume; SF-36, Short Form 36 Health Survey; SGRQ, St. George's Respiratory Questionnaire; TLC, total lung capacity.
Hospitalizations and Mortality
Thirty-five hospitalizations occurred in the intervention group compared with 44 in the control group (p=0.31). There were no differences in hospitalization rates (number of hospitalizations/study observation days) (intervention group versus control group, 35/10,951 versus 44/12,012, respectively; p=0.63) or hospitalization durations (intervention group versus control group, 392±30 versus 463±32 days, respectively; p=0.8). Six intervention group patients had multiple hospitalizations for exacerbations compared with 11 control group patients (p=0.27). There was no significant difference in time to first hospitalization or mortality between groups (intervention group versus control group, n=6 versus n=2, respectively; p=0.25).
Acute Exacerbation Symptoms
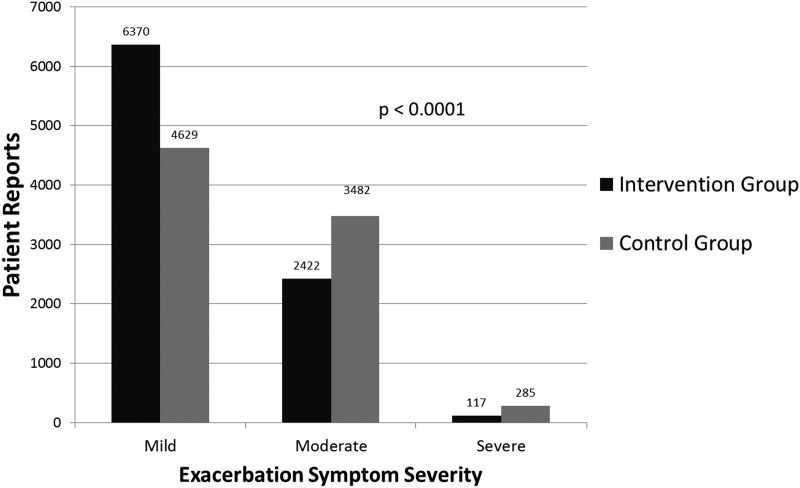
The intervention group submitted 8,909 symptom reports over a total of 10,951 patient-days of participation, and the control group submitted 8,396 symptom reports over a total of 12,012 patient-days of participation. All patients reported the presence of at least one COPD symptom daily. The distribution of symptom severity was different between the intervention group and the control group, with the intervention group reporting significantly fewer moderate and severe symptom days (p<0.0001) (Fig. 2).
Fig. 2.
Symptom index reports by study group.
Longitudinal Symptom and Activity Reports
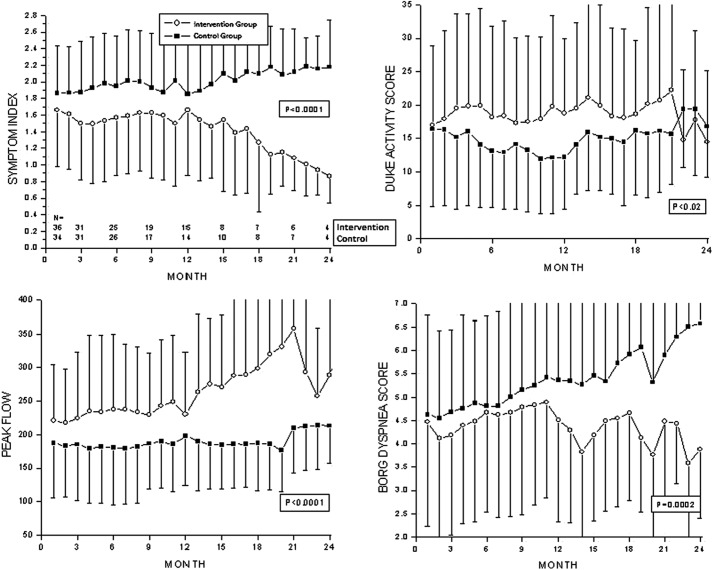
Symptom index scores, Duke Activity Scores, peak flow rates, and Borg dyspnea scores were compared over the 24-month reporting period (Fig. 3). Intervention group patients had significantly lower symptom index scores compared with control group patients. The decrease in the intervention group symptom index score was sustained up to 24 months. Average peak flow improved in intervention group patients over time compared with baseline but remained unchanged in control group patients. Improvements in intervention group peak flow were sustained up to 24 months (p<0.0001). The Borg dyspnea score in the intervention group declined significantly over time compared with values observed at study entry compared with the control group. The improvements in intervention group dyspnea were sustained up to 24 months (p<0.0006). Following randomization, the Duke Activity Status Index was significantly higher in the intervention group compared with the control group and was sustained over the 24-month period of follow-up (p<0.02).
Fig. 3.
Longitudinal symptom and activity reports.
SGRQ
There were no differences between the intervention and control groups in baseline SGRQ total scores (59±11 versus 59±12, respectively; p=0.72) and its three component scores: Symptom (67±17 versus 70±12, respectively; p=0.46), Activity (82±11 versus 85±11, respectively; p=0.32), and Impact (43±15 versus 43±19, respectively; p=0.96). At 24 months, the Symptom score was lower in the intervention group (67±15; n=5) compared with the control group (78±14; n=3) (p=0.009). There were no differences in the Activity, Impact, or total scores between groups.
SF-36
Main SF-36 summary scores were not different between groups. SF-36 Reported Health Transition Score (improvement in health over last 12 months) showed that the intervention group was significantly better at 12 and 18 months compared with baseline, whereas the control group remained unchanged (p=0.04).
Shortness of Breath Questionnaire and Quality of Life Assessment
The Shortness of Breath Questionnaire and Quality of Well-being assessments were similar for both groups at all study times.
Timeliness of Medical Interventions in the Intervention Group Arm
The call center team performed 142 interventions in the intervention group. Interventions included addition or increase in bronchodilators (n=10), antibiotics (n=43), systemic corticosteroids (n=40), and antibiotics and systemic corticosteroids (n=49). In 100% of the interventions, patients began therapy the same day their symptom index was ≥1 over the initial value.
Correlation of Symptom Index and SGRQ Symptom Score
To determine its clinical utility, the symptom index score was related to the SGRQ symptom score. A significant correlation was found (r=0.5, p<0.05).
Discussion
We failed to enroll the number of patients needed to show either a mortality benefit or reduction in hospitalization days prior to the end of study funding. An unexpected barrier to recruitment was the unwillingness of many eligible patients to participate in a trial that was expected to last for 2 years. We also observed a lower rate of overall hospitalization in both the control group and the intervention group than what we had originally predicted, possibly due to the fact that all patients were being followed up by pulmonologists and had had their medication regimens optimized. Even so, this was not an entirely negative study.
In many disease states the early recognition of a decline in health is essential to timely intervention. A heart failure telemedicine intervention failed to improve patient outcomes.4 In this study, six of the seven daily-reported parameters were subjective, with the exception being body weight. The remaining parameters asked the patient to compare his or her symptoms to those of the previous day. Unfortunately, patients may find it difficult to recognize small day-to-day variations in symptoms that might benefit from treatment. In COPD, symptoms may not be at a level considered to be an exacerbation, yet a minor worsening of pulmonary symptoms may represent an opportunity where treatment could lead to the avoidance of further declines in lung function and quality of life. As with the heart failure study, all of the symptoms reported by our patients were subjective, with the exception of the peak flow readings. The major difference was the use of a computerized algorithm that identified the variability of a patient's symptoms in an objective manner relative to his or her initial values. Based on the symptom reports we were not only able to detect worsening symptoms, but also to identify the specific symptoms that resulted in an alert being generated. With this information treatment approaches could range from intensification of bronchodilator therapy, the addition of oral steroids if the peak flow dropped to less than 80% of initial values, and the addition of antibiotics if sputum was purulent. After treatment was initiated continued symptom reporting served as a tool to assess response to treatment.
A prospective randomized controlled trial reported the effects of a single 1.5-h education session, an action plan for self-treatment of exacerbations, and monthly nurse case management phone follow-ups on repeat COPD hospitalizations and emergency visits in 743 veterans with COPD at five regional medical centers.21 The interventional group had a 41% reduction (p<0.001) in COPD hospitalizations or emergency room visits compared with standard care over 1 year. In this study the time between symptom onset and initiation of therapy with antibiotics or steroids was not mentioned. However, when a multicenter cooperative trial involving 20 Veterans Administration hospitals attempted a similar study, the study was stopped prematurely due to excessive mortality in the comprehensive case management group.22 The reason for this difference is unclear, but the study did show that the time from the onset of symptoms to the initiation of antibiotics or prednisone was not different between the groups. The average time before receiving prednisone was 6.4 days in the intervention group versus 7.7 days in the usual care group. The time to receiving antibiotics was also similar (7.0 versus 6.8 days, respectively). In contrast, patients in the intervention group in our study received antibiotics, prednisone, or both as directed by reported symptoms within 24 h of their COPD symptom score increasing more than 1 point from the initial values.
Data on the utility of telemedicine-based management in COPD are limited. Published studies regarding telemedicine in COPD have involved small patient numbers, used variable intensities of monitoring, and lacked predetermined treatment plans. In a randomized controlled trial involving 240 patients (101 with COPD) with chronic respiratory failure, those randomized to receive tele-assistance had fewer hospitalizations, fewer urgent calls, and decreased acute exacerbations.23 Pare et al.24 reported a decrease in home visits and hospitalizations in COPD patients who were treated with the telehomecare model and substantial cost savings at 6 months of follow-up. Others, however, have found no benefit of home-based COPD telemonitoring programs in decreasing hospital or homecare costs.8 A retrospective study by Alrajab et al.25 attributed reduced rates of COPD exacerbation and healthcare utilization to the use of a telemonitoring program. Unfortunately, they did not provide patient reporting compliance or the criteria used to “flag” a report as a possible exacerbation that needed clinician follow-up.
Our telemedicine program was focused on monitoring of respiratory symptoms and peak flow and early intervention of acute COPD exacerbations. Treatments used in our study were not novel but were those endorsed by guideline recommendations. Our scheme of telemedicine symptom reporting allowed us to initiate treatments directed at patient-specific symptoms on the same day COPD symptoms worsened. We believe that timely, focused intervention was the primary reason for improvements in patient outcomes. Early intervention decreased the frequency and magnitude of subsequent exacerbation symptoms, decreased dyspnea, and improved peak flow and daily activity status. Long-term electronic daily monitoring of respiratory symptoms was well accepted by moderate to severe COPD patients as evidenced by the high rate of reporting compliance in both the control and intervention groups. In addition to its prospective, randomized, and controlled design, other strengths of our study include the utilization of a novel daily electronic diary and a composite symptom score that detected patient-specific symptom worsening. Our study also used evidence-based medical interventions and covered a prolonged follow-up period.
Limitations and Bias
Our study was able to overcome some, but not all, of the limitations identified by Goldstein and O'Hoski.11 Our patient population was well defined, and we used a randomized controlled design. The interventions used were those recommended by consensus guidelines and could be tailored to the symptoms reported by the patient. The period of follow-up exceeded 9 months for the majority of patients, with some patients being followed up for up to 2 years. However, our sample size was too small to detect a difference in the combined primary outcome of hospitalization or death between the intervention and control groups. Another limitation is the possibility of data entry errors, particularly for the peak flow meter readings.
As suggested in the CONSORT diagram (Fig. 1), there is the possibility of recruitment bias. One hundred eighty-three patients were referred to the study as potential participants. All had severe, symptomatic COPD; however, slightly more than 40% of these (75) declined participation. There is the possibility therefore that those patients who chose to enroll do not reflect the overall COPD patient population. Because data could not be collected on those who chose not to participate, we could not compare the clinical characteristics of the enrollers versus the nonenrollers. However, the demographics and clinical information of the participants are consistent with severe, symptomatic COPD.
Conclusions
Daily monitoring and early treatment of worsening respiratory symptoms that herald the onset of an acute exacerbation using a telemedicine-based home management program decreased the frequency and severity of COPD exacerbation symptoms, which led to an improvement in daily symptom control, lung function, and functional status. Future studies are required to demonstrate if such programs will result in a reduction in hospitalizations or improvement in mortality.
Acknowledgments
This study was supported by the Pennsylvania Department of Health (grant RFA 02-07-20).
Disclosure Statement
G.J.C. has served on Advisory Committees for Bayer, Dey Pharmaceuticals, and Phillips-Respironics and as a consultant for Johnson & Johnson, Glaxo-Smith-Kline, PortAERO, Pulmonx, and Uptake Medical, Inc. All of these sums are less than $2,500. G.J.C. has received research grants from Boehringer Ingelheim, Actelion, Forest, Glaxo-Smith-Kline, InterMune, the National Institutes of Health, Novartis, Phillips-Respironics, Roche Pharmaceuticals, and Spectral Diagnostics, Inc. All research grant monies are deposited and controlled by Temple University. F.C.C., D.C., C.G., J.G., K.B., F.G., and M.R.J. declare no competing financial interests exist.
References
- 1.Farmer AJ, Gibson OJ, Dudley C, Bryden K, Hayton PM, Tarassenko L, Neil A. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446). Diabetes Care 2005;28:2697–2702 [DOI] [PubMed] [Google Scholar]
- 2.Montori VM, Helgemoe PK, Guyatt GH, Dean DS, Leung TW, Smith SA, Kudva YC. Telecare for patients with type 1 diabetes and inadequate glycemic control: A randomized controlled trial and meta-analysis. Diabetes Care 2004;27:1088–1094 [DOI] [PubMed] [Google Scholar]
- 3.Logan AG, McIsaac WJ, Tisler A, Irvine MJ, Saunders A, Dunai A, Rizo CA, Feig DS, Hamill M, Trudel M, Cafazzo JA. Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens 2007;20:942–948 [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastrogiannis DS, Igwe E, Homko CJ. The role of telemedicine in the management of the pregnancy complicated by diabetes. Curr Diab Rep 2013;13:1–5 [DOI] [PubMed] [Google Scholar]
- 6.Boaz M, Hellman K, Wainstein J. An automated telemedicine system improves patient-reported well-being. Diabetes Technol Ther 2009;11:181–186 [DOI] [PubMed] [Google Scholar]
- 7.Joseph AM. Care coordination and telehealth technology in promoting self-management among chronically ill patients. Telemed J E Health 2006;12:156–159 [DOI] [PubMed] [Google Scholar]
- 8.Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, Stoddart A, van der Pol M, MacNee W, Sheikh A, Pagliari C, McKinstry B. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: Researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitacca M, Rossin M, Assoni G, Baratti D, Zanardini M, Ruocco G, Quinto T, Bernasconi A, Scalvini S, Colombo F. Tele-assistance respiratory card: Feasibility of self-reporting in patients with severe COPD. Telemed J E Health 2013;19:99–103 [DOI] [PubMed] [Google Scholar]
- 10.de Toledo P, Jimenez S, del Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed 2006;10:567–573 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RS, O'Hoski S. Telemedicine in COPD: Time to pause. Chest 2014;145:945–949 [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005;26:153–161 [DOI] [PubMed] [Google Scholar]
- 13.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 15.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther 2000;22:1121–1145 [DOI] [PubMed] [Google Scholar]
- 16.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619–624 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis 1984;37:85–95 [DOI] [PubMed] [Google Scholar]
- 18.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–654 [DOI] [PubMed] [Google Scholar]
- 19.Carter R, Holiday DB, Grothues C, Nwasuruba C, Stocks J, Tiep B. Criterion validity of the Duke Activity Status Index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2002;22:298–308 [DOI] [PubMed] [Google Scholar]
- 20.2004 update. Workshop report, global strategy for the diagnosis, management, and prevention of COPD. Available at http://www.goldcopd.org/Guidelines/guidelines-global-strategy-for-diagnosis-management-2004-3.html (last accessed July28, 2015)
- 21.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, Kumari S, Thomas M, Geist LJ, Beaner C, Caldwell M, Niewoehner DE. Disease management program for chronic obstructive pulmonary disease: A randomized controlled trial. Am J Respir Crit Care Med 2010;182:890–896 [DOI] [PubMed] [Google Scholar]
- 22.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, Thwin SS, Huang GD, Robbins R, Sriram PS, Sharafkhaneh A, Mador MJ, Sarosi G, Panos RJ, Rastogi P, Wagner TH, Mazzuca SA, Shannon C, Colling C, Liang MH, Stoller JK, Fiore L, Niewoehner DE. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: A randomized, controlled trial. Ann Intern Med 2012;156:673–683 [DOI] [PubMed] [Google Scholar]
- 23.Vitacca M, Bazza A, Bianchi L, Gile S, Assoni G, Porta R, Bertella E, Fiorenza D, Barbano L, Comini L, Scalvini S. Tele-assistance in chronic respiratory failure: Patients' characterization and staff workload of 5-year activity. Telemed J E Health 2010;16:299–305 [DOI] [PubMed] [Google Scholar]
- 24.Pare G, Sicotte C, St-Jules D, Gauthier R. Cost-minimization analysis of a telehomecare program for patients with chronic obstructive pulmonary disease. Telemed J E Health 2006;12:114–121 [DOI] [PubMed] [Google Scholar]
- 25.Alrajab S, Smith TR, Owens M, Areno JP, Caldito G. A home telemonitoring program reduced exacerbation and healthcare utilization rates in COPD patients with frequent exacerbations. Telemed J E Health 2012;18:772–776 [DOI] [PubMed] [Google Scholar]