Abstract
Efficient and effective growth factor (GF) delivery is an ongoing challenge for tissue regeneration therapies. The accurate quantification of complex molecules such as GFs, encapsulated in polymeric delivery devices, is equally critical and just as complex as achieving efficient delivery of active GFs. In this study, GFs relevant to bone tissue formation, vascular endothelial growth factor (VEGF) and bone morphogenetic protein 7 (BMP-7), were encapsulated, using the technique of electrospraying, into poly(lactic-co-glycolic acid) microparticles that contained poly(ethylene glycol) and trehalose to assist GF bioactivity. Typical quantification procedures, such as extraction and release assays using saline buffer, generated a significant degree of GF interactions, which impaired accurate assessment by enzyme-linked immunosorbent assay (ELISA). When both dry BMP-7 and VEGF were processed with chloroform, as is the case during the electrospraying process, reduced concentrations of the GFs were detected by ELISA; however, the biological effect on myoblast cells (C2C12) or endothelial cells (HUVECs) was unaffected. When electrosprayed particles containing BMP-7 were cultured with preosteoblasts (MC3T3-E1), significant cell differentiation into osteoblasts was observed up to 3 weeks in culture, as assessed by measuring alkaline phosphatase. In conclusion, this study showed how electrosprayed microparticles ensured efficient delivery of fully active GFs relevant to bone tissue engineering. Critically, it also highlights major discrepancies in quantifying GFs in polymeric microparticle systems when comparing ELISA with cell-based assays.
Introduction
Formulations for the controlled release of therapeutics have been in the spotlight of the biotechnology industry for many years, and while robust drugs with low molar mass have extensively been addressed, developing similar systems for proteins has proven much more challenging.1 Protein therapeutics are increasingly being explored and utilized within the field of tissue engineering (TE) since many mechanisms involved in tissue regeneration are driven by proteins, which need to be delivered efficiently for maximum benefit.2,3
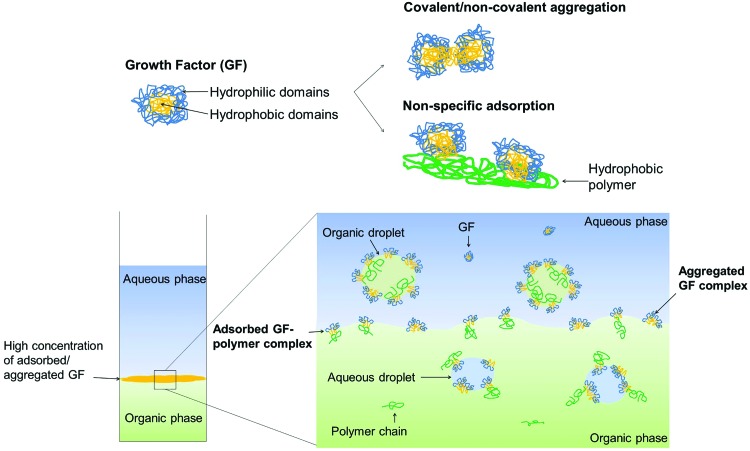
However, the development of controlled release formulations for proteins faces many challenges due to the relatively large and complex architecture of most proteins, which incorporate hydrophilic and hydrophobic domains with numerous reactive groups.4,5 The unfolding of polypeptide chains can expose hydrophobic groups, which can interact with other molecules (aggregation) and with hydrophobic matrices (nonspecific adsorption) (Fig. 1, top).6–8 As a consequence, proteins are challenging molecules to encapsulate and deliver from polymeric carriers, but likewise they are also difficult to quantify after the encapsulation process.
FIG. 1.
Schematic description of growth factor interactions in solution through hydrophobic domains (top, adapted from Wu and Jin4) and concentration of growth factor (GF) complexes at the water-in-oil interface upon an extraction procedure (bottom). Color images available online at www.liebertpub.com/tec
It is paramount to appreciate that protein encapsulation and in vivo delivery may be affected by protein denaturation, but in vitro experimental conditions and characterization methods also account for denaturation, a phenomenon which often leads to underestimated protein quantification, a vast issue in the field.5 With this in mind, two objectives were set in the present study: one was to provide a sustained release formulation with full preservation of the protein's native state and the other was to use representative assays that accurately assess the formulation, both ambitious goals, which will be addressed hereafter.
To address the first objective, it was important to consider that protein–carrier formulations require strategies to preserve the native state of proteins.1 When encapsulating in biodegradable polymeric particles, dry encapsulation has become a preferred option compared with aqueous incorporation, which requires a water-in-oil (w/o) emulsion, a potent cause of protein denaturation9 due to the w/o interfacial tension causing protein molecules to unfold.4 By using dry encapsulation, such as in spray-drying or with solid emulsions, the bioactivity of proteins can be improved or maintained.10,11
Stabilizers can also be used to protect proteins,12 by increasing the energy barrier between the native and denatured states of protein molecules, for example.4 Size reduction of protein aggregates (or micronization) with poly(ethylene glycol) (PEG) upon colyophilization13 can also effectively lead to a more favorable state of the protein,5,14,15 as seen for nerve growth factor (GF)16 and albumin,14 and may also reduce protein adsorption to the polymer matrix.5,7 Alternatively, the use of saccharides is another approach to protect proteins4,6,17 by stabilizing proteins through preferential hydration of the native form during lyophilization.1,6 Sugars were already shown to protect several types of GFs18 and proteins.19 However, while many alternative approaches to GF bioactivity protection have been sought, protein denaturation often remains a key limitation of traditional encapsulation techniques, which has limited the clinical translation.
Electrospraying of polymeric microparticles that contain therapeutic molecules is an emerging technique that has been shown to adequately maintain the bioactivity of some proteins and GFs, including insulin-like GF 1 (IGF-1),20 platelet-derived GF (PDGF),21 transforming GF β-3 (TGFβ-3), and bone morphogenetic protein (BMP) 6.19 Electrospraying is a one-step technique using high voltage that can be used for dry encapsulation of proteins and drugs.22 It can provide tight control over particle characteristics,23 generating narrow size distributions of submicrometric particles, with limited agglomeration of particles and high yields.24
Electrospraying is particularly appealing for tissue engineering applications due to the coating nature of the technique. For instance, melt electrospun microfiber scaffolds were homogenously coated with electrosprayed poly(lactic-co-glycolic acid) (PLGA) loaded with serum albumin,25 providing constructs for TE with a tailorable protein delivery function. In terms of reducing denaturation, electrospraying holds great potential considering the limited exposure to organic solvents and the possibility to use dry encapsulation.22 Although sugars blended into the polymer matrix have been widely used for improving protein bioactivity in traditional encapsulation techniques,4,5,14–16,26 to our knowledge, no studies have investigated their use in electrospraying and hence this possibility represented an avenue of interest.
Further to providing an optimized protein–carrier formulation, accurate quantification of active proteins was the next challenge. Several techniques can assess protein denaturation and inform on structural and conformational changes and size and shape distributions.27 When encapsulating a protein in a polymeric device, however, it becomes difficult to assess the protein conformation after extraction or release in buffer since irreversible changes can be induced by the extraction/release processes themselves (Fig. 1, bottom).28 This has been extensively demonstrated in the literature where polyester-based microparticles almost always present incomplete release profiles5 when assessed with enzyme-linked immunosorbent assay (ELISA) and other protein assays. The study of GF activity is arguably more relevant when cell-based assays are used, which are the best indicators of GF bioactivity, and which will be utilized in this study in a head-to-head comparison with the more traditional assays.
In summary, to address the two important challenges of encapsulation and quantification, we have evaluated two complementary GFs used in bone tissue engineering, BMP-7 and vascular endothelial growth factor (VEGF), for encapsulation using an electrospraying technique. We furthermore assessed the potential of protective additives within the formulation. GF bioactivity was assessed at various stages of the encapsulation process with typical buffer assays using ELISA and the results were compared with bioactivity results from cell assays. A direct contact assay of GF-loaded particles with cells was also used to evaluate the efficiency of the delivery system and provided key findings regarding the quantification of GFs in microparticle systems, using fundamentally different assays. Collectively, this highlighted the discrepancies found between in vitro characterization assays and encourages careful consideration of the most appropriate future techniques.
Materials and Methods
Materials
PLGA 85:15 DLG 4.5E with ester end groups (Mn 41.3 kDa, PDI 1.6) was purchased from Evonik Industries. PEG with Mn = 35 kDa, trehalose, chloroform, dichloromethane (DCM), polysorbate 20 (PS20), sodium dodecyl sulfate (SDS), human serum albumin (SA), and fluorescein isothiocyanate-SA (FITC-SA) were purchased from Sigma-Aldrich. Recombinant VEGF was purchased from ProsSpec-Tany TechnoGene Ltd., through BioNovus Life Sciences. Recombinant human BMP-7 was generously donated by Stryker.
Particle fabrication
Either SA alone or GF:SA were first micronized to ensure protein particle size reduction before electrospraying.12,13,29 Protein loadings were set to less than 1 wt% of the final microparticle weight, as shown previously to be ideal for loaded electrospraying particles, in terms of release and protein distribution.23 Aqueous solutions were prepared, containing SA and PEG, with and without GF and with and without trehalose addition. The GF:SA and (GF:SA):PEG part ratios were maintained at 1:9 and 1:10, respectively (Table 1), to obtain ideal GF protection by SA and ideal micronization by PEG, as previously shown.13,23
Table 1.
Summary of Microparticle Formulations
| Formulation name | GF | GF (μg) | SA (mg) | Trehalose (mg) | PEG (mg) | PLGA (mg) | Loading (ng GF/mg microparticles) |
|---|---|---|---|---|---|---|---|
| Blank | — | — | 1.8 | — | 33.3 | 299.7 | — |
| VEGF | VEGF | 10 | 0.09 | — | 16.7 | 150.3 | 60 (Low) |
| BMP | BMP-7 | 200 | 1.8 | — | 33.3 | 299.7 | 600 (High) |
| Blank-T | — | — | 1.8 | 3.3 | 33.3 | 299.7 | — |
| VEGF-T | VEGF | 10 | 0.09 | 1.67 | 16.7 | 150.3 | 60 (Low) |
| BMP-T | BMP-7 | 200 | 1.8 | 3.3 | 33.3 | 299.7 | 600 (High) |
BMP, bone morphogenetic protein; VEGF, vascular endothelial growth factor.
Final protein contents were set to 0.06 and 0.6 wt% for VEGF and BMP-7 formulations, respectively, which were shown to be ideal to obtain effective, yet nontoxic, in vitro cellular responses from human umbilical vein endothelial cells (HUVECs) in a proliferation cell assay and from C2C12 in a differentiation assay (see the Growth factor bioactivity section and Supplementary Data; Supplementary Data are available online at www.liebertpub.com/tec). Samples were dissolved in 0.2 μm filtered doubly distilled water (1 mL) and frozen by immersion in liquid nitrogen. After freeze-drying, PLGA was dissolved in chloroform and added to the lyophilized GF under magnetic stirring. The resultant dispersions were probe sonicated for 1 min at 0.5 W (Misonix 3000). The final polymer (PLGA:PEG) content was 11% wt/v for a PLGA:PEG part ratio of 9:1.
The dispersions were immediately loaded into a 1-mL glass syringe, fitted with a 21 G stainless steel nozzle, and electrosprayed according to an established electrospraying configuration (Supplementary Fig. S1).23,24 Briefly, the dispersions were extruded at a rate of 0.8 mL/h using a syringe pump (World Precision Instruments) and a voltage of 10 kV was applied to the needle tip. The tip-to-collector (TTC) distance was 15 cm and collectors consisted of aluminum foils (15 × 15 cm2) sterilized with 70% ethanol. After electrospraying, collectors were placed under vacuum for a further 72 h. The microparticles were transferred into glass vials and stored at −20°C until further analysis.
Particle characterization
Particle morphology was characterized with an FEI Quanta 200 scanning electron microscope (SEM) operating at 5 kV in high vacuum mode. Microparticle samples were taped on aluminum stubs and gold coated at 30 mA (SC500 sputter coater; Bio-Rad). Particle size was assessed with ImageJ analysis software by automated measurements of particle diameter (National Institutes of Health) based on light micrographs (AxoVision, Carl Zeiss MicroImaging GmbH).
The dispersion of GFs within electrosprayed particles was assessed by viewing encapsulated albumin, which had been prelabeled with a fluorescent dye (FITC). The distribution of FITC-SA within microparticles was visualized using confocal laser scanning microscopy (CLSM). To randomly collect FITC-SA-loaded particles, a microscope glass slide was introduced in the electrospraying apparatus housing and held in contact with the collector, in the center of the spraying zone for 5 min, while electrospraying. The slide was then removed and fluorescence images were captured using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems), 63× objective with a 9.7× zoom. Excitation was 488 nm and emission was captured between 495 nm and 633 nm.
In vitro characterization
Encapsulation efficiency and loading
Particles (5 mg) were loaded into 15-mL Falcon tubes (Fisher Scientific) and dissolved in DCM (1 mL), n = 4, and vortexed for 30 s. PBS (1 mL) was added and tubes were vortexed for 30 s to extract GFs into the aqueous phase. The aqueous phase was analyzed using a human BMP-7 or human VEGF ELISA from R&D systems, according to the manufacturer's protocol, upon sample dilution to fit the detection range of the assay.
In vitro release
Particles (10 mg) were placed in 2-mL screw-capped high-purity polypropylene microtubes (Sarstedt), supplemented with the release solution: PBS + PS20 (0.05%), (1.5 mL). Tubes were agitated at a speed of 8 rpm at 37°C. At specific time points, microtubes were removed from the incubator and agitation was stopped. After allowing for natural particle settlement at the bottom of microtubes, the supernatant (1.3 mL) was collected and replaced by the same amount of fresh release solution. Supernatants were immediately stored at −20°C for further analysis using ELISA.
GF recovery through in vitro processing
Using the extraction procedure stated above, the recovery of VEGF with and without the presence of unloaded PLGA microparticles was investigated to check whether the released VEGF was adsorbing back to the polymer matrix, impairing detection. A 250 ng/mL VEGF solution (1 mL, containing 0.1% wt SA) was lyophilized with and without unloaded microparticles (5 mg), n = 3. For BMP-7, the recovery after freeze-drying and extraction procedure, containing contained surfactants, was assessed. Both a nonionic surfactant, polysorbate 20 (PS20), and a less commonly used anionic surfactant, SDS, were used here. Three aqueous solutions were prepared: PBS, PBS+PS20 (0.05%), and PBS + SDS (0.05%). A 600 ng/mL BMP-7 solution (1 mL) (containing 0.1% wt SA) was lyophilized with and without the presence of unloaded PLGA microparticles (5 mg), n = 3. All mixtures were then subjected to the extraction procedure and the aqueous phases were analyzed using ELISA after sample dilution. Controls consisted of reconstituted GFs following freeze-drying in the same aqueous solutions.
Growth factor bioactivity
HUVEC proliferation assay
VEGF bioactivity after micronization with PEG, SA, trehalose, and contact with organic solvent was studied in vitro with an optimized HUVEC proliferation assay (Supplementary Data). The growth medium consisted of Dulbecco's modified Eagle's medium (DMEM) F12-K nutrient mixture (Invitrogen), 0.1 mg/mL heparin (Sigma), 0.05 mg/mL endothelial cell growth supplement (ECGS) (Millipore), 10% fetal calf serum (FCS), and 1% penicillin/streptomycin (P/S) (both from Invitrogen). Each processed VEGF group was reconstituted in PBS before assessment with HUVECs, except for one treatment that included vortexing the VEGF lyophilizate with chloroform to mimic the process of solid GF dispersion in organic solvent that is undertaken before electrospraying. The solvent was left to evaporate overnight before redissolution in PBS.
A 96-well plate was gelatin coated and 8000 cells were seeded per well. The culture medium contained no ECGS and contained only 5% FCS. On day 1 (24 h after initial cell seeding and culture in growth medium), 150 μL of culture medium and 50 μL of VEGF treatments or controls were added to each well, equating to a VEGF concentration of 12 ng/mL per well. Cells were then cultured for 3 days without media change, followed by medium removal and freezing for 48 h, n = 6. After treatment with proteinase K (Sigma), DNA content was analyzed with the PicoGreen® assay (Invitrogen), according to the manufacturer's protocol. In parallel, VEGF concentrations were also measured by ELISA (n = 4).
C2C12 differentiation assay
The bioactivity of BMP-7 was evaluated in vitro using a mouse myoblast cell (C2C12) differentiation assay. Cells were cultured in DMEM (Invitrogen) supplemented with 10% FCS and 1% P/S. Concentrations of 5000 cells/well were seeded in a 48-well plate and incubated for 24 h. The effect of BMP-7 dose on cell proliferation and cell differentiation was optimized in Supplementary Data). Subsequently, BMP-7 bioactivity after micronization with PEG, SA, trehalose, and contact with organic solvent was assessed with the differentiation assay, using a BMP-7 concentration of 1.4 μg/mL. Again, in the case of chloroform vortexing, the solvent was left to evaporate overnight before redissolution in PBS and then injection on C2C12 cells.
The various treatments were given on day 1, which were replaced by fresh medium containing identical treatments on day 3. All treatments were removed on day 5 and cells frozen for 48 h. Twelve replicates were used per condition, 6 were used for DNA content quantification by a PicoGreen assay (Invitrogen) after proteinase K treatment (Sigma) and six were used for quantification of alkaline phosphatase (ALP) expression. The latter was measured by adding paranitrophenyl phosphate (pNPP) (Sigma) to cells, which was converted to p-nitrophenol (pNP). The absorbance of pNP was measured at 405 nm with a spectrophotometer (Bio-Rad). In parallel, the concentrations of BMP-7 were also measured by ELISA (n = 3).
In vitro microparticle direct culture
The ability of PLGA microparticle formulations to deliver active BMP-7 was assessed through an in vitro direct contact culture with murine calvaria preosteoblast (MC3T3-E1) cells over 3 weeks. BMP-7 formulations were selected (BMP and BMP-T, Table 1) to probe for an effect from the trehalose addition. Microparticles that did not contain BMP-7 were used as a negative control (NC). Cells were cultured in α-minimum essential medium (α-MEM) supplemented with 10% FCS and 1% P/S as the standard growth media for all experimental conditions, except the positive control 1 (PC1), which contained osteogenic media (containing 10 mM β-glycerophosphate, 0.1 mM ascorbate-2-phosphate, and 100 nM dexamethasone in standard growth media).
Concentrations of 20,000 cells/well were seeded in 24-well plates and incubated with growth media for 24 h. On day 1, media were aspirated and cells were treated with different conditions (1 mL, n = 36, N = 288) summarized in Table 2. After optimizing the appropriate amount of particles to be used in direct contact culture (Supplementary Data), 2.5 mg of microparticles/well was selected, representing a total of 1.5 μg BMP-7. The positive control hence consisted of a bolus delivery of 1.5 μg BMP-7 administered once at the start of the experiment on day 1 (directly added to the cells of the PC2 group).
Table 2.
Experimental Conditions for In Vitro Microparticle Culture (n = 36 Per Group, i.e., n = 12 Per Time Point)
| NC | Negative control | Standard media |
|---|---|---|
| PC1 | Positive control 1 | Osteogenic media |
| PC2 | Positive control 2 | Bolus delivery of fresh BMP-7 (1.5 μg/well at start) |
| C1 | Condition 1 | 2.5 mg/well BMP-7-loaded microparticles (BMP) |
| C2 | Condition 2 | 2.5 mg/well BMP-7-loaded microparticles (with 1% trehalose) (BMP-T) |
| C3 | Condition 3 | 2.5 mg/well unloaded particles (Blank) |
| C4 | Condition 4 | 2.5 mg/well unloaded particles (with 1% trehalose) (Blank-T) |
Cells were assayed at days 7, 14, and 21. Twelve replicates per group were selected for each time point, six were used for DNA content quantification using a PicoGreen assay after proteinase K treatment and six were used for quantification of ALP expression as described previously. Optical microscopy (Nikon Eclipse TS100-PixeLINK) was used to assess cell morphology and interactions with microparticles. For SEM, the microparticle–cell sheet was recovered and centrifuged at 1500 rpm for 10 min at each time point. Media were removed and the microparticles were rinsed twice with doubly distilled water. After final centrifugation, microparticles were freeze-dried and imaged with SEM according to the Particle characterization section.
Statistical analysis
Statistical analysis was performed with PASW Statistics 18 (IBM Corp). For particle size, analysis was undertaken using medians with a Mann–Whitney nonparametric test after Levene's test confirmed inequality of variances. Elsewhere, analysis was performed with a two-way analysis of variance (ANOVA) and post hoc tests were performed using Games–Howell, assuming unequal variances. The significance level was determined for p < 0.05.
Results
Particle microstructure
Polymeric microparticles encapsulating BMP-7 or VEGF were prepared by electrospraying (Supplementary Fig. S1). The colyophilization of GFs with PEG before electrospraying was used to form micron-sized GF particles, as described in our previous study.23 Upon dispersion of the lyophilized protein mixture in PLGA 85:15 solution and further electrospraying with optimized parameters, spherical and narrowly dispersed microparticles were obtained with an average size of 5.0 ± 1.3 μm. After micronization with 1% wt trehalose, a similar size and size distribution were observed due to the minimal increase of overall concentration of solids and presence of the additive, as expected.22 Morphologies were similar, spherical and smooth, and were not affected by incorporation of trehalose (Supplementary Fig. S2).
GF encapsulation efficiency, loading, and in vitro release
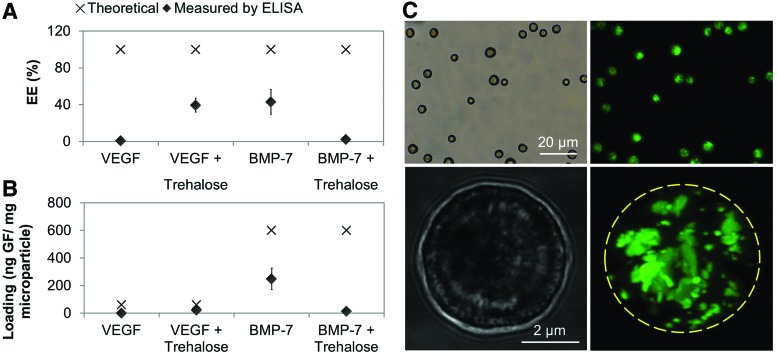
The results of GF encapsulation efficiency (EE) and GF loading into electrosprayed microparticles are presented in Figure 2 (details of microparticle formulations can be found in Table 1). During the process of electrospraying, solid proteins were dispersed in a polymer solvent, thus there was no protein dissolution into an aqueous phase and high EE values are expected. However, all results, as measured by ELISA after extraction, were below 50%, with high dispersity and with no clear trend with the addition of trehalose (Fig. 2A). Similarly, the loading was also significantly smaller than theoretical predictions (Fig. 2B). This result highlighted the first in vitro discrepancy. Indeed, when confocal scanning microscopy was used, full encapsulation of GF was observed, as seen in Figure 2C, with a homogenous distribution of FITC-SA within the particles. SA is an excipient used to protect GF bioactivity and is used in excess compared with GF (1:9 ratio), hence its distribution is representative of GF distribution within the particles.
FIG. 2.
(A) Encapsulation efficiencies. (B) Loading, theoretical, and as measured by enzyme-linked immunosorbent assay (ELISA). Means ± standard errors (SE), n = 4. (C) Microscopy images of electrosprayed particles loaded with 1% wt serum albumin labeled with fluorescein isothiocyanate (FITC), bright-field, and maximum projection of the confocal laser scanning microscopy z-stacks. Color images available online at www.liebertpub.com/tec
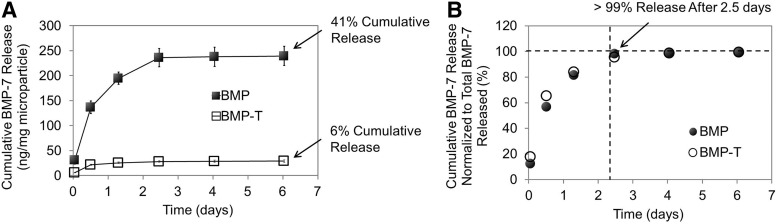
Due to the nature of the slow-degrading PLGA used in the formulation, which generates a lag phase after initial release, we have assessed and presented only the first phase of the release, as measured by ELISA (Fig. 3A), which is also relevant since initial GF release is the most critical one for tissue regeneration. According to the results, the presence of trehalose in the microparticle formulation was revealed to have a significant effect on measured GF with lower overall cumulative release of BMP-7 (p < 0.001). The maximum amount released was measured as 240 ng BMP-7 per mg of particle for BMP-7 only and 29 ng/mg for BMP-7 + trehalose and this was achieved only after 2.5 days (Fig. 3B). However, from Figure 2B, the amount of BMP-7 supposedly encapsulated in the trehalose formulation was measured as only 12.6 ng/mg microparticles, clearly indicating that the extraction procedure did not provide accurate EE values. Approximately three times more BMP-7 was actually measured during the release assay, compared with what was predicted by the EE assay, measured by extraction and ELISA.
FIG. 3.
Release profiles of bone morphogenetic protein 7 (BMP-7)-loaded electrosprayed microparticles as measured by ELISA. (A) Cumulative BMP-7 amount released per mg of microparticle. (B) Cumulative BMP-7 amount released compared with total released. Mean ± SE, n = 3.
These results openly demonstrate the sensitivity of processed growth factors and their inability to be processed and subsequently detected after harsh techniques such as extraction.
GF recovery through in vitro processing
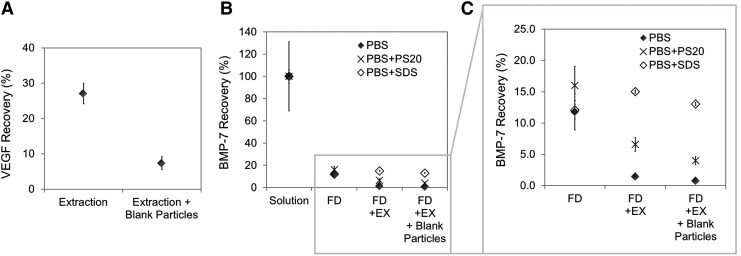
From the previous section, it was evident that the extraction process was affecting the detection quality of GF encapsulated in microparticles, hence a substudy was designed to further assess the impact of the extraction technique. First, a VEGF solution (250 ng/mL) was lyophilized and subjected to the extraction procedure. After measurement by ELISA, only 27% VEGF was recovered (68 ng/mL), suggesting GF aggregation (Fig. 4A). Next, when the same experiment was repeated in the presence of unloaded particles (i.e., VEGF solution + blank polymer particles), only 7% VEGF was recovered (18.4 ng/mL), significantly lower than without particles (p = 0.004). This result particularly highlights the negative impact of polymer matrix in solution in recovering GFs, which had nonspecifically adsorbed to the matrix, impeding detection.
FIG. 4.
GF recovery after in vitro processing, as measured by ELISA. (A) Vascular endothelial growth factor (VEGF) recovery after extraction in the presence and absence of unloaded microparticles in PBS. (B, C) BMP-7 recovery after different treatments and in different media. Mean ± SE, n = 3.
The experiment was then repeated for BMP-7 and expanded to include two possible surfactants, polysorbate 20 (PS20) and SDS, to attempt to dissociate aggregated GFs and separate adsorbed GFs from the polymer should those interactions be reversible. The results are presented in Figure 4B and C. The first striking result was the low recovery of BMP-7 (12%), as measured by ELISA, after only freeze-drying and redissolution (Fig. 4B), identifying freeze-drying as a significant issue for GF detection. Next, similar to the results attained for VEGF, BMP-7 recovery was lower after the extraction procedure and after contact with unloaded particles (Fig. 4C). While this lower recovery was statistically significant compared with lyophilized and resuspended BMP-7, values were similar for the extracted BMP-7 with and without particles (p = 0.16), suggesting that aggregation phenomena were more critical here than nonspecific adsorption to the matrix (Fig. 4C).
In vitro GF bioactivity
HUVECs are known to proliferate in a dose-dependent manner upon exposure to VEGF up to 20 ng/mL,30 and hence are used as a means of determining VEGF bioactivity in vitro. Similarly, BMP-7 is an effective inducer of C2C12 myoblast cell differentiation into osteoblast cells at more than 200 ng/mL.31 In this case, the extent of differentiation can be correlated to the expression of ALP, an early marker of osteogenic differentiation, so that the bioactivity of BMP-7 may be determined. Hence, as a first step, the appropriate culture conditions and GF concentration ranges to be used were determined and optimized. Results are presented in Supplementary Data.
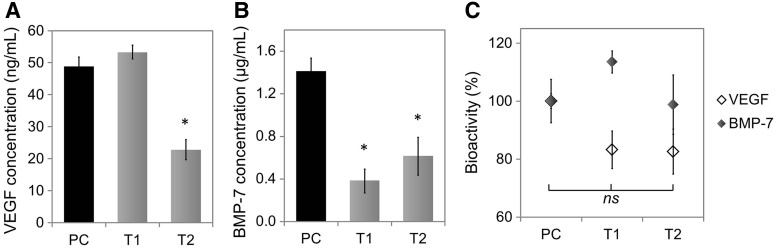
The next step was to assess GF bioactivity after the most critical steps of microparticle processing where GFs may become denatured, involving micronization with the polymer formulation additives (PEG, SA, and trehalose) and further vortexing with chloroform (Table 3 for details). GF concentrations were measured by ELISA in parallel and results are presented in Figure 5.
Table 3.
Summary of Treatments
| Name | Description |
|---|---|
| PC | Fresh GF after freeze-drying |
| T1 | Micronized GF with PEG, SA, and trehalose |
| T2 | Micronized GF with PEG, SA, and trehalose, subjected to chloroform |
GF, growth factor; PEG, polyethylene glycol; SA, serum albumin.
FIG. 5.
Effect of micronization and vortexing with chloroform on the concentrations of VEGF and BMP-7 measured in vitro and their effective bioactivities. (A) VEGF quantification by ELISA (n = 4). (B) BMP-7 quantification by ELISA (n = 3). (C) GF bioactivity, normalized to the positive control PC and measured by cell-based assays; VEGF bioactivity is expressed as DNA content after 3 days of culture with treatments (n = 6); BMP-7 activity is measured by the ALP expression (pNP absorbance), normalized by DNA content, after 5 days of culture with treatments (n = 6). Mean ± SE. (* and ns indicate statistical and nonstatistical significance, respectively, compared with the positive control [PC]).
First, upon micronization, and unlike BMP-7, it appeared that VEGF was fully detectable according to ELISA analysis (Fig. 5A, PC vs. T1). After contact with chloroform (T2), however, only 47% VEGF was detected, suggesting GF aggregation. Strikingly, when compared with the bioactivity results, although bioactivity was slightly lower compared with the control, there was no statistical difference (p = 0.15) between any groups. Importantly, bioactivity was identical for micronized VEGF before (T1) and after contact with chloroform (T2), with around 83% bioactivity (Fig. 5C) in both cases.
A similar discrepancy between ELISA and cell assays—and even more pronounced—was observed for BMP-7, where bioactivity was unaltered upon any of the two treatments compared with the control (Fig. 5C). Up to 98% bioactivity after vortexing with chloroform was obtained (T2), hence demonstrating the nondenaturing effect of organic solvent on BMP-7 as well. Conversely, again, the micronization of BMP-7 and subjection to chloroform were detrimental to BMP-7 detection by ELISA (Fig. 5B), with 27% and 43% detection compared with the control, respectively.
Further to this last experiment, it also appeared from the Particle characterization section, in the case of BMP-7, that even the simple freeze-drying of a BMP-7 solution and further redissolution in PBS itself lowered the detection of reconstituted BMP-7 by ELISA analysis. This was observed in the presence and absence of surfactant (Fig. 4B), suggesting GF aggregation. The experiment was thus repeated so that the bioactivity of reconstituted BMP-7 could be assessed in parallel. Results are shown in Supplementary Data, Supplementary Figure S7. Briefly, ELISA analysis detected significantly less BMP-7 after freeze-drying (64%, p = 0.048), but no differences in bioactivity were observed (p = 0.08). This indicated that once in solution with C2C12 cells, freeze-dried BMP-7 performed in a similar way as unprocessed BMP-7.
In vitro microparticle direct culture
In this experiment, known amounts of BMP-7-loaded microparticles were placed in direct contact with MC3T3-E1 preosteoblast cells since these cells differentiate into osteoblasts upon stimulation with BMP-7. The maximum amount available per well (based on total amount of BMP-7 contained in microparticles) was 1.5 μg BMP-7, hence the positive control was 1.5 μg fresh BMP-7 delivered as bolus (one-time injection at the beginning of experiment), analogous to the surgical procedure routinely employed in the clinic for OP-1, for example.
During the entire 3-week culture, MC3T3s proliferated extensively and DNA content was similar across all groups (within each time point) except for the positive control PC1 (osteogenic media), which triggered more proliferation than all the other groups at day 7 (data not shown), due to the fundamentally different composition of media, which boosted proliferation at this early time point. Neither formulation of BMP-7-loaded or unloaded microparticles had any negative impact of proliferation compared with all the other controls, indicative of the cytocompatibility of electrosprayed PLGA particles in contact with MC3T3s.
All wells showed confluent monolayers of cells at every time point and particles fully covered the monolayers (Supplementary Fig. S9). Cells had a similar morphology (independently of BMP-7 or presence of trehalose) and showed positive interactions with the microparticles by forming multiple attachment points to them. In fact, at the first analysis point (day 7), it was already impossible to recover any particles without detaching the entire cell monolayer, which had established good adhesion with the microparticles. After culture, microparticles were recovered for SEM imaging. Results are presented in Figure 6. At all time points and for all formulations, cells spread around particles, covering several particles simultaneously and spanning to adjacent particles until a micropatterned cell monolayer incorporating all particles was established (Fig. 6, right).
FIG. 6.
Scanning electron microscopy (SEM) images of BMP-7-loaded electrosprayed microparticles after in vitro culture with MC3T3-E1 cells.
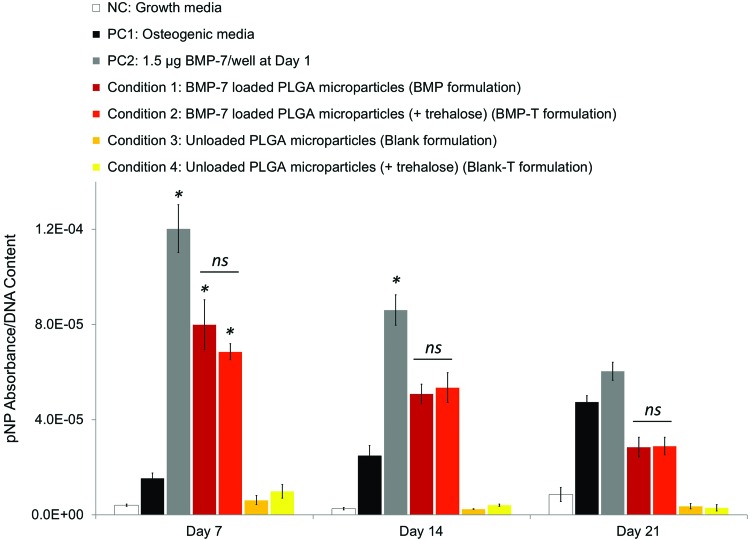
The results of ALP expression of MC3T3-E1 over time are presented in Figure 7. As expected, all positive controls + treatments were superior to the NC (p < 0.001) and there were no significant differences with unloaded particles (p > 0.8 for Blank and Blank-T, see Table 1 for details). Both BMP-7 formulations provoked enhanced ALP expression compared with the NCs at all time points, implying that both formulations were having a positive effect on the differentiation of MC3T3-E1 cells up to 3 weeks. More specifically, according to the release data (Fig. 3A), no more than 6% and 41% of the loaded BMP-7 would have been released from the trehalose and trehalose-free formulations, respectively, after 7 days (i.e., 28 and 238 ng BMP-7/mg of particles). Since 2.5 mg of particles and 1 mL solution were used in the cell assay, there was a theoretical cumulative amount of 70 and 595 ng/mL available, respectively, which according to32 should have led to significant differences in the differentiation of MC3T3-E1 cells into osteoblasts, as measured by an ALP assay, but which were not observed here (both formulations were effective and nonstatistically different). Hence, it could be concluded that there were clearly different amounts of BMP-7 released and likely higher than predicted by the release assay. Furthermore, also contrary to the release profiles, there were no statistical differences between the trehalose-free formulation (BMP) and the formulation with trehalose (BMP-T) seen in the direct contact assay and both formulations performed in a similar way at all time points.
FIG. 7.
ALP activity of MC3T3-E1 cells during a 3-week culture with various treatments. Results were divided by the DNA content measured for each group separately. Mean ± SE (n = 6 for ALP, n = 6 for DNA content). (* indicates statistical local significance compared with PC1 (osteogenic media) for PC2, C1, and C2, ns indicates local nonsignificance between groups). Color images available online at www.liebertpub.com/tec
Discussion
Presently, the field of particle–protein delivery systems used in tissue engineering is still of tremendous interest clearly indicated by the considerable number of publications that address or attempt to address the topic each year. The lack of translation of these technologies, however, should not be ignored and should rather serve to highlight the gaps felt in the field; a prevailing one being the inadequacy of the quantification methodologies being used. As useful and as necessary as in vitro experiments may be–as a prelude to any expensive and ethically involved animal experiments−, the results of this present study demonstrate the inefficiency of the gold standard methodologies in quantifying microparticle-based systems that incorporate large and delicate molecules such as growth factors.
In polymer–protein-based systems, which properly and reproducibly encapsulate the proteins, as can be achieved by the electrospraying technique (Fig. 2C), subsequent quantification attempts are challenging partly due to the intimate entanglement of the protein in the polymer network. Such a configuration is ideal in terms of reproducible delivery (controlled mostly by polymer degradation) for the release of the entrapped content; however, saline-based assessment assays and further biochemical assays will remain unrepresentative of the true quality of these systems.
As seen in this present study, the presence of stabilizers in polymer formulations (such as SA) or surfactants in solution (PS20, SDS) cannot counterbalance the fact that proteins act as strong surfactant-like compounds. As a consequence, a highly used process like the extraction process results in proteins being trapped at the interfacial layer through their hydrophobic domains (Fig. 1, top) and will in turn provide underestimated EE values (Figs. 1, bottom, and 2), an issue which is always vaguely eluded in the field. In this study, it was further proven that even when nonencapsulated VEGF and BMP-7 solutions were subjected to the extraction process, GF aggregation was observed as well as nonspecific adsorption to the polymer once unloaded particles were added to both GF solutions (Fig. 4).
Importantly, BMP-7 showed extensive aggregation during the freeze-drying step (only 20% recovery) even before any extraction treatment (Fig. 4), as measured by ELISA. A similar result was previously observed by Lochmann et al. where one single freeze/thaw cycle caused a loss of about one third in ELISA detection of BMP-2, clearly suggesting that the freezing step was a significant issue.26 Additionally, even during assays, which are gentler than the extraction methods, such as in vitro release assays in saline buffer, in the absence of any surrounding cells, which would otherwise rapidly utilize the released GFs/signaling molecules, these molecules are invariably and rapidly adsorbed back to the dissolving polymer matrix, in turn biasing the release results. Surfactants are commonly used in this respect by effectively raising the energy barrier for intermolecular interactions between proteins by surrounding them with their hydrophilic ends.14
In this study, two types of surfactants (nonionic and anionic) were investigated in GF recovery and release assays. When a BMP-7 solution was subjected to the extraction procedure with and without the presence of unloaded particles, both surfactants were beneficial in dissociating noncovalent BMP-7 aggregates and displacing adsorbed BMP-7 from the polymer matrix (Fig. 4). Similarly, when PS20 was added to the release medium, up to 16% more cumulative release was observed for BMP-T particles compared with the same particles in PBS only (not shown).
These results proved that both the extraction, involving GF dispersion in organic solvent and a w/o interface, and the in vitro release in saline buffer generated some reversible conformational changes, which could be somewhat addressed by the use of additives. Furthermore, while the extraction process is the gold standard method for quantifying proteins in polymeric systems, it may be irrelevant when used with hydrophobic polymers, such as polyester-based polymers and derivatives. Radiolabeling may be an acceptable alternative, although the prelabeling chemical reaction is an extra step, which itself may partly denature the GF due to undesired side reactions because of sensitive amino acid residues, as well as the radioactive isotopes can denature proteins over prolonged exposure of time.33 For release assays, while most strategies have aimed at avoiding the formation of the destabilizing environment, it has been shown that different protein–polymer formulations require different stabilization approaches.5 As stated by Giteau et al., an optimal strategy, which protects the protein during the different stages of release and which is simple and transposable to any protein, would be optimal, but is yet to be invented.
Instead of tuning in vitro assays, another strategy involves the modulation of the polymer formulations themselves to both protect the GFs and facilitate their detection. For instance, the use of sugars as stabilizing agents of therapeutic molecules into polymer particles has been often used and has been well covered over the last 20 years.1,28,34,35 Like other stabilizers, sugars protect proteins by preferential interactions.6,27,28 However, it is clear from the literature that there is not always a beneficial effect in using any sugar with any protein, and sugars tend to work on a protein-to-protein basis, leading to different results for different proteins. Bilati et al. have summarized some recent studies and concluded that trehalose was statistically more effective than other sugars, as opposed to mannitol, which was largely inefficient.34
The ability of trehalose to stabilize biological molecules is generally highly valued; in fact, some animals release significant levels of trehalose in response to environmental stresses, as a protection mechanism, and hence many pharmaceutical drugs use trehalose as a stabilizer.36 Trehalose has shown great advantages as an excipient in linear polymers for proteins37 and enzymes36 or as a conjugate to stabilize proteins and retain protein activity against high heat conditions and lyophilization.38 However, while many studies report protein stability data, less information is found upon sugar effect on the actual encapsulation efficiency and release of proteins from polymeric particles.
Compared with traditional techniques, the use of trehalose is advantageous in electrospraying since its addition did not significantly affect the properties of the final polymer particles in terms of size and size distribution (Supplementary Data, Fig. S2 and as seen in Sukarto and Amsden19). Besides, the ability of electrospraying to process proteins through nonaqueous routes allows protein lyophilization with trehalose before mixing with organic solution,22 maximizing protein protection compared with delivery systems using aqueous encapsulation processes, and also increases the osmotic activity of the solution created by dissolution of the particles.19 However, when 1% wt trehalose was present here in the final BMP-7 formulations, reduced EE (Fig. 2) and burst release, followed by incomplete release, were measured through ELISA (Fig. 3). This can be expected since some stabilizers are compromised with burst release or aggregation upon release.4
Similarly, in the only other study, to date, assessing the use of additives in electrospraying microparticles,19 when the amount of trehalose was increased from 3% to 4.5 wt% in P(TMC-CL)2-PEG microspheres, the release of lysozyme (a model protein) went from a similar low burst to more sustained release profile, although EE was reduced by 20%. Of note, these profiles were measured by a nonspecific protein assay (calorimetric bicinchoninic acid protein assay) and the release of actual GFs (BMP-6 and TGF-β3) and their direct bioactivies with/without trehalose were not studied.19
However, in our study, although reduced EE and release profiles were observed as measured by ELISA, we proved that there were no statistical differences between the trehalose-loaded and trehalose-free formulations with BMP-7 when cultured with MC3T3-E1 cells. The formulations presented similar ALP values over time and triggered differentiation for up to 3 weeks (Fig. 7), contrary to what both the encapsulation efficiency and release assay in solution (no cells) had predicted. Hence, trehalose in this system was shown here to promote GF noncovalent aggregation during lyophilization, which impaired accurate quantification, but did not impede effectiveness of the delivery system.
This finding may be explained by the fundamental differences between biochemical- and cell-based assays. When using a sandwich ELISA, monoclonal capture and detection antibodies with binding sites that are specific to the protein are used, hence they bind on the protein of interest to either one sequence in a linear configuration (2D) or to a local conformation of two domains (contiguity of two protein parts) in a 3D configuration. During GF processing as it is the case here and in general when encapsulating GFs in polymeric devices for tissue regeneration, GFs may aggregate during the manufacturing process or during the characterization assays. Hence, the specific sequence or specific conformation required for capture or detection by the binding sites of the antibodies may be masked.
In cell-based assays, however, functional mechanisms are much more complex, where numerous membrane receptors at the surface of the cell (such as tyrosine-specific kinase receptors for VEGF39 or serine-specific kinase receptors for BMPs40) can recognize several different sequences in the protein, causing binding and subsequent cellular response stimulation, hence the masking of one specific sequence is not an issue. Similarly, some proteins dimerize, that is, two molecules form one compound, further activating cellular responses.41 Hence, the specific sequence or conformations required for ELISA (which capture only free GFs) may as well be masked in the dimer configuration, while dimers may precisely be needed for cellular activation. Hence, more studies may be needed to conclude the effect of additives in electrosprayed particles, yet our results highlight the importance of not only addressing additives in solution but also to always compare results with actual cell-based assays. Finally, as stated by Walle et al., while the use of additives is very popular, a clear need for these additives has not yet emerged and case-by-case basis studies are required.35
In terms of microparticle preparation, we have already established that electrospraying is an innovative emerging technique with enhanced capacity for reproducible protein encapsulation and delivery compared with traditional encapsulation techniques.22–25 Electrosprayed particles present superior characteristics compared with particles from traditional water-in-oil emulsion-based techniques due to a gentler technique and superior control over the particles' features.22 In terms of bioactivity, proteins (SA) and growth factors (IGF-1, PDGF, VEGF, BMP-7, TGF-β3) immediately released from electrosprayed particles were highly bioactive (80–90%), as assessed with cell proliferation assays.20,21,42 Interestingly, it is found in the literature that over time and when assessed by cell-free assays, the bioactivity of released VEGF and PDGF decreased (less than 21% final bioactivity after 21 days), which the authors attributed to the assay conditions, denaturing the GFs prone to oxidation and pH-dependent deamidation.21 This result, in particular, underlines the unsuitability of saline buffer assays, where cells are not present, for assessing released GFs.
In this study, when encapsulating GFs into electrosprayed particles, there are two steps that may potentially denature GFs: first the micronization with additives, then the mixing with organic solvent before electrospraying. It is thus necessary to assess the bioactivity of GFs after those two steps independently rather than after release in buffer, which in itself involves denaturing factors. From the results presented in this study (Fig. 5 and Supplementary Figure S7, Supplementary Data), it was proven for the first time that the most critical steps of GF processing in a polyester-based system, involving freeze-drying, micronization with PEG, or vortexing with organic solvent, generated a significant degree of GF aggregation, which impaired accurate detection by ELISA. However, all bioactivity assays used during this study confirmed that the interactions were reversible in nature since both VEGF and BMP-7 performed equally as well as before processing in all cases.
In particular, these results underpin the limitations of the ELISA for quantifying GFs that have been processed with polymers since they risk possible aggregation. This leads to incorrect use of ELISA as means of GF quantification, as seen in the field, considering that ELISA readings may give lower concentrations to what is effectively present and biologically active in a system, potentially leading to GF overdosing at dangerous levels. For instance, Wang et al. showed that after GF lyophilization, they had lost 75% of the BMP-2 detectable by ELISA and thus increased their in vivo dose for compensation, stating that 75% of the bioactivity was lost.43 This approach is erroneous considering that the ELISA is steadily proven here to be a misleading means of GF detection and that cell assays remain a superior method for assessing the effectiveness of processed GFs.
The previous sections shine a definitive light on the difficulties of GF quantification and at the same time also validated the electrospraying process as an effective technique for encapsulating two major GFs relevant in bone tissue formation. Next, GF bioactivity postprocessing was assessed by means of a direct contact assay with cells. Cells indeed actively consume GFs either released in the medium or by direct contact with the microparticles; hence, an in vitro context with cells has certain similarities with the in vivo context. Such an assay is fundamentally better than simple saline buffer assays where no cells are present to take up released GFs and so GFs may detrimentally become adsorbed or aggregated in solution before analysis. This was investigated here with the BMP-7 electrosprayed formulations in contact with MC3T3-E1 cells.
The size of electrosprayed particles produced was within the order of size of MC3T3-E1 cells, thus it was suitable for cellular stimulation by topographical cues.44 Positive interactions were expected considering that we showed in previous studies that unloaded and electrosprayed microparticles on the 5–10 μm size range had positive effects on several cell types, including fibroblasts24 and preosteoblasts,25 but such positive effects were investigated and obtained here for the first time with growth factor-loaded electrosprayed particles. Bolus delivery gave a higher effect, as explained by the particles not being fully degraded after 21 days and evidenced by SEM analysis (Fig. 6), considering the slow-degrading polymer used here (PLGA 85:15), which contains a high lactide fraction.
Due to the short-term nature of the cell assays and the unsuitability of release assays in saline buffer assays, only the first phase of the release pattern was investigated in this study and GF molecules may still be released in the third part of the release pattern following the lag phase, which may happen after several months for PLGA 85:15. However, as previously demonstrated, polymer–protein interactions in solution would also happen at this stage, with released proteins adsorbing back onto the degrading polymer. Such issue impairs in any way the accurate quantification of release profiles in saline buffer, making it pointless to measure those profiles for longer amounts of time. The most sensible studies were hence to look at the effect of released BMP-7 onto cells when compared with the NCs. These showed that released BMP-7 was still active and triggered significant cell differentiation at all time points during the 3-week period (Fig. 7). Taken together, the results presented here emphasized how the use of an appropriate release system, such as the novel electrosprayed GF-polymer particles produced here, can enable a lower dose of active GFs to be released when adequate quantification means using cell-based assays are used in conjunction.
Conclusions
In this study, GFs for bone tissue engineering, VEGF and BMP-7, were encapsulated into electrosprayed microparticles and assessed by saline buffer-based assays and cellular assays to enable a direct comparison of assays. Fundamental differences were observed; when cell-free quantification procedures or stabilizers in the polymer formulations were used, reduced ELISA detection was systematically obtained, providing biased results. Conversely, when the processed GFs were tested with cells, GF bioactivity was verified at all stages of microparticle processing (involving micronization and contact with organic solvent) and in the presence of stabilizer in the formulation, showing the reversible nature of the interactions observed during encapsulation and quantification. The positive effect of BMP-7-loaded electrosprayed particles in stimulating differentiation of preosteoblasts up to 3 weeks provided further proof of the discrepancies between release assays in saline buffer and measured by ELISA and the actual effectiveness seen with direct contact with cells.
Taken together, these results highlight the importance of using appropriate analysis techniques for GF delivery, which is a complex undertaking with multiple interactions. A major change in the assessment of microparticle systems containing intricate molecules such as GFs may be needed, hopefully paving the way to further development in the field. Electrosprayed particles have therefore shown promise in developing active formulations of GFs, but the use of cell-based assays to accurately evaluate any protein–carrier formulations remains paramount.
Supplementary Material
Acknowledgments
The authors wish to thank Prof. George Muscat from the University of Queensland for providing C2C12 cells, Dr. Mary Wang for help with handling, and the Australian Research Council (ARC), LP130100945, for financial support. N.B. also acknowledges the financial support from QUT in the form of an Australian Postgraduate Award scholarship and top-up from the Deputy Vice Chancellor. M.A.W. acknowledges support from the ARC LP100200084.
Disclosure Statement
No competing financial interests exist.
References
- 1.Putney S.D., and Burke P.A. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol 16, 153, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Biondi M., Ungaro F., Quaglia F., and Netti P.A. Controlled drug delivery in tissue engineering. Adv Drug Deliver Rev 60, 229, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Babensee J.E., McIntire L.V., and Mikos A.G. Growth factor delivery for tissue engineering. Pharm Res 17, 497, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Wu F., and Jin T. Polymer-Based Sustained-Release Dosage Forms for Protein Drugs, Challenges, and Recent Advances. AAPS Pharm Sci Tech 9, 1218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giteau A., Venier-Julienne M.C., Aubert-Pouëssel A., and Benoit J.P. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm 350, 14, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Arakawa T., Prestrelski S.J., Kenney W.C., and Carpenter J.F. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliver Rev 46, 307, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Pean J.M., Boury F., Venier-Julienne M.C., Menei P., Proust J.E., and Benoit J.P. Why does PEG 400 co-encapsulation improve NGF stability and release from PLGA biodegradable microspheres? Pharm Res 16, 1294, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Lu L., Stamatas G.N., and Mikos A.G. Controlled release of transforming growth factor beta 1 from biodegradable polymer microparticles. J Biomed Mater Res 50, 440, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ye M., Kim S., and Park K. Issues in long-term protein delivery using biodegradable microparticles. J Control Release 146, 241, 2010 [DOI] [PubMed] [Google Scholar]
- 10.King T.W., and Patrick C.W. Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J Biomed Mater Res 51, 383, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Mok H., and Park T.G. Water-free microencapsulation of proteins within PLGA microparticles by spray drying using PEG-assisted protein solubilization technique in organic solvent. Eur J Pharm Biopharm 70, 137, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Morita T., Sakamura Y., Horikiri Y., Suzuki T., and Yoshino H. Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant. J Control Release 69, 435, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Morita T., Horikiri Y., Yamahara H., Suzuki T., and Yoshino H. Formation and isolation of spherical fine protein microparticles through lyophilization of protein-poly(ethylene glycol) aqueous mixture. Pharm Res 17, 1367, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Rawat S., Kohli N., Suri C.R., and Sahoo D.K. Molecular mechanism of improved structural integrity of protein in polymer based microsphere delivery system. Mol Pharm 9, 2403, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Carpenter J.F., and Crowe J.H. The mechanism of cryoprotection of proteins by solutes. Cryobiology 25, 244, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Johnson P.J., Skornia S.L., Stabenfetdt S.E., and Willits R.K. Maintaining bioactivity of NGF for controlled release from PLGA using PEG. J Biomed Mater Res A 86A, 420, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Cleland J.L., Duenas E.T., Park A., Daugherty A., Kahn J., Kowalski J., and Cuthbertson A. Development of poly-(D,L-lactide-coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release 72, 13, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Amsden B.G., Timbart L., Marecak D., Chapanian R., Tse M.Y., and Pang S.C. VEGF-induced angiogenesis following localized delivery via injectable, low viscosity poly(trimethylene carbonate). J Control Release 145, 109, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Sukarto A., and Amsden B.G. Low melting point amphiphilic microspheres for delivery of bone morphogenetic protein-6 and transforming growth factor-β3 in a hydrogel matrix. J Control Release 158, 53, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Wang F., Li Z.Q., Tamama K., Sen C.K., and Guan J.J. Fabrication and Characterization of Prosurvival Growth Factor Releasing, Anisotropic Scaffolds for Enhanced Mesenchymal Stem Cell Survival/Growth and Orientation. Biomacromolecules 10, 2609, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Ekaputra A.K., Prestwich G.D., Cool S.M., and Hutmacher D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ɛ-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 32, 8108, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Bock N., Dargaville T.R., and Woodruff M.A. Electrospraying of polymers with therapeutic molecules: state of the art. Prog Polym Sci 37, 1510, 2012 [Google Scholar]
- 23.Bock N., Dargaville T.R., and Woodruff M.A. Controlling microencapsulation and release of micronized proteins using poly(ethylene glycol) and electrospraying. Eur J Pharm Biopharm 87, 366, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Bock N., Woodruff M.A., Hutmacher D.W., and Dargaville T.R. Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers 3, 131, 2010 [Google Scholar]
- 25.Bock N., Woodruff M.A., Steck R., Hutmacher D.W., Farrugia B.L., and Dargaville T.R. Composites for Delivery of Therapeutics: combining melt electrospun scaffolds with loaded electrosprayed microparticles. Macromol Biosci 14, 202, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Lochmann A., Nitzsche H., von Einem S., Schwarz E., and Mäder K. The influence of covalently linked and free polyethylene glycol on the structural and release properties of rhBMP-2 loaded microspheres. J Control Release 147, 92, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm 289, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 28.van de Weert M., Hennink W.E., and Jiskoot W. Protein Instability in Poly(Lactic-co-Glycolic Acid) Microparticles. Pharma Res 17, 1159, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Morita T., Horikiri Y., Suzuki T., and Yoshino H. Preparation of gelatin microparticles by co-lyophilization with poly(ethylene glycol): characterization and application to entrapment into biodegradable microspheres. Int J Pharm 219, 127, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Rui J., Dadsetan M., Runge M.B., Spinner R.J., Yaszemski M.J., Windebank A.J., and Wang H. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: in vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater 8, 511, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh L.C.C., Tsai A.D., and Lee J.C. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem 87, 292, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Zhang F., Ren L.-f., Lin H.-s., Yin M.-n., Tong Y.-q., and Shi G.-s. The optimal dose of recombinant human osteogenic protein-1 enhances differentiation of mouse osteoblast-like cells: an in vitro study. Arch Oral Biol 57, 460, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Schumacher T.N.M., and Tsomides T.J. In Vitro Radiolabeling of Peptides and Proteins. Current Protocols in Protein Science. Hoboken, NJ: John Wiley & Sons, Inc., 2001 [DOI] [PubMed] [Google Scholar]
- 34.Bilati U., Allemann E., and Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur J Pharm Biopharm 59, 375, 2005 [DOI] [PubMed] [Google Scholar]
- 35.van der Walle C.F., Sharma G., and Kumar M.N.V.R. Current approaches to stabilising and analysing proteins during microencapsulation in PLGA. Exp Op Drug Deliv 6, 177, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Ko J.H., Lin E.-W., Wallace P., Ruch F., and Maynard H.D. Trehalose hydrogels for stabilization of enzymes to heat. Polym Chem 6, 3443, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J., Lin E.W., Lau U.Y., Hedrick J.L., Bat E., and Maynard H.D. Trehalose glycopolymers as excipients for protein stabilization. Biomacromolecules 14, 2561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mancini R.J., Lee J., and Maynard H.D. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J Am Chem Soc 134, 8474, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Hoeben A., Landuyt B., Highley M.S., Wildiers H., Van Oosterom A.T., and De Bruijn E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56, 549, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Hogan B.L.M. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10, 1580, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Marianayagam N.J., Sunde M., and Matthews J.M. The power of two: protein dimerization in biology. Trends Biochem Sci 29, 618, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Xie J.W., and Wang C.H. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor Cone-Jet mode. Biotechnol Bioeng 97, 1278, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Wang C.-K., Ho M.-L., Wang G.-J., Chang J.-K., Chen C.-H., Fu Y.-C., and Fu H.-H. Controlled-release of rhBMP-2 carriers in the regeneration of osteonecrotic bone. Biomaterials 30, 4178, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Miyaki M., Fujimoto K., and Kawaguchi H. Cell response to micropatterned surfaces produced with polymeric microspheres. Colloid Surface A 153, 603, 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.