Abstract
Mesenchymal stem cells, precursors that can differentiate into osteoblasts, chondrocytes, and adipocytes, have tremendous potential for derivation of cells with specific (e.g., osteogenic) phenotypes for tissue engineering and tissue regeneration applications. To date, the predominant strategy to achieve directed differentiation of MSCs into osteoblasts was to recapitulate the normal developmental ontogeny of osteoblasts using growth factors (e.g., bone morphogenetic proteins). In contrast, the effects of biophysical stimuli alone on such outcomes remain, at best, partially understood. This in vitro study examined and optimized the effects of alternating electric current alone on the differentiation of adult human mesenchymal stem cells (hMSCs) at the cell population and single-cell levels. hMSCs, cultured on flat, indium-tin-oxide-coated glass in the absence of supplemented exogenous growth factors were exposed to alternating electric current (5–40 μA, 5–10 Hz frequency, sinusoidal waveform), for 1–24 h daily for up to 21 consecutive days. Compared to results obtained from the respective controls, hMSC populations exposed to the alternating electric current alone (in the absence of exogenous growth factors) expressed genes at various stages of differentiation (specifically, TAZ, Runx-2, Osterix, Osteopontin, and Osteocalcin). Optimal osteogenic differentiation was achieved when hMSCs were exposed to a 10 μA, 10 Hz alternating electric current for 6 h daily for up to 21 days. Exclusive osteodifferentiation was observed since genes for the chondrocyte (Collagen Type II) and adipocyte (FABP-4) lineages were not expressed under all conditions of the biophysical stimulus tested. Single cell mRNAs for 45 genes (indicative of hMSC differentiation) were monitored using Fluidigm Systems. Homogeneous expression of the early osteodifferentiation genes (specifically, TAZ and Runx-2) was observed in hMSCs exposed to the alternating electric current at 7 and 21 days. Heterogeneity for all other genes monitored was observed in hMSCs exposed to alternating electric current and in their respective controls. These results provide the first glimpse of gene expression in differentiating hMSCs at the cell population and single-cell levels and represent novel approaches for stem cell differentiation pertinent to new tissue formation.
Introduction
The present in vitro study was motivated by scientific literature reports of enhanced healing of bone fractures in experimental animals in response to electrical stimulation. These early studies used the methodologies and instrumentation available at those times and focused on tissue-level healing outcomes using direct electric current,1,2 electromagnetic fields,3 and pulsed electric fields.4
Availability of in vitro cell models enabled examination of alternating electric current at the cell-level, specifically, select functions of osteoblasts pertinent to new tissue formation.5,6 Investigations at this level addressed the effects of various aspects of this biophysical stimulus on functions (including differentiation) of mesenchymal stem cells, the multipotent stem cells that have the ability to differentiate into osteoblasts, chondrocytes, and adipocytes.7 Pulsed electric magnetic fields,8 biphasic electric current,9 and alternating electric current10 were examined in conjunction with select biochemical compounds as stimuli to induce osteodifferentiation of these stem cells. It should be noted that osteodifferentiation of mesenchymal stem cells also has been achieved in the presence of exogenous osteogenic factors (such as dexamethasone and/or bone morphogenetic proteins [BMPs]) in the absence of biophysical stimuli.11,12
To date, only one study examined and reported that, in the absence of exogenous osteogenic factors, alternating electric current induces exclusive mesenchymal stem cell differentiation along the osteogenic pathway.13 The optimal conditions that induce this remarkable result and pertinent aspects at the gene-level were not addressed at that time.
Undoubtedly elucidation of the underlying events at the cellular- and molecular- levels and optimization of the osteodifferentiation of mesenchymal stem cell populations under alternating electric current, require further investigation. Furthermore, recent advances in technologies and methodologies have expanded the scope of scientific investigations to include single-cell analysis.
This study was motivated by the aforementioned scientific needs and utilized interdisciplinary approaches, novel laboratory setups, cellular models, in addition to biochemical and molecular assays to examine human mesenchymal stem cells (hMSCs) differentiation in response to alternating electric current at the population level and, for the first time, at the single-cell level.
In addition to providing fundamental information pertinent to stem cell physiology, the unique biophysical stimulus examined provides an untapped (to date) alternative approach to obtain critically needed differentiated cell (specifically, osteoblasts, the bone-forming cells) supplies for cell-based assays and/or therapies needed for regeneration/repair of damaged tissues in the clinical milieu.
Materials and Methods
Cells and cell culture
Adult, hMSCs were obtained commercially (Lonza Walkersville, Inc.). These cells, characterized by the vendor, were used in this study without any further characterization. For passaging, the hMSCs were treated with trypsin/EDTA obtained from, and according to protocols provided by, the vendor (Lonza Walkersville, Inc.), and were cultured under standard cell culture conditions (i.e., a sterile, humidified, 37°C, 5% CO2/95% air environment) in mesenchymal stem cell growth medium (consisting of mesenchymal stem cell basal medium supplemented with serum, l-glutamine, and gentamicin/amphotericin-B). The concentrations of all supplements in this medium were considered proprietary information and were not disclosed by the vendor (Lonza Walkersville, Inc.). hMSCs of passage number 3–5 were used for all experiments.
Alternating electric current stimulation laboratory setup
hMSCs were exposed to alternating electric current using a custom-made laboratory setup adapted from Ulmann5 and Supronowicz et al.6 The system consisted of five components: (i) a function generator; (ii) a multimeter; (iii) an oscilloscope; (iv) a 1000 Ω resistor; and (v) a cathode. Coaxial cables connected the positive output of the function generator to the positive end of a 1000 Ω resistor, and the negative output of the function generator to the current-conducting cathode substrate; this arrangement assured that electric current stimulation was delivered to the cells cultured on the indium tin oxide (ITO)-coated glass. To monitor the signal output from the function generator, another coaxial cable connected the positive output of the oscilloscope to the positive end of the resistor, and the negative output from the oscilloscope to the current-conducting cathode substrate. A multimeter recorded readings of the alternating current as voltage difference across the 1000 Ω resistor.
The alternating electric current stimulation system delivered a sinusoidal waveform output with a frequency of either 5 or 10 Hz. Additionally, the system delivered a voltage (peak to peak) corresponding to a current in the range of 5–40 μA.
Exposure of adult hMSCs to alternating electric current
hMSCs in mesenchymal stem cell growth medium (as described in the Cells and Cell Culture section) cultured on flat, ITO-coated substrates (precoated with 1 mg/mL fibronectin) were exposed to an alternating electric current regime consisting of the following parameters: (i) alternating electric current in the ranges of 5–40 μA; (ii) frequency of either 5 or 10 Hz; (iii) sinuosoidal waveform; and (iv) duration of exposure of either 1, 3, 6, or 24 h/day for up to 21 consecutive days. In this study, the hMSCs were exposed to alternating electric current alone, that is, in the absence of exogenous growth factors. The supernatant medium was changed every 3 days for the duration of the experiments (up to 21 consecutive days).
Controls were hMSCs cultured in parallel under similar conditions but not exposed to alternating electric current.
Differentiation of adult hMSCs in response to alternating electric current
Differentiation of hMSCs cultured on flat, ITO-coated glass substrates in response to the chosen alternating electric current conditions (described in the Exposure of Adult hMSCs to Alternating Electric Current section) after 1, 2, 3, 5, 7, 14, and 21 consecutive days of culture was determined by monitoring the expression profiles of select genes.
Determination of select gene expression by adult hMSC populations
At the prescribed time points, total RNA was isolated from the hMSC populations cultured on ITO-coated glass slides using TRIzol Reagent (Life Technologies) following standard laboratory techniques. RNA from the aqueous phase was further purified (to remove impurities) using the RNeasy® Mini kit (Qiagen) and following protocols supplied by the vendor. RNA from hMSCs either exposed to alternating electric current or respective controls were converted to complementary DNA (cDNA) using a commercially available reverse-transcriptase kit (Finnzymes; Thermo Scientific) and protocols provided by the vendor. Quantitative real-time polymerase chain reaction (qRT-PCR) of the cDNA products from the hMSCs was performed using a commercially available DyNAmo SYBR green qRT-PCR kit (Finnzymes; Thermo Scientific) and the DNA Engine Opticon II continuous fluorescence detection system (Bio-Rad).
Expression of genes indicative of the lineage-specific, osteoblastic pathway (specifically, TAZ, Runx-2, Osterix, Osteopontin, and Osteocalcin), and the chondrogenic (Collagen Type II) and adipogenic (FABP-4) lineages were also monitored.
Relative gene expression (fold change) was calculated using the 2−ΔΔCT method14; ribosomal protein L13a was used as a housekeeping gene to ensure equal loading of RNA into all qRT-PCR reactions. The results obtained from hMSCs exposed to alternating electric current were compared to those obtained from the respective controls, that is., cells cultured in parallel under similar conditions but not exposed to alternating electric current.
Determination of select gene expression by adult hMSCs at the single-cell level
Following exposure of hMSCs to 10 μA, 10 Hz, alternating electric current for 6 h daily for 7 and 21 consecutive days, or their respective controls, a single-cell suspension in hMSC medium was obtained and used for qRT-PCR measurement of mRNA levels in individual cells using the C1 Single-Cell Autoprep System and BioMark HD instruments (Fluidigm) as described in literature reports.15 Briefly, individual hMSCs were captured on a C1 Integrated Fluidic Circuit (17–25 μm cells) using the Fluidigm C1 Single-Cell Autoprep System, stained using LIVE/DEAD Cell Viability/Cytotoxicity Kit (Life Technologies), and imaged using an AxioImager M1 microscope (Zeiss).
Subsequently, on the C1 chip, preamplified cDNA was generated from each cell using the Single Cells-to-CT Kit (Life Technologies), pooled qRT-PCR primers (Table 1), and Fluidigm specific target amplification reagents according to the manufacturer's (Fluidigm) recommendations. Preamplified cDNA harvested from the C1 was then used for high-throughput qRT-PCR measurement of each amplicon using a BioMark HD system as described in literature reports,15–17 with modifications. Briefly, a 2.25 μL aliquot of each amplified cDNA was mixed with 2.5 μL of 2X SsoFast EvaGreen Supermix with Low ROX (Bio-Rad) and with 0.25 μL of 20X DNA Binding Dye Sample Loading Reagent (Fluidigm). Each sample mix was then pipetted into one sample inlet in a Dynamic Array IFC chip (Fluidigm). Individual qRT-PCR primer pairs (100 mM, Table 1) were diluted 1:10 with Tris-EDTA (2.5 μL total volume), mixed with 2.5 μL Assay Loading Reagent (Fluidigm), and then individually pipetted into one assay inlet in the same Dynamic Array IFC chip. Subsequent sample/assay loading was performed with an IFC Controller HX (Fluidigm), and qRT-PCR was performed on the BioMark HD real-time polymerase chain reaction (PCR) reader (Fluidigm) following the manufacturer's instructions using standard fast cycling conditions and melt-curve analysis, generating an amplification curve for each gene of interest in each sample.
Table 1.
Primer Sequences Used to Monitor Adult Human Mesenchymal Stem Cell Differentiation Using Quantitative Real-Time Polymerase Chain Reaction
| Gene (abbreviation/alternative name) | Forward primer | Reverse primer |
|---|---|---|
| aggrecan (ACAN) | 5′-GAC TTC CGC TGG TCA GAT GG-3′ | 5′-GTT TGT AGG TGG TGG CTG TG-3′ |
| Bone gamma-carboxyglutamate (gla) protein (BGLAP; Osteocalcin) | 5′-ATG AGA GCC CTC ACA CTC CT-3′ | 5′-CTT GGA CAC AAA GGC TGC AC-3′ |
| Bone morphogenetic protein 2 (BMP2) | 5′-ACT CGA AAT TCC CCG TGA CC-3′ | 5′-CCA CTT CCA CCA CGA ATC CA-3′ |
| Bone morphogenetic protein 6 (BMP6) | 5′-GAT AAG GAC TGA GGG CCA GG-3′ | 5′-CCC TTG GCG TGA GCA GTC-3′ |
| Bone morphogenetic protein 7 (BMP7) | 5′-CAG GCC TGT AAG AAG CAC GA-3′ | 5′-CAC AGT AGT AGG CGG CGT AG-3′ |
| Collagen, type I, alpha 1 (COL1A1) | 5′-AGT GGT TTG GAT GGT GCC AA-3′ | 5′-GCA CCA TCA TTT CCA CGA GC-3′ |
| Collagen, type II, alpha 1 (COL2A1; Collagen Type II) | 5′-GAG CAG GAA TTC GGT GTG GA-3′ | 5′-GCC ATT CAG TGC AGA GTC CT-3′ |
| Epidermal growth factor (EGF) | 5′-TGA CAC TTG GGA GCC TGA TG-3 | 5′-CTA CAG GGC ACG TGC AGT AA-3′ |
| Fatty acid-binding protein 4, adipocyte (FABP4; FABP-4) | 5′-GGA ATG CGT CAT GAA AGG CG-3′ | 5′-GCG AAC TTC AGT CCA GGT CA-3′ |
| Fibroblast growth factor 2 (basic) (FGF2) | 5′-CCC CAG AAA ACC CGA GCG A-3′ | 5′-CGT CCG CTA ATC TGG CAC C-3′ |
| Insulin-like growth factor 1 (somatomedin C) (IGF1) | 5′-ACC CAG AAG TAT CAG CCC CC-3′ | 5′-AGG TAA CTC GTG CAG AGC AAA-3′ |
| Integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12) (ITGB1) | 5′-CCG CGC GGA AAA GAT GAA TTT-3′ | 5′-CCA CAA TTT GGC CCT GCT TG-3′ |
| Integrin-binding sialoprotein (IBSP; Bonesialoprotein) | 5′-GAC ACC ACA GAG ACC GGA AG-3′ | 5′-CCA AAA GGT GGG GAA GTG GT-3′ |
| Runt-related transcription factor 2 (RUNX2; Runx-2) | 5′-TCG GAG AGG TAC CAG ATG GG-3′ | 5′-CAT TCC GGA GCT CAG CAG AA-3′ |
| Secreted phosphoprotein 1 (SPP1; Osteopontin) | 5′-GAA CAT GAA ATG CTT CTT TCT CAG T-3′ | 5′-CAG GGA GTT TCC ATG AAG CCA-3′ |
| Secreted protein, acidic, cysteine-rich (osteonectin) (SPARC; Osteonectin) | 5′-TTG CAA TGG GCC ACA TAC CT-3′ | 5′-GGG CCA ATC TCT CCT ACT GC-3′ |
| Sp7 transcription factor (SP7; Osterix) | 5′-AAA GGG TTA AAG CCG CTG GA-3′ | 5′-ACT CCA CAA GGG CAT GAT CC-3′ |
| SRY (sex determining region Y)-box 9 (SOX9) | 5′-CGA AAG CGG AGC TCG AAA CT-3′ | 5′-GTT TCC GGG GTT GAA ACT GG-3′ |
| Transforming growth factor, beta 1 (TGFB1) | 5′-TGG TGG AAA CCC ACA ACG AA-3′ | 5′-GAG CAA CAC GGG TTC AGG TA-3′ |
| Vascular endothelial growth factor A (VEGFA) | 5′-CTT GCC TTG CTG CTC TAC CT-3′ | 5′-GCA GTA GCT GCG CTG ATA GA-3′ |
| Vimentin (VIM) | 5′-AAC TTA GGG GCG CTC TTG TC-3′ | 5′-CCT GCT GTC CCG CCG-3′ |
| Vitronectin (VTN) | 5′-CGC TTT GAG GAT GGT GTC CT-3′ | 5′-ACT GTA GCT ATG GGC AGG GA-3′ |
| WW domain-containing transcription regulator 1 (WWTR1; TAZ) | 5′-CCA CAA CTC CGG AGG ACT TC-3′ | 5′-GAA ACG GGT CTG TTG GGG AT-3′ |
Data were analyzed using real-time PCR analysis software (Fluidigm) with the following settings: curve quality threshold 0.65, linear derivative baseline correction, automatic thresholding by assay, and manual melt curve exclusion. Cycle threshold (Ct) values for each reaction from live single cells were exported and further analyzed using an R-script package, SINGuLar Analysis Toolset 2.1 (Fluidigm), with a limit of detection of 24 and default outlier exclusion; this analysis generated the violin plots of Log2-transformed Ct values for each gene of interest in live, single, hMSCs.
Results
Adult hMSC differentiation at the cell population level
Differentiation of adult hMSCs was determined, in the absence of exogenous growth factors, after cell exposure to a sinusoidal alternating electric current in the ranges of 5–40 μA, frequency either at 5 or 10 Hz, for various durations (1–24 h) daily, for up to 21 consecutive days.
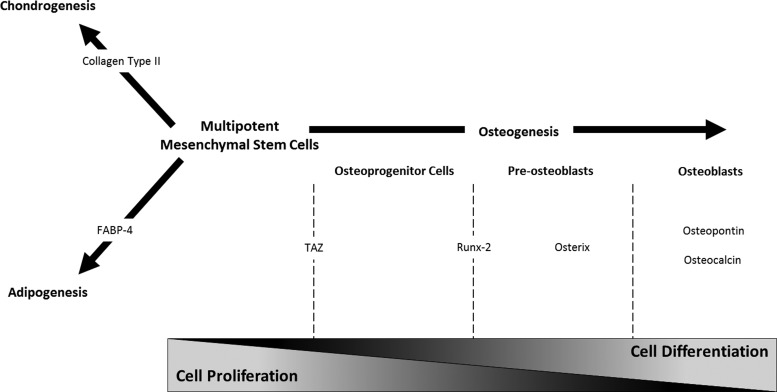
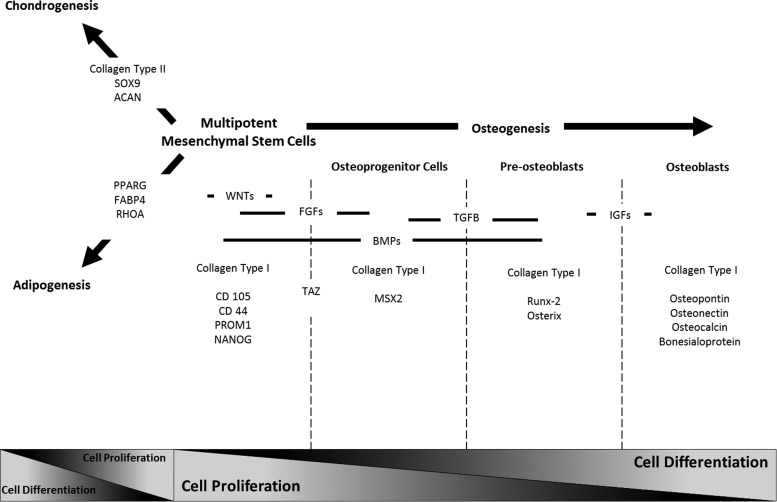
Figure 1 illustrates the sequence of targeted genes, expressed by differentiating hMSCs and monitored in this study.
FIG. 1.
Sequence of targeted genes expressed by differentiating adult human mesenchymal stem cells (hMSCs) and monitored at the cell population level. Schematic representation of the time course of genes expressed during adult hMSC differentiation into the adipogenic, chondrogenic, and osteogenic lineages. During osteogenesis, mesenchymal stem cells go through four major phases: multipotent mesenchymal stem cells, committed osteoprogenitor cells, pre-osteoblasts, and osteoblasts. Genes indicative of the respective early, middle, and late stages of the osteogenic phenotype pathway are expressed by the differentiating mesenchymal stem cells. FABP-4, fatty acid-binding protein 4. Adapted from Hughes et al.11
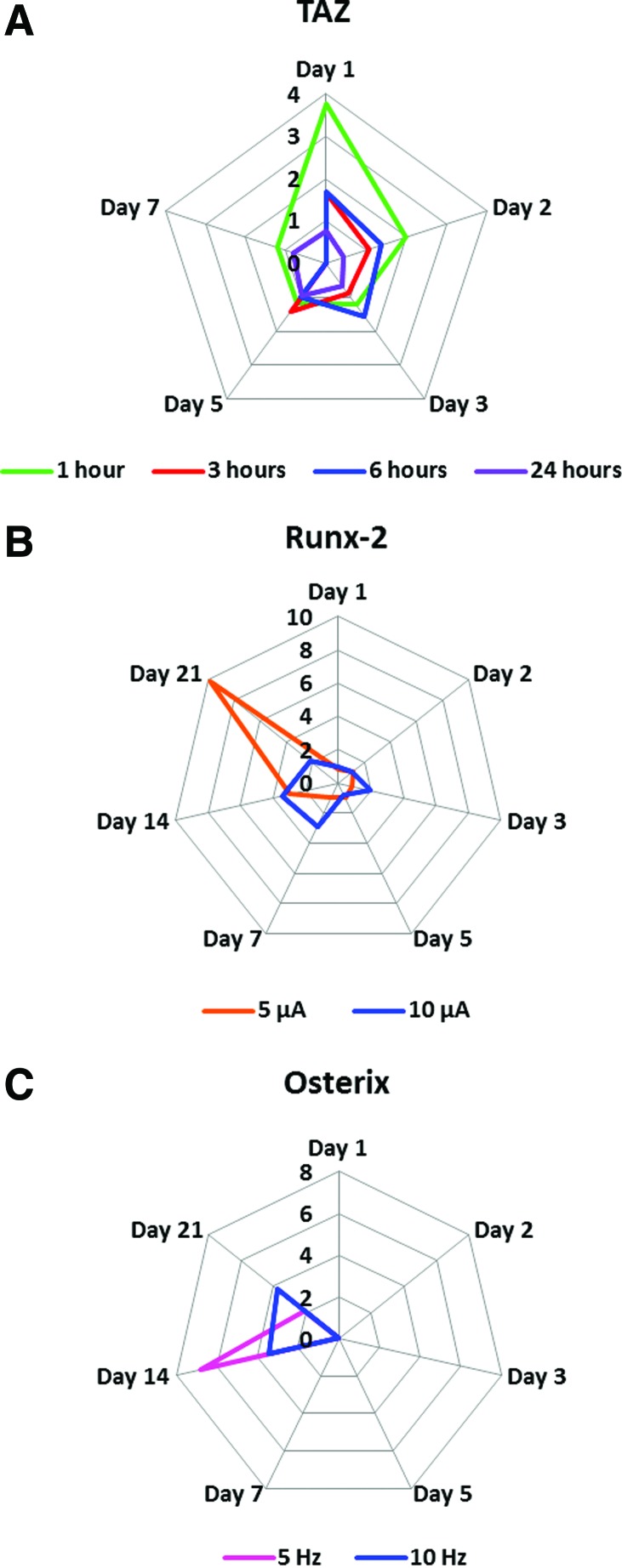
The experimental parameters tested affected expression of the monitored differentiation-related genes differently. TAZ, an early gene, exhibited the highest expression after the shortest (i.e., 1 h) duration of hMSC exposure to 10 μA, 10 Hz alternating electric current for 1 day (Fig. 2A). The level of electric current affected expression of Runx-2, which was maximally expressed when hMSCs were exposed to 5 μA (rather than 10 μA), 10 Hz alternating electric current for 6 h daily for 21 consecutive days (Fig. 2B). Variation in the tested frequency (5 Hz versus 10 Hz) affected expression of Osterix (a gene expressed in the middle of the osteodifferentiation pathway) when hMSCs were exposed to 10 μA alternating electric current for 6 h daily for 14 consecutive days (Fig. 2C).
FIG. 2.
Effect of alternating electric current on the expression of select “Early” and “Middle” osteodifferentiation genes by adult hMSCs. For the three frames of Figure 2: The axes indicate fold change compared to the “baseline,” that is, results obtained from adult hMSCs in numbers similar to those seeded on ITO-glass (on day 0), but not exposed alternating electric current. (A) Effect of the duration of adult hMSC exposure to electric current on the expression of TAZ. Adult hMSCs were exposed to 10 μA, 10 Hz alternating electric current for various durations (hours) daily for up to seven consecutive days of culture. (B) Effect of the current level on the expression of Runx-2. Adult hMSCs were exposed to either 5 μA (orange line) or 10 μA (blue line), 10 Hz alternating electric current for 6 h daily for up to 21 consecutive days of culture. (C) Effect of electric current frequency on the expression of Osterix. Adult hMSCs were exposed to 10 μA alternating electric current at either 5 Hz (pink line) or 10 Hz (blue line) frequency, for 6 h daily for up to 21 consecutive days of culture. ITO, indium tin oxide. Color images available online at www.liebertpub.com/tec
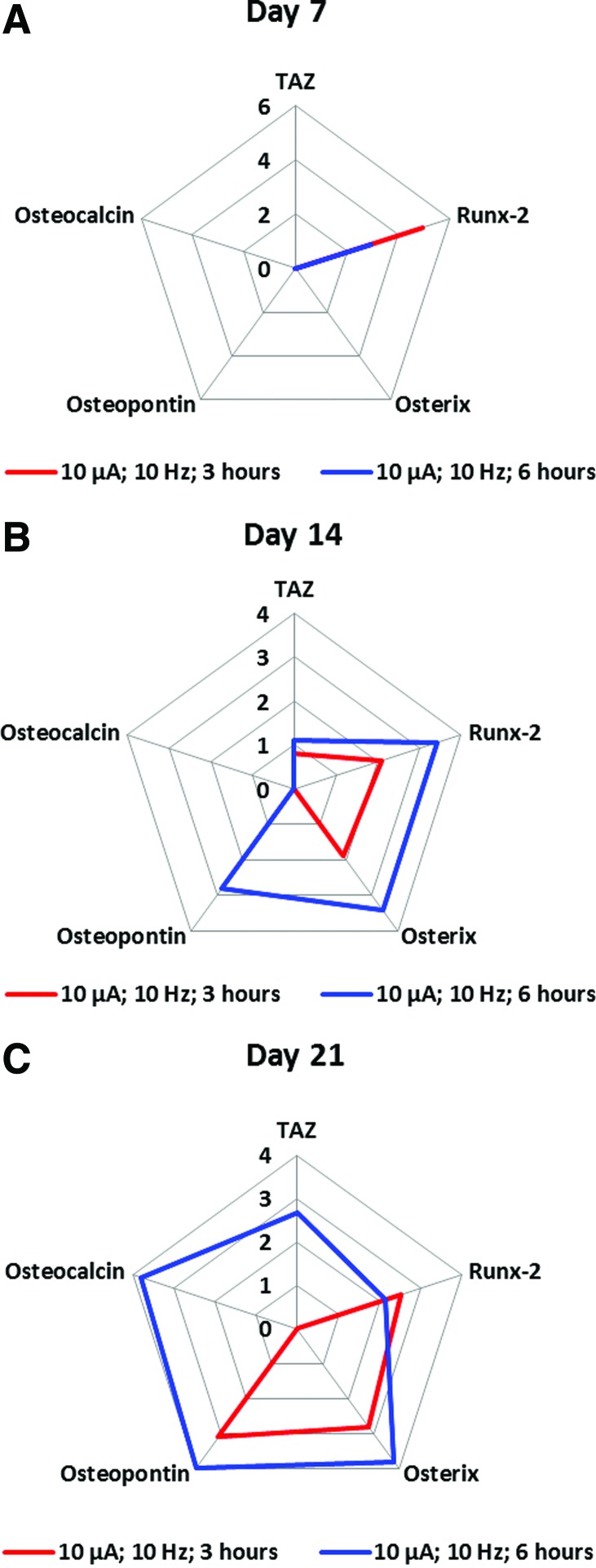
Exposure of hMSCs to a sinusoidal, 10 μA, 10 Hz alternating electric current for 3 h daily for up to 21 consecutive days of cell culture resulted in a similar trend of gene expression compared to results obtained under the optimal (i.e., 10 μA, 10 Hz for 6 h daily) electric current conditions (Fig. 3).
FIG. 3.
Time course of the effect of alternating electric current duration of exposure on the osteodifferentiation of adult hMSC. For the three frames of Figure 3: The axes indicate fold change compared to the “baseline,” that is, results obtained from adult hMSCs in numbers similar to those seeded on ITO-glass (on day 0), but not exposed alternating electric current. (A) Effect of the duration of exposure on the osteodifferentiation of adult hMSCs at 7 days of culture. Adult hMSCs were exposed to 10 μA, 10 Hz alternating electric current for either 3 h (red line) or 6 h (blue line) daily at 7 days of culture. (B) Effect of the duration of exposure on the osteodifferentiation of adult hMSCs at 14 days of culture. Adult hMSCs were exposed to 10 μA, 10 Hz alternating electric current for either 3 h (red line) or 6 h (blue line) daily at 14 days of culture. (C) Effect of the duration of exposure on the osteodifferentiation of adult hMSCs at 21 days of culture. Adult hMSCs were exposed to 10 μA, 10 Hz alternating electric current for either 3 h (red line) or 6 h (blue line) daily at 21 days of culture. Color images available online at www.liebertpub.com/tec
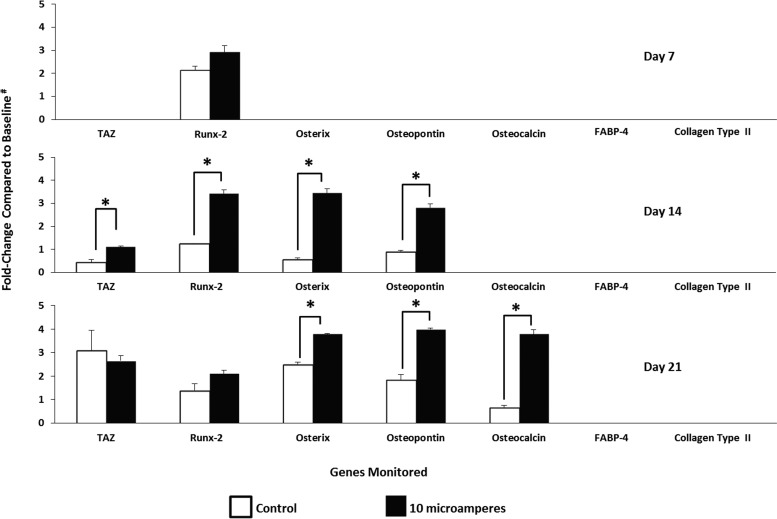
This study determined the optimal osteodifferentiation of hMSCs under the electric current conditions tested. Compared with results obtained from the respective controls, exposure of hMSCs to a sinusoidal, 10 μA, 10 Hz alternating electric current for 6 h daily (in the absence of supplemented exogenous growth factors) induced statistically significant (p < 0.05) upregulation of TAZ, Runx-2, Osterix, and Osteopontin after 14 consecutive days, and of Osterix, Osteopontin, and Osteocalcin after 21 consecutive days of exposure to the biophysical stimulus tested (Fig. 4).
FIG. 4.
Time course of gene expression by adult hMSCs exposed to sinusoidal, 10 μA, 10 Hz alternating electric current for 6 h daily for up to 21 consecutive days. “Baseline #” refers to the results obtained from adult hMSCs in numbers similar to those seeded on indium tin oxide-glass (on day 0), but not exposed to alternating electric current. n = 4 for data presented by the white; n = 3 for data presented by the black bars; *p < 0.05 compared to the respective controls at each time point tested.
Adult hMSCs differentiation at the single-cell level
Since, it was not possible, to determine the uniformity of hMSC-directed differentiation using the bulk cell approaches of the cell population level analysis, a series of single-cell gene expression experiments were performed to determine how hMSCs similarly responded to the optimal alternating electric current (i.e., sinusoidal, 10 μA, 10 Hz, 6 h daily for up to 21 consecutive days in the absence of exogenous growth factors) conditions during osteodifferentiation. To define the degree of gene expression heterogeneity among individual cells, mRNA levels of a panel of 45 genes in individual cells were determined by examining their expression (and that of their respective controls) levels (log2-transformed Ct values) after 7 and 21 days of exposure to the alternating electric current.
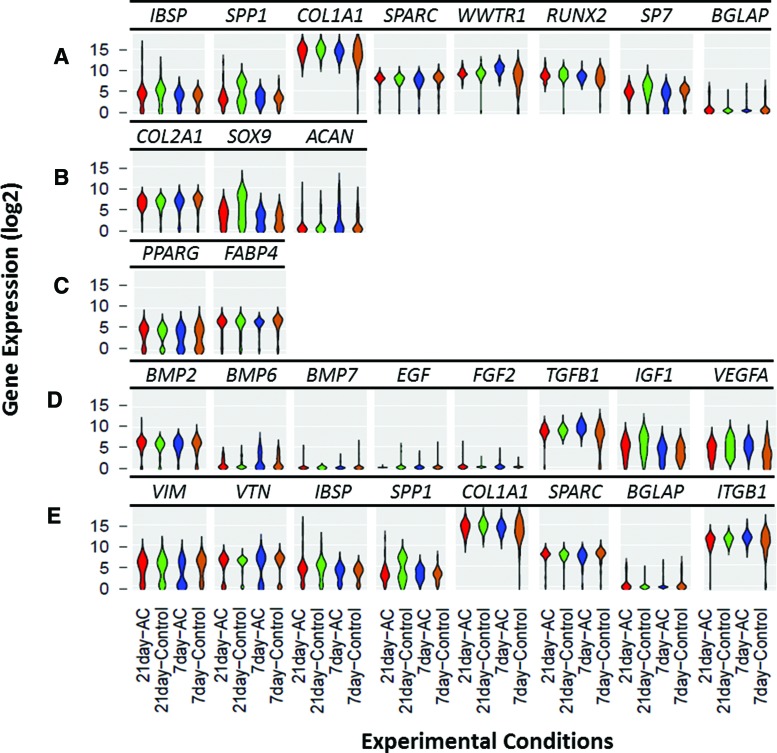
These data (Fig. 5) are presented as violin plots, which are two-dimensional histograms in which the vertical position denotes the relative expression level for a given sample set (i.e., cells exposed to alternating electric current and their respective controls); the width of the histogram reflects the relative proportion of cells in a given sample set, which have the same gene expression level. Differences in mRNA abundance patterns between the treatment groups were largely quantitative (differences in mRNA levels), rather than qualitative (on/off or detectable/undetectable).
FIG. 5.
Single-cell gene expression by mesenchymal stem cells exposed to sinusoidal, 10 μA, 10 Hz, alternating electric current (AC) for 6 h daily for 7 and 21 consecutive days. (A) osteogenic genes; (B) chondrogenic genes; (C) adipogenic genes; (D) growth factors; (E) extracellular matrix proteins and adhesion molecules. Data are presented as violin plots of the log2-transformed cycle threshold values of the cells analyzed. Curve height, mRNA levels; Width, relative cell number. Color images available online at www.liebertpub.com/tec
Four general patterns of mRNA abundance were observed: (i) genes in which mRNA levels were detectable and normally distributed in a relatively uniform fashion (e.g., COL1A1 in 7 day alternating electric current; Fig. 5A); (ii) genes that were largely undetectable (e.g., BGLAP; Fig. 5A); (iii) genes with a bimodal distribution in which two groups of cells had mRNA levels that were different and normally distributed (e.g., SPP1 at 21 days control; Fig. 5A); and (iv) heterogeneous patterns of mRNA abundance spanning multiple levels (e.g., SOX9 and IGF1 in 21 days control; Fig. 5B, D). Since different genes exhibited different expression profiles among individual cells, this result underscored the importance of examining gene expression as a readout of cellular differentiation both at the cell population and single-cell levels for understanding the differentiation process and pertinent characteristics of the differentiated cells.
At early (7 days) stages of osteodifferentiation, some osteogenic genes, including collagen type I (COL1A1; the predominant protein in the bone extracellular matrix), WW domain-containing transcription regulator 1 (TAZ), and runt-related transcription factor 2 (RUNX2) were more homogeneous among the cells exposed to alternating electric current than the respective controls (Fig. 5A), suggesting more uniformity in initial osteodifferentiation under the electric current stimulus. Levels of mRNA for tissue growth factor β 1 (TGFB1; which is a mediator of the osteoprogenitors to the preosteoblast stages of hMSCs osteodifferentiation11; Fig. 6), the matrix adhesion molecule and integrin β 1 (ITGB1), and to a lesser extent vascular endothelial growth factor A, were also more homogeneous at 7 days of cell exposure to alternating electric current (Fig. 5D, E). Not all osteogenic genes, however, followed this pattern: the results obtained for Osterix (SP7) and Osteopontin (SPP1) suggest that exposure to alternating electric current did not affect similarly all aspects of hMSC osteogenic induction.
FIG. 6.
Sequence of genes expressed at the single-cell level by differentiating adult hMSCs. Schematic representation of the genes expressed (and monitored) during adult hMSC differentiation into the adipogenic, chondrogenic, and osteogenic lineages. SOX9, SRY (sex determining region Y)-box 9; ACAN, aggrecan; PPARG, peroxisome proliferator-activated receptor gamma; FAB4, fatty acid-binding protein 4; RHOA, ras homolog family member A; PROM1, prominin 1; NANOG, Nanog homeobox; TAZ, WW domain-containing transcription regulator 1; MSX2, msh homeobox 2; WNTs, a group of extracellular signaling molecules; FGFs, fibroblast growth factors; TGFB, transforming growth factor-beta; BMPs, bone morphogenetic proteins; IGFs, insulin-like growth factors.
Of the five genes examined, which mark chondrogenic and adipogenic lineages, only sex determining region Y-box 9 (SOX9, a chondrogenic marker) was differentially expressed but showed a muted expression profile at 21 days (Fig. 5B) in cells exposed to alternating electric current in comparison to the respective controls; this outcome may indicate a reduced level of chondrogenic differentiation under the biophysical stimulus tested.
Discussion
This in vitro study investigated the effects of alternating electric current alone, that is, in the absence of exogenous osteogenic chemical promoters (such as dexamethasone and/or BMPs) on adult hMSC differentiation, and was the first to (i) identify the alternating electric current experimental conditions (specifically, 10 μA, 10 Hz, sinusoidal alternating electric current and cell exposure for 6 h daily for several [and up to 21] consecutive days) that optimize osteodifferentiation of mesenchymal stem cell populations; and (ii) investigate the effects of alternating electric current on mesenchymal stem cell differentiation at the single-cell level.
The results provided evidence that exposure of hMSC populations under all conditions of the alternating electric current regime tested, specifically, to sinusoidal alternating electric current in the ranges of 5–40 μA, 5–10 Hz, for 1–24 h daily for up to 21 consecutive days (in the absence of exogenous growth factors), and at all time points tested did not induce expression of genes associated with either the chondrogenic (Collagen Type II) or adipogenic (FABP-4) lineages (Figs. 2–4).
The results obtained under the established optimal conditions in which alternating electric current induced osteodifferentiation of hMSC populations are in agreement with the only other pertinent data published in the literature to date.13 Both the Creecy et al.13 and the present study used hMSCs that exhibited specific osteodifferentiation in the presence of select bone morphogenetic proteins (BMP-2 and BMP-6) alone. It should be noted, however, that the Creecy et al. study did not optimize the conditions that result in osteodifferentiation of these cells under electric current stimulation. In this respect, this study expanded the scope of the investigation to include a range of current levels (5–40 μA), frequency (5 and 10 Hz), and duration (1–24 h daily) of cell exposure to the alternating electric current regime tested in search of the optimal experimental conditions that lead to osteodifferentiation of hMSC populations.
Osteodifferentiation of hMSCs was determined by monitoring expression of targeted select osteogenic genes (namely, “early” TAZ, Runx-2, “middle” Osterix, and “late” Osteopontin, and Osteocalcin genes) along the various stages of the time course of this process (Figs. 1 and 6). Collagen type I, a hallmark of the osteoblast phenotype, however, was not used as an indicator of osteodifferentiation in this study because it is expressed by both undifferentiated mesenchymal stem cells and osteoblasts.13,18
Exposure to low frequency (5 Hz), low current level (5 μA), and short duration (1 h) daily to the alternating electric current for up to 7 days, induced expression of early (specifically, TAZ and Runx-2) and middle (specifically, Osterix) osteodifferentiation genes by hMSCs (Fig. 2). Exposure of hMSCs to the alternating electric current tested at 10 μA, 10 Hz for 3 h daily for 21 days resulted in a similar trend of gene expression compared to cells exposed to the optimal electric current conditions (Fig. 3).
Specific gene expression was dependent on the time course of cell exposure to the biophysical stimulus; for example, compared to results obtained from the respective controls (i.e., cells cultured in parallel under similar conditions but not exposed to alternating electric current), exposure of hMSCs to the optimal electric current regime tested induced statistically significant (p < 0.05) upregulation of TAZ, Runx-2, Osterix, and Osteopontin after 14 consecutive days, and of Osterix, Osteopontin, and Osteocalcin after 21 consecutive days (Fig. 4B); these genes represent all stages (i.e., early, middle, and late) of osteodifferentiation under the optimal regime tested.
Furthermore, this study was the first to utilize the Fluidigm analysis method to investigate, at the single-cell level, aspects of the differentiation of hMSCs exposed to alternating electric current, and provided information regarding (i) the homogeneity/heterogeneity of hMSCs and (ii) the genes expressed by these cells under the conditions of interest to this study.
To date, the Fluidigm single-cell analysis method has been used with various stem cells types, such as, mouse hematopoietic stem cells,19,20 mouse embryonic stem,21 human cancer stem cells,16 and human22 and mouse23 mesenchymal stem cells mostly in the presence of bioactive chemicals but not under a biophysical stimulus. Exposure of hMSCs to the optimal alternating electric current conditions examined in the present study provided evidence of homogeneous (defined as the uniform expression of a specific gene between 5 and 15; Fig. 5) expression of transforming growth factor-beta 1(TBFB1; a growth factor associated with stem cell proliferation), integrin β1 (ITGB1; associated with the early differentiation phases of stem cells) (Fig. 5D, E), of WW domain-containing transcription regulator 1 (TAZ), and of runt-related transcription factor 2 (RUNX2; an early osteodifferentiation gene) (Fig. 5A). In other words, the biophysical stimulus tested promoted homogeneous expression of genes pertinent to the proliferative and early stages of the osteodifferentiation of hMSCs.
In contrast, genes (specifically, Osterix [SP7], Osteopontin [SPP1], and Osteocalcin [BGLAP]) indicative of the “middle” and “late” stages of osteogenic differentiation were expressed heterogeneously, but at similar levels under both the control and alternating electric current conditions at days 7 and 21 (Fig. 5). Heterogeneity (defined as the varying expression of a specific gene between 0 and 20; Fig. 5) was also observed for the other genes monitored, specifically, select adipogenic (specifically, peroxisome proliferator-activated receptor gamma [PPARG] and fatty acid-binding proetin-4 [FABP4]; Fig. 5C) and chondrogenic (e.g., [SRY] sex determining region Y-box 9 [SOX9], and Aggrecan [ACAN]) related genes (Fig. 5B). The heterogeneity observed at the single-cell level in hMSCs maintained under control conditions in this study is in agreement with literature reports regarding hMSC heterogeneity.24,25 Such an outcome may reflect the presence of different precursors of distinct mesenchymal lineages, for example, cells of either the osteogenic, adipogenic, or chondrogenic lineages/phenotypes.26
The differences in the results regarding the lineage-specific differentiation of hMSCs obtained at the population and at the single-cell levels are intriguing. Since this study is the first to use single-cell analysis to investigate bone marrow-derived mesenchymal stem cell responses to a biophysical stimulus, definitive conclusions are not yet possible; for example, the differences in gene expression at the cell population and single-cell levels cannot be explained based on the current limited knowledge. The cell population analysis revealed that, compared to the respective controls, genes (such as SPP1 and BGLAP) associated with the latter stages of osteodifferentiation of hMSCs were upregulated at statistically (p < 0.05) significant levels after 21 consecutive days of cell exposure to the optimal electric current regime tested (Fig. 4).
The single-cell analysis, however, revealed homogeneous expression of the early (TAZ and RUNX2) osteodifferentiation genes, and heterogeneous expression of the late (SPP1 and BGLAP) osteodifferentiation genes when these cells were exposed to the optimal electric current regime tested at 7 and 21 days (Fig. 5). A possible explanation for these results is that, the single-cell level analysis revealed differentiation of the hMSCs under the electric current milieu was delayed.
Another explanation for the observed difference between the results of the two approaches used in this study may be due to the different sensitivities of the two analyses methods used. Since typical cellular and molecular biology approaches for measuring gene expression yield averaged values for cell populations, it is possible that, at the population level, expression of one gene by mesenchymal stem cells is dominant and, under these conditions, other genes may not be expressed. Furthermore, paracrine cell communications needed to “drive” exclusive osteodifferentiation of hMSCs is active in the population but not in the single cell milieu.
As far as the differences in adipogenic and chondrogenic gene expression obtained at the single-cell level versus the exclusive osteogenic gene expression at the cell population level are concerned, one possible explanation is that these stem cells express the same genes at the early stages (specifically at the osteoprogenitor stage) with adipocytes (specifically, preadipocytes) and chondrocytes (specifically, chondroprogenitors). Since the implications of mesenchymal stem cell differentiation at the population and single-cell levels for biomedical applications (e.g., tissue engineering and regenerative medicine) are possible, but unknown at this time, further research is needed to establish pertinent correlations in this field.
In summary, one of the key contributions of this study was establishing the optimal experimental conditions needed to induce exclusive osteodifferentiation of hMSC populations exposed to alternating electric current alone. In addition, the present study provided interesting and intriguing results regarding gene expression of bone marrow-derived adult hMSCs exposed to alternating electric current at the population and, for the first time, at the single-cell levels. Knowledge of the pertinent similarities and differences is needed for insightful implementation of stem cells in successful tissue engineering and regenerative medicine applications.
Acknowledgments
The authors wish to thank Mr. Senthlinath Laksmanachetty, Dr. John McCarrey, Dr. Jaime E. Ramírez-Vick, and Ms. Vanessa Wechsler for assistance with various aspects of this project.
Financial support for this study was provided in part by the Peter T. Flawn Professorship (R.B.), NIH Grant HD062687 (BPH), NSF Grant 1337513 (B.P.H. and R.B.), The University of Texas at San Antonio (UTSA) College of Sciences (B.P.H.), and to UTSA RISE (NIH Grant R25GM060655) and UTSA MARC U-STAR (NIH Grant T34GM007717), which provided support to M.E.W.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friedenberg Z.B., Andrews E.T., Smolenski B.I., Pearl B.W., and Brighton C.T. Bone reactions to varying amounts of direct current. Surg Gynecol Obstet 131, 894, 1970 [PubMed] [Google Scholar]
- 2.Hagiwara T., and Bell W.H. Effect of electrical stimulation on mandibular distraction osteogenesis. J Craniomaxillofac Surg 28, 12, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bassett C.A.L., Pawluk R.J., and Pilla A.A. Acceleration of fracture repair by electromagnetic fields: a surgically noninvasive method. Ann N Y Acad Sci 238, 242, 1974 [DOI] [PubMed] [Google Scholar]
- 4.McLeod K.T., and Rubin C.T. The effect of low-frequency electrical fields on osteogenesis. J Bone Joint Surg Am 74, 920, 1992 [PubMed] [Google Scholar]
- 5.Ulmann K. The effects of alternating current stimulation on select osteoblast functions [M.S. thesis]. Department of Biomedical Engineering, Renesselaer Polytechnic Institute, Troy, NY, 2000 [Google Scholar]
- 6.Supronowicz P.R., Ajayan P.M., Ullmann K.R., Arulanandam B.P., Metzger D.W., and Bizios R. Novel currentconducting composite substrates for exposing osteoblasts to alternating current stimulation. J Biomed Mater Res 59, 499, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Baksh D., Song L., and Tuan R.S. Adult mesenchymal stem cells: characterization, differentiation, and applications in cell and gene therapy. J Cell Mol Med 8, 301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai M., Li W., Tuan R.S., and Chang W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res 27, 1169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim I.S., Song J.K., Song Y.M., Cho T.H., Lee T.H., Lim S.S., Kim S.J., and Hwang S.J. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng Part A 15, 2411, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hronik-Tupaj M., Rice W.L., Cronin-Golomb M., Kaplan D.L., and Georgakoudi I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed Eng Online 10, 9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes F.J., Turner W., Belibasakis G., and Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontology 2000 41, 48, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Jäger M., Fischer J., Dohrn W., Li X., Ayers D.C., Czibere A., Prall W.C., Lensing-Höhn S., and Krauspe R. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. J Orthop Res 26, 1440, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Creecy C.M., O'Neill C.F., Arulanandam B.P., Sylvia V.L., Navara C.S., and Bizios R. Mesenchymal stem cell osteodifferentiation in response to alternating electric current. Tissue Eng Part A 19, 467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hermann B.P., Mutoji K.N., Velte E.K., Ko D., Oatley J.M., Geyer C.B., and McCarrey J.R. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol Reprod 95, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalerba P., Kalisky T., Sahoo D., Rajendran P.S., Rothenberg M.E., Leyrat A.A., Sim S., Okamoto J., Johnston D.M., Qian D., Zabala M., Bueno J., Neff N.F., Wang J., Shelton A.A., Visser B., Hisamori S., Shimono Y., van de Wetering M., Clevers H., Clarke M.F., and Quake S.R. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol 29, 1120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benavides-Garcia R., Joachim R., Pina N.A., Mutoji K.N., Reilly M.A., and Hermann B.P. Granulocyte colony-stimulating factor prevents loss of spermatogenesis after sterilizing busulfan chemotherapy. Fertil Steril 103, 270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva W.A., Jr., Covas D.T., Panepucci R.A., Proto-Siqueira R., Siufi J.L.C., Zanette D.L., Santos A.R.D., and Zago M.A. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 21, 661, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Swiers G., Baumann C., O'Rourke J., Giannoulatou E., Taylor S., Joshi A., Moignard V., Pina C., Bee T., Kokkaliaris K.D., Yoshimoto M., Yoder M.C., Frampton J., Schroeder T., Enver T., Göttgens B., and de Bruijn M.F. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat Commun 4, 2924, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moignard V., Macaulay I.C., Swiers G., Buettner F., Schütte J., Calero-Nieto F.J., Kinston S., Joshi A., Hannah R., Theis F.J., Jacobsen S.E., de Bruijn M.F., and Göttgens B. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nat Cell Biol 15, 363, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong D.E., Hahn-Windgassen A., Foygel K., Jun S., Behr B., and Yao M.W.M. Morpholino-mediated gene knockdown in the early mouse embryo. Nat Protoc 2009. DOI: 10.1038/nprot.2009.110 [DOI] [Google Scholar]
- 22.Spitzer T.L.B., Rojas A., Zelenko Z., Aghajanova L., Erikson D.W., Barragan F., Meyer M., Tamaresis J.S., Hamilton A.E., Irwin J.C., and Giudice L.C. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 86, 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duscher D., Rennert R.C., Januszyk M., Anghel E., Maan Z.N., Whittam A.J., Perez M.G., Kosaraju R., Hu M.S., Walmsley G.G., Atashroo D., Khong S., Butte A.J., and Gurtner G.C. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 4, 7144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem 113, 2806, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Zhang C., Weiner L.P., Zhang Y., and Zhong J.F. Molecular characterization of heterogeneous mesenchymal stem cells with single-cell transcriptomes. Biotechnol Adv 31, 312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieker M., Pautke C., Haasters F., Schieker J., Docheva D., Böcker W., and Mutschler W. Human mesenchymal stem cells at the single-cell level: simultaneous seven-colour immunofluorescence. J Anat 210, 592, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]