Abstract
Although altered resting-state functional connectivity (FC) is a characteristic of many chronic pain conditions, it has not yet been evaluated in patients with chronic fatigue. Our objective was to investigate the association between fatigue and altered resting-state FC in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Thirty-six female subjects, 19 ME/CFS and 17 healthy controls, completed a fatigue inventory before undergoing functional magnetic resonance imaging. Two methods, (1) data driven and (2) model based, were used to estimate and compare the intraregional FC between both groups during the resting state (RS). The first approach using independent component analysis was applied to investigate five RS networks: the default mode network, salience network (SN), left frontoparietal networks (LFPN) and right frontoparietal networks, and the sensory motor network (SMN). The second approach used a priori selected seed regions demonstrating abnormal regional cerebral blood flow (rCBF) in ME/CFS patients at rest. In ME/CFS patients, Method-1 identified decreased intrinsic connectivity among regions within the LFPN. Furthermore, the FC of the left anterior midcingulate with the SMN and the connectivity of the left posterior cingulate cortex with the SN were significantly decreased. For Method-2, five distinct clusters within the right parahippocampus and occipital lobes, demonstrating significant rCBF reductions in ME/CFS patients, were used as seeds. The parahippocampal seed and three occipital lobe seeds showed altered FC with other brain regions. The degree of abnormal connectivity correlated with the level of self-reported fatigue. Our results confirm altered RS FC in patients with ME/CFS, which was significantly correlated with the severity of their chronic fatigue.
Key words: : chronic fatigue, connectivity, fMRI, ICA
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) affects over 1.3 million individuals in the United States and reduces the quality of life to levels comparable to people with congestive heart failure (Komaroff et al., 1996). Treatments for ME/CFS remain largely ineffective at reducing illness-related symptoms. To improve the care of these individuals, a greater understanding of the pathophysiology of ME/CFS is needed. Recent work in this area has examined the role of abnormal immunity as a pathway leading to ME/CFS (Hornig et al., 2015), while less attention has focused on the central mechanisms of persistent fatigue.
Fatigue is a common symptom in the general population, but usually resolves with sleep (Akerstedt et al., 2014). ME/CFS, however, is a condition characterized by disabling fatigue and cognitive abnormalities that seem to be unresponsive to rest. Similar but often less severe fatigue has been reported in other chronic conditions, such as depression, arthritis, and chronic pain, where symptoms persist for long periods of time (Katz et al., 2015; Kroenke et al., 2011; Maes et al., 2012).
With advances in neuroimaging, the brain's role in persistent conditions, like ME/CFS, has become an important focus of research. Previous investigations of brain abnormalities in ME/CFS were mostly inconclusive (Lange et al., 1998). There is conflicting evidence for altered brain structure in this illness, with some studies reporting either globally decreased gray matter or local abnormalities in several brain regions (Puri et al., 2012 and Okada et al., 2004), while others report no such findings (Perrin et al., 2010).
Global or regional reductions in cerebral blood flow (CBF) at rest have been reported in ME/CFS (Biswal et al., 2011; Cope and David, 1996; Tirelli et al., 1998; Yoshiuchi et al., 2006), but some investigators found no group differences in CBF compared to normal controls (Perrin et al., 2010). However, functional MRI (fMRI) has shown altered cortical activation during cognitive challenges in ME/CFS (De Lange et al., 2004; Tanaka et al., 2006).
Resting-state functional connectivity (FC) is often disrupted in chronic conditions, such as fibromyalgia, back pain, and temporomandibular joint disorder, and may contribute to prolonged symptoms (Baliki et al., 2011; Ichesco et al., 2014; Schmidt-Wilcke et al., 2014; Tagliazucchi et al., 2010). However, FC is yet to be investigated in patients with ME/CFS. Examining FC between brain areas in the absence of a task (i.e., resting state [RS]) may offer some insights as to why such patients have considerable difficulty in performing challenging tasks, such as the Psychomotor Vigilance Test (Lim et al., 2010).
Two general approaches can be used to evaluate the FC among brain regions. One requires prior knowledge of seed regions (i.e., model based), while the other is driven by the acquired data (i.e., data driven). The results of these approaches are complementary and, when combined, may provide powerful insights about the neural underpinnings of clinical disorders, like ME/CFS.
In this study, we used both approaches to assess FC in patients with ME/CFS. Our data-driven approach was similar to the one used in patients diagnosed with fibromyalgia (Napadow et al., 2010). We investigated FC among brain regions in ME/CFS patients and normal nonfatigued healthy controls (HC) in five resting-state networks (RSN): the default mode network (DMN), salience network (SN), sensory motor network (SMN), and the left and right frontoparietal networks (LFPN, RFPN). We hypothesized that self-reported fatigue would be associated with altered FC within these networks. Moreover, compared to HC, we expected the RS among ME/CFS patients to connect to additional brain regions such as the insula, parietal, and frontal lobes.
For the second set of analyses, we used the model-based approach to test FC among brain regions, using seed regions that had demonstrated decreased resting regional cerebral blood flow (rCBF) in ME/CFS patients through arterial spin labeling (ASL). Those regions were located in the parahippocampal and occipital lobes. Given the reductions in rCBF within these brain clusters, we expected the FC with other brain regions to be disrupted and associated with reports of fatigue in patients with ME/CFS.
Methods and Procedures
Participants
Individuals with chronic fatigue who fulfilled the 1994 Case Definition (Fukuda et al., 1994) as well as the 2003 Canadian Criteria for ME/CFS were recruited from outpatient clinics at the University of Florida. Only female subjects were enrolled because of the preponderance of women in ME/CFS. Following the recruitment of clinical subjects, nonfatigued healthy individuals were invited to participate through local advertising to serve as age- and sex-matched controls. Subjects with a history of heart disease, COPD, malignancy, or other systemic disorders were excluded, including those with psychiatric illnesses that would be exclusionary for a diagnosis of ME/CFS (Reeves et al., 2003). The work described in this article was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants provided informed consent before participation. The University of Florida Institutional Review Board approved all study procedures before the start of study enrollment.
Multidimensional fatigue inventory
The multidimensional fatigue inventory (MFI) is a self-reported instrument that contains 20 statements covering different aspects of fatigue (Smets et al., 1995). The 20 items are organized into 5 scales: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. Higher scores on the questionnaire indicate more fatigue.
Neuroimaging
Acquisition
All neuroimaging data were acquired with a research-dedicated Philips Achieva 3T whole body MRI scanner using a 32-channel head coil. Three types of data were obtained during a single scanning session: (A) A high-resolution 3D anatomic structural scan; (B) a resting-state ASL scan; and (C) a blood oxygen level-dependent (BOLD) resting-state scan. The parameters for the anatomical T1-weighted MPRAGE structural scan were as follows: 176 slices in sagittal orientation, TR = 7 msec, TE = 3.2 msec, acquisition matrix = 240 mm × 240 mm, and voxel size =1 mm3. The ASL sequence used a pseudocontinuous ASL design with the following parameters: 20 slices in transverse orientation, slice gap = 1 mm, TR = 4000 msec, TE = 11 msec, label duration = 1500 msec, postlabel delay = 1800 msec, acquisition matrix = 72 mm × 72 mm, voxel size = 3.19 mm ×3.19 mm × 6 mm, and number of dynamic scans (pairs) = 45. Finally, the resting-state BOLD scans were acquired using an echo planar acquisition protocol with the following parameters: 42 slices in transverse orientation, slice gap =0 mm, TR = 2250 msec, TE = 30 msec, acquisition matrix =80 mm × 80 mm, and 3 mm isotropic voxels. The RS ASL scans were obtained to identify brain areas demonstrating rCBF differences between ME/CFS subjects and NC to be subsequently used as seeds in our model-based approach.
Resting-state CBF
Statistical Parametric Mapping 8 (SPM8) (Wellcome Department of Imaging Neuroscience, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm) software was used for ASL data preprocessing. Label and control ASL images were independently processed through SPM8's realign motion correction algorithm, and a combined mean realigned image was created. The raw ASL images were then resliced with the motion correction parameters generated by the previously described step. The realigned resliced images were then smoothed with a 6 mm full-width half-maximum (FWHM) Gaussian kernel. Using the MATLAB toolbox ASLtbx (Wang et al., 2008), subtractions between labeled and controlled images were performed resulting in 45 perfusion images (one for each dynamic scan). CBF values were then calculated on a per voxel basis for each perfusion image and then averaged (over the 45 images) to create one mean CBF image. For a detailed description of the CBF calculation, see Wang et al., 2008. The mean CBF image was then normalized to the Montreal Neurologic Institute (MNI) standardized space using the parameters generated by SPM's segmentation normalization procedure and resliced to 2 mm3 voxels. Finally, the normalized mean CBF image was masked with SPM's default brain mask with a 50% threshold to exclude outlier voxels. This process was repeated for each participant.
SPM12 was used to perform independent sample t-tests to compare CBF between the ME/CFS and HC groups. Contrasts were created to identify cortical regions where CBF was increased or decreased in clinical subjects compared to controls. These contrasts produce maps with a t-statistic for every voxel in the brain. The resulting t maps were thresholded at a t-statistic of p < 0.001 and a cluster size of 120 mm3 to control for the large number of multiple comparisons associated with voxelwise analyses.
BOLD data preprocessing
Functional imaging data were preprocessed in SPM12 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). Images were (1) slice-time corrected, (2) realigned and resliced into 3 mm isotropic voxels, (4) coregistered to the high resolution 3D anatomic volume, and (5) warped into MNI standard space using the deformations used to realign the 3D anatomical data into MNI space.
Data-based approach to RS network analysis
For the data driven analyses, an additional preprocessing step included smoothing the fMRI data with a 6 mm FWHM Gaussian kernel. The next step in the data-driven approach was done with the Group independent component analysis (ICA) of fMRI Toolbox (GIFT v3.0a; http://icatb.sourceforge.net/). ICA is a data-driven statistical analysis technique that yields independent components (ICs), which isolate sources of variance within the data. Each estimated IC represents a group of brain regions with a unique pattern of synchronized neural activity (i.e., time course) and can be conceptualized as an RSN (Calhoun et al., 2004). The GIFT IC estimation procedure occurs in three stages: (1) reduction of data dimensionality, (2) estimation of group signal sources and reduction of mutual information among those sources, and (3) back reconstruction of group-level ICs to a single-subject level. We estimated 25 IC, from which we used a spatial matching procedure to select the 5 IC that best fit the previously published RSN templates for the DMN, SMN, SN, and the RFPN, LFPN (Smith et al., 2009). SPM12 was used to perform independent t-tests with each IC to identify significant group differences in spatial recruitment for each network. These contrasts were used to investigate group differences in the intrinsic connectivity of an RSN and brain regions that showed disrupted connectivity with the RSN. RSNs are associated with particular brain regions. For example, the posterior cingulate cortex (PCC) is a part of the DMN. Using the DMN as an example, should the DMN contrast show a cluster of voxels within the PCC as significantly different between groups, the interpretation would be disrupted intrinsic connectivity within the DMN. Alternatively, using the same DMN contrast, should a cluster of voxels within the insular cortex show significant group differences, the interpretation would be disrupted FC between the DMN and the insular cortex. To keep the familywise error rate at 5%, a permutation-based extent and cluster thresholding method was used, see Correction for Multiple Comparisons section.
Model-based approach to RS network analysis
For the model-based approach, additional preprocessing steps included spike correction to reduce the impact of artifacts using the postprocessing artifact detection tool (ART) toolbox for fMRI data (http://www.nitrc.org/projects/artifact_detect). Time points were identified as artifacts and later removed during the first-level general linear model, where the mean global signal changed by above three standard deviations (SD), translation movement exceeded 0.5 mm, or rotational movement exceeded 0.01°. The final preprocessing steps were then carried out using the FC toolbox Conn version 14 (http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012) that implements the component-based noise correction method (CompCor) strategy for physiological and other noise source reduction, which included (1) temporal (band-pass) filtering set between 0.01 and 0.1 Hz and (2) removal of several sources of nonspecific variance by regression of nuisance variables. Those nuisance variables included (1) the signal averaged over the lateral ventricles, (2) the signal averaged over the deep cerebral white matter, (3) the six parameters obtained by motion correction, and (4) the artifact data points identified with the ART toolbox.
Selection of a priori seed regions is a critical step in the model-based approach. For this study, we used brain regions where there was a significant reduction in CBF identified by ASL scanning, as our seeds, see Table 2. Binary masks of clusters (identified by ASL) were created and used as our seed region of interest (ROI) and overlaid with a gray matter mask to include only gray matter voxels. The time course was then estimated by the spatial average of the BOLD signal over all voxels at each time point within each ROI. Zero-order Cross Correlation coefficients (CC) (i.e., bivariate correlation) for each ROI's time course between the ROI time course and the rest of the brain were estimated using the Conn_v14 toolbox.
Table 2.
Brain Regions Used as Seeds for Functional Connectivity Analysis (Model-Based Approach)
| Seed name | X | y | z | K |
|---|---|---|---|---|
| RH cuneus (seed 1) | 10 | −84 | 22 | 64 |
| RH parahippocampus (seed 2) | 28 | −52 | 0 | 29 |
| LH lingual gyrus (seed 3) | −20 | −78 | 4 | 52 |
| RH inferior occipital gyrus (seed 4) | 32 | −94 | 0 | 42 |
| RH cuneus (seed 5) | 24 | −64 | 18 | 37 |
Threshold t-statistic p < 0.001 and a cluster size (k) of 120 mm3.
x, y, and z coordinates are in Montreal Neurologic Institute (MNI) space.
RH, right hemisphere; LH, left hemisphere.
Group-level differences between seed and voxel-based FC
Contrasts for each of the previously identified seed regions were created to distinguish brain regions of ME/CFS subjects showing differential connectivity from those of controls. Each contrast produced a map with a t-statistic for every voxel in the brain. These contrasts were used to identify brains regions, where the strength of FC (CC) differed between groups. The strength of FC estimates the extent to which fluctuations in BOLD signal (interpreted as brain activity) between two regions are coupled. Typically, values range from −1 to 1. Values closer to 1 (or −1) suggest greater coupling, while values closer to 0 suggest less coupling. Negative values suggest anticoupling. To keep the familywise error rate at 5%, a permutation-based extent and cluster thresholding method was used, see Correction for Multiple Comparisons section.
Correction for multiple comparisons
The statistical result of each method (i.e., data driven and seed based) was corrected for multiple comparisons using AlphaSim (see the AlphaSim command description at http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim) available within AFNI (http://afni.nimh.nih.gov/afni/). AlphaSim uses an estimation of the spatial correlation across voxels to generate clustering thresholds. We set the voxel puncorected <0.001, which is the recommended default lower limit (Woo et al., 2014). The resulting pair of uncorrected statistical threshold and cluster size threshold together then corrects a whole-brain map to a familywise error-corrected threshold of α <0.05. For the data-driven approach, a combination threshold of voxel puncorrected <0.001 and cluster size of 20 voxels was considered significant, which corresponds with a corrected p < 0.05. For the seed-based approach, a combination threshold of voxel puncorrected <0.001 and cluster size of 15 voxels was considered significant, which corresponds with a corrected p < 0.05. Two separate corrections were used because the estimated spatial correlation across voxels was different for the data-driven and seed-based approach. There was a higher spatial correlation within the data-driven method.
Correlations between clinical fatigue and FC
The Pearson's CC was used to characterize the relationship between fatigue and the strength of connectivity between seed regions and clusters of voxels that showed significant group differences. To extrapolate each subjects' mean strength of connectivity within a significant cluster, we used the response exploration software (REX, alpha 0.5) (available at http://www.nitrc.org/projects/rex/) (Duff et al., 2007). A similar procedure was used for the data-based method. Pearson's CC was utilized to estimate the relationship between the strength of FC within each RSN and ratings of fatigue within domains of the MFI. Analyses were performed using SPSS Statistics version 22.0 (IBM, Inc. Chicago IL). Significance levels were set at the alpha level 0.05. The appropriateness of conducting these assessments was done by visually inspecting the distribution of all variables. In addition, skewness and kurtosis values for each variable were determined to confirm that the assumption of normality was not violated. For this purpose, estimated values of skewness and kurtosis were divided by the respective standard error. Values greater than 1.96 were considered to violate normality.
Results
Subject characteristics
Thirty-six females (19 ME/CFS, 17 HC) with a mean age of 48.75 years (SD = 11.75 years) volunteered for this study. Table 1 provides descriptive data on the sample, including the five domains of fatigue identified by the MFI: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. Significant group differences were found for all fatigue measures (Table 1).
Table 1.
Descriptive Statistics of Participants
| ME/CFS (n = 19) | HC (n = 17) | Group difference | p-Value | |
|---|---|---|---|---|
| Age | 52.33 (10.63) | 48.75 (11.75) | 3.73 | 0.34 |
| General fatiguea | 16.82 (2.10) | 7.26 (2.41) | 9.56 | >0.01 |
| Physical fatiguea | 14.36 (3.05) | 6.35 (2.39) | 8.01 | >0.01 |
| Reduced activitya | 12.76 (3.87) | 5.65 (1.79) | 7.11 | >0.01 |
| Reduced motivationa | 11.77 (3.59) | 5.79 (2.15) | 5.98 | >0.01 |
| Mental fatiguea | 13.70 (3.56) | 7.38 (2.48) | 6.32 | >0.01 |
All participants were female; variables are presented as mean and standard deviation.
Item of the multidimensional fatigue inventory (MFI).
HC, healthy controls; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
Resting-state CBF
Using ASL, subjects with ME/CFS demonstrated reduced rCBF at rest when compared to control subjects in several clusters located within the occipital (right cuneus, inferior occipital gyrus, and left lingual gyrus) and temporal lobes (right parahippocampal gyrus) (Table 2). No cortical regions had increased rCBF in subjects with ME/CFS compared to control subjects. There was, however, no significant difference in global CBF between groups.
Data-based FC
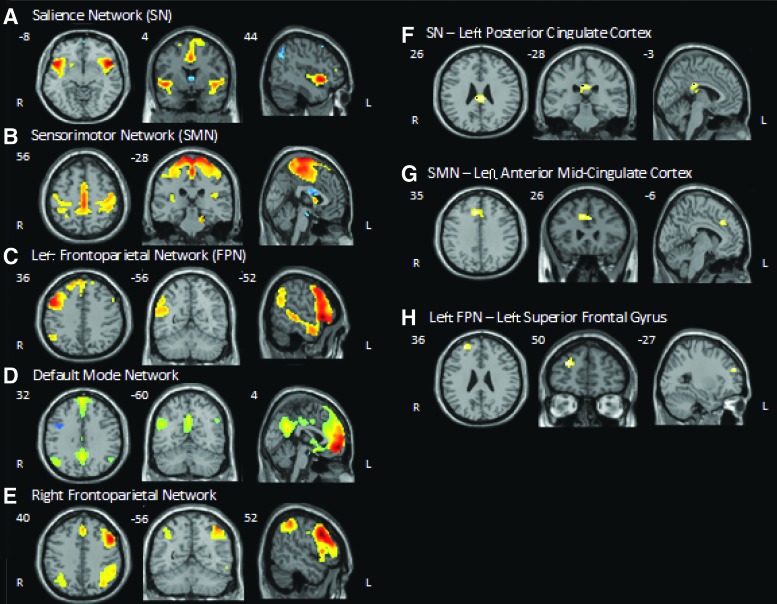
A group-level ICA was used for the evaluation of each of the five RSNs (Fig. 1). Compared to HC, ME/CFS was associated with disrupted intrinsic connectivity within the left frontoparietal network. More specifically, a region in the left superior frontal gyrus, which is a major node of this network, showed reduced coupling of activity with the rest of the network. ME/CFS was also associated with decreased connectivity between the SN and the left PCC; similarly, the SMN demonstrated decreased connectivity with the left anterior midcingulate cortex (aMCC) compared to HC (Fig. 1). No differences in FC were found within the DMN or RFPN (Table 3).
FIG. 1.
Spatial maps of resting-state networks and regional group differences in functional connectivity threshold t > 4.0; k > 15. R = Right, L = Left, x, y, and z coordinates are in Montreal Neurologic Institute space. (A–E) Representative group-level independent component for each of the five resting-state networks. (F–G) Regions of the cingulate cortex where myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients showed less connectivity with the salience and sensory motor networks. (H) Region of the left frontal gyrus where ME/CFS patients showed decreased intrinsic connectivity in the left frontoparietal network. Color images available online at www.liebertpub.com/brain
Table 3.
Brain Regions Showing Reduced Functional Connectivity (Data-Based Approach) in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subjects
| RSN–brain region | x | y | z | k | t |
|---|---|---|---|---|---|
| SN—left posterior cingulate cortex | −3 | −28 | 26 | 32 | 4.14 |
| SMN—left anterior midcingulate cortex | −6 | 26 | 35 | 42 | 4.26 |
| Left FPN—left superior frontal gyrus | −27 | 50 | 26 | 25 | 4.67 |
Threshold t-statistic of 4.0 and a cluster size (k) of 60 mm3.
x, y, and z coordinates are in MNI space.
FPN, frontoparietal network; RSN, resting-state network; SMN, sensory motor network; SN, salience network.
Data-based FC as predictor of fatigue
As all data met normalcy criteria (see Correlations Between Clinical Fatigue and FC section), Pearson's product moment correlations were used. The strength of connectivity between each of the disrupted regions was significantly correlated with self-reported fatigue ratings of the MFI, including general fatigue, physical fatigue, motivation, and mental fatigue (Table 4). The direction of all three relationships was negative, suggesting stronger FC is associated with less severe symptoms of fatigue.
Table 4.
Correlations Between Degree of Functional Connectivity and Self-Reported Fatigue in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subjects
| Functional connectivity | |||
|---|---|---|---|
| Intrinsic L FPN | SN–L PCC | SMN—aMCC | |
| General fatiguea | −0.60b | −0.65b | −0.61b |
| Physical fatiguea | −0.57b | −0.64b | −0.58b |
| Reduced activitya | −0.51b | −0.58b | −0.50b |
| Reduced motivationa | −0.46b | −0.54b | −0.42c |
| Mental fatiguea | −0.50b | −0.68b | −0.61b |
Item of the MFI.
p < 0.01.
p < 0.05.
aMCC, anterior midcingulate cortex; L, left; FPN, frontoparietal network; PCC, posterior cingulate cortex.
Model-based FC
Brain areas demonstrating rCBF abnormalities in ME/CFS subjects during RS ASL were used as a priori seeds for this analysis. Of the five seed regions, all but the right inferior occipital seed (seed 4) showed altered FC with other regions of the brain (Fig. 1). ME/CFS was associated with decreased FC between the right cuneus (seed 1) and a region in the right superior temporal gyrus; the left lingual gyrus (seed3) and regions located in the left occipital lobe and right precuneus; and the second right cuneus seed (seed 5) and regions within the right superior midfrontal gyrus and left midfrontal gyrus. Increased FC was found between the right parahippocampus (seed 2) and the right inferior parietal lobe (Table 5).
Table 5.
Brain Clusters Demonstrating Altered Functional Connectivity with Seed Regions in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subjects
| Seed—cluster | x | y | z | k | t | Direction of difference |
|---|---|---|---|---|---|---|
| RH cuneus (seed 1)—RH superior temporal gyrus (cluster 1) | 58 | −20 | −4 | 15 | 4.23 | ME/CFS<HC |
| RH parahippocampus (seed 2)—RH Inferior parietal Lobe BA 40 (cluster 1) | 42 | −44 | 40 | 15 | 4.58 | ME/CFS>HC |
| LH lingual gyrus (seed 3)—LH occipital lobe (cluster 1) | −36 | −68 | −2 | 20 | 4.12 | ME/CFS<HC |
| LH lingual gyrus (seed 3)—RH precuneus BA 7 (cluster 2) | 12 | −70 | 34 | 15 | 4.03 | ME/CFS<HC |
| RH cuneus (seed 5)—RH superior midfrontal gyrus (cluster 1) | 34 | 46 | 32 | 16 | 4.01 | ME/CFS<HC |
| RH cuneus (seed 5)—LH middle frontal gyrus BA6 (cluster 2) | −38 | −2 | 52 | 16 | 4.00 | ME/CFS<HC |
Threshold uncorrected p < 0.001; k > 15.
x, y, and z coordinates are in MNI space.
BA, Brodman area; k, cluster size; t, t-value; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
Model-based connectivity as predictor of fatigue
As all data met normalcy criteria (see Correlations Between Clinical Fatigue and FC section), Pearson's product moment correlations were used. A significant relationship was found between the strength of connectivity and self-reports of fatigue obtained with the MFI for all disrupted seed-to-brain region pairs (Table 6). For the majority of seed-to-brain region pairs, ME/CFS subjects showed negative correlations with FC. Only for the seed-to-brain region pair (right parahippocampus to right inferior parietal lobe) a positive relationship with fatigue was observed in ME/CFS subjects.
Table 6.
Correlation Between Degree of Functional Connectivity and Self-Reported Fatigue in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subjects
| Functional connectivity | ||||||
|---|---|---|---|---|---|---|
| Seed 1: cluster 1 | Seed 2: cluster 1 | Seed 3: cluster 1 | Seed 3: cluster 2 | Seed 5: cluster 1 | Seed 5: cluster 2 | |
| General fatiguea | −0.61b | 0.61b | −0.62b | −0.46b | −0.59b | −0.66b |
| Physical fatiguea | −0.50b | 0.60b | −0.59b | −0.44b | −0.56b | −0.64b |
| Reduced activitya | −0.39c | 0.57b | −0.59b | −0.44b | −0.58b | −0.62b |
| Reduced motivationa | −0.41b | 0.52b | −0.50b | −0.29 | −0.51b | −0.69b |
| Mental fatiguea | −0.53b | 0.44b | −0.57b | −0.40c | −0.57b | −0.46b |
Item of the MFI.
p < 0.01.
p < 0.05.
Seed 1: cluster 1, RH cuneus—RH superior temporal gyrus; Seed 2: cluster 1, RH parahippocampus—RH inferior parietal lobe; Seed 3: cluster 1, LH lingual gyrus—LH occipital lobe; Seed 3: cluster 2, LH lingual gyrus—RH precuneus; Seed 5: cluster 1, RH cuneus—RH superior midfrontal gyrus; Seed 5: cluster 2, RH cuneus—LH middle frontal gyrus.
Discussion
To identify neural correlates of self-reported fatigue, we compared patterns of resting-state FC of ME/CFS patients with age–sex-matched normal control subjects. To obtain complementary information on FC of multiple brain networks in ME/CFS, two different methods of analysis were used: (1) ICA depends on statistical associations of neural activity between brain areas and (2) a seed-based approach exploring the associations of brain activity with a priori determined brain areas. Our results demonstrate that ME/CFS is associated with altered resting-state FC of several brain networks, and the degree of altered connectivity is significantly related to self-reported fatigue. Both approaches showed ME/CFS to be associated with decreased intrinsic connectivity in the LFPN and with decreased connectivity between regions in the cingulate cortex and the SMN and SN.
Neural networks encompassing multiple brain regions show synchronized activity at rest (Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; Salvador et al., 2005; van den Heuvel and Hulshoff Pol, 2010). The FPN is one of these networks that is composed of lateral prefrontal regions and inferior parietal cortices and often shows lateralization into right and left components (Beckmann et al., 2005; Damoiseaux et al., 2006; Di and Biswal, 2015; Smith et al., 2009). This RSN is implicated in cognitive control, attention, language processing, and working memory (Lois et al., 2014) and links the bilateral insular regions and anterior cingulate cortex (Dosenbach et al., 2007). Coherent activation between the FPN and the DMN has been suggested to play an important role in salience processes of human cognition (Fox and Raichle, 2007; Gusnard et al., 2001), including mind wandering (Mason et al., 2007), goal-directed behavior (Dosenbach et al., 2007), and relating oneself to the outside world (Gusnard et al., 2001). Considerable evidence suggests that the FPN, in particular the left FPN, plays a pivotal gate-keeping role in goal-directed cognition, mediating the dynamic balance between DMN and dorsal attention networks (Spreng et al., 2013; Vallesi et al., 2015; Vincent et al., 2008). Less FC within the left FPN, which was seen in ME/CFS patients, may be an important feature of the condition that needs more research to fully understand its role.
The SN mainly consists of the insular cortex and anterior cingulate cortex. This RSN is involved in a wide range of functions such as detection of salient stimuli, interoception, audition, pain, deception, music, and classical conditioning (Borsook et al., 2013; Kucyi and Davis, 2015). Because of the heterogeneous range of functions, the SN has been considered a transitional network linking cognition and emotion/interoception (Lois et al., 2014; Smith et al., 2009). The SN causally influences the DMN and FPN. It also mediates the “switching” between activation of the DMN and of the FPN and executive control networks to guide appropriate responses to salient stimuli (Uddin, 2015). In our study, there was decreased FC of ME/CFS patients between the SN and PCC, which is a key node of the DMN. Less effective connectivity between these regions was strongly associated with more fatigue. Therefore, it appears that decreased FC between these regions may be indicative of decreased drive to guide thoughts away from self-referential experience of fatigue toward externally directed cognition.
The SMN includes primary sensory motor cortices and is associated with action and somesthesis (Lois et al., 2014). ME/CFS participants showed reduced connectivity between this RSN and the aMCC. The aMCC has a key role for cognitive aspects of movement generation, that is, intentional motor control (Hoffstaedter et al., 2014). We found that fatigue strongly predicted an inverse relationship of FC between this RSN and the aMCC. Further research is required to better understand the altered relationship of the SMN and aMCC with ME/CFS. Taking into account ME/CFS patients' impaired physical, affective, and cognitive functions and the decreased connectivity between the spatial maps of these RSNs, it can be argued that patterns of FC within or between these networks may be impaired in ME/CFS (Laird et al., 2011). We believe that our findings shed new light on the understanding of chronic fatigue across individuals and how the intrinsic connectivity of the brain is influenced by ME/CFS patients' symptoms.
Our seed-based approach was predicated on brain regions that had demonstrated altered function during ASL imaging, as evidenced by reduced rCBF. It demonstrated that the parahippocampus and occipital lobes of ME/CFS patients have altered FC. Although we are the first to report abnormal resting-state FC in patients with ME/CFS, others have described altered structure and task-related dysfunction within some of the same regions using other imaging modalities (Cook et al., 2007; De Lange et al., 2005; Okada et al., 2004). In addition, utilizing diffusion tensor imaging researchers found disrupted white matter connectivity along the right inferior fronto-occipital fasciculus in veterans with gulf war syndrome, which was marked by severe levels of fatigue and malaise (Rayhan et al., 2013). The right inferior fronto-occipital fasciculus connects posterior brain structures such as the parahippocampus and occipital lobe with anterior brain structures. Our results would support such disrupted tracts because the relationship of brain activity between posterior brain structures and intermediate structures is decreased in ME/CFS patients. Similarly, a previous study reported that multiple sclerosis patients who reported significant levels of fatigue had decreased cortical activity within the precuneus, cuneus, and middle frontal gyrus compared to MS patients without fatigue (Filippi et al., 2002).
The DMN, the most commonly studied RSN, comprises the precuneus/PCC, the medial frontal cortex, and bilateral inferior parietal regions (Fox and Raichle, 2007; Greicius et al., 2009; Gusnard et al., 2001). Activity and connectivity of the DMN have been linked to central processes of human cognition, including integration of cognitive as well as emotional brain activity (Greicius et al., 2009), monitoring of the environment (Gusnard et al., 2001), and mind wandering (Mason et al., 2007). Abnormalities of DMN connectivity have been reported in attention deficit disorder, multiple sclerosis, and Alzheimer's disease, all of which demonstrate overlapping clinical features with ME/CFS, including attention and memory difficulties. Surprisingly, we did not find fatigue-related DMN abnormalities. The DMN can be represented as a single IC or it can split into two or three components. Such a decomposition is frequently observed with separation into anterior and posterior networks (Uddin et al., 2009 and Laird et al., 2009). In our study, we choose the components that best fit the spatial template of a typical DMN. Retrospective examination of all components, however, showed three that resembled aspects of the DMN. The ability of networks to split into more than one component is a limitation of our applied methods and may explain why the DMN did not show altered connectivity between ME/CFS and HC groups.
Limitations
Some caution needs to be exercised when interpreting our results, as our sample only included female participants. Although ME/CFS affects more women than men, our results may not be applicable to males. Second, our model-based results are dependent on our seed selection method. While other researchers may use alternative seed regions for connectivity analyses, we empirically chose our seeds according to a priori detected rCBF abnormalities in ME/CFS. This approach of seed selection allowed us to focus on brain regions clearly associated with ME/CFS. Group-dependent rCBF findings provide a rationale to explore FC between those regions and the rest of the brain.
Conclusions
ME/CFS appears to be a chronic illness that affects several different brain areas and is associated with abnormal neuronal connectivity. Using two different methods of analysis, significant differences in resting-state FC were detected between ME/CFS and HC. Furthermore, these changes were significantly correlated with self-reported levels of fatigue. Additional neuroimaging studies will be necessary to better understand the contributions of these brain regions to ME/CFS.
Acknowledgments
The expert assistance of Ricky Madhavan and Yesenia M. Lucas is gratefully acknowledged.
This study was supported by NIH grant R01 NR014049-01 and NIH/NCATS Clinical and Translational Science grants UL1 TR000064. CWG was supported by NIH training grant F32AT007729.
Author Disclosure Statement
None of the authors have any financial or other relationships that might result in a conflict of interest.
References
- Akerstedt T, Axelsson J, Lekander M, Orsini N, Kecklund G. 2014. Do sleep, stress, and illness explain daily variations in fatigue? A prospective study. J Psychosom Res 76:280–285 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. 2011. The cortical rhythms of chronic back pain. J Neurosci 31:13981–13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Kunwar P, Natelson BH. 2011. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci 301:9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. 2013. Pain and analgesia: the value of salience circuits. Prog Neurobiol 104:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pekar JJ. 2004. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn Reson Imaging 22:1181–1191 [DOI] [PubMed] [Google Scholar]
- Cook DB, O'Connor PJ, Lange G, Steffener J. 2007. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36:108–122 [DOI] [PubMed] [Google Scholar]
- Cope H, David AS. 1996. Neuroimaging in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 60:471–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, et al. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103:13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange FP, et al. 2004. Neural correlates of the chronic fatigue syndrome—an fMRI study. Brain 127:1948–1957 [DOI] [PubMed] [Google Scholar]
- De Lange FP, et al. 2005. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage 26:777–781 [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. 2006. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29:1359–1367 [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB. 2015. Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct Funct 220:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, et al. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff EP, Cunnington R, Egan GF. 2007. REX: response exploration for neuroimaging datasets. Neuroinformatics 5:223–234 [DOI] [PubMed] [Google Scholar]
- Filippi M, et al. 2002. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15:559–567 [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Fukuda K, et al. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 121:953–959 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. 2009. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. 2001. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694 [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, et al. 2014. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, et al. 2015. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv 1:e1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E, et al. 2014. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain 15:815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P, et al. 2015. Sleep disturbance, depression, obesity, and physical inactivity explain a significant portion of fatigue in rheumatoid arthritis. Arthritis Care Res (Hoboken), doi: 10.1002/acr.22577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff AL, et al. 1996. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med 101:281–290 [DOI] [PubMed] [Google Scholar]
- Kroenke K, et al. 2011. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain 12:964–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2015. The dynamic pain connectome. Trends Neurosci 38:86–95 [DOI] [PubMed] [Google Scholar]
- Laird AR, et al. 2009. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci 29:14496–14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, et al. 2011. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange G, Wang S, DeLuca J, Natelson BH. 1998. Neuroimaging in chronic fatigue syndrome. Am J Med 105:50S–53S [DOI] [PubMed] [Google Scholar]
- Lim J, et al. 2010. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage 49:3426–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois G, Linke J, Wessa M. 2014. Altered functional connectivity between emotional and cognitive resting state networks in euthymic bipolar I disorder patients. PLoS One 9:e107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, et al. 2012. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, et al. 2007. Wandering minds: the default network and stimulus-independent thought. Science 315:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, et al. 2010. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheumatol 62:2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. 2004. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R, Embleton K, Pentreath VW, Jackson A. 2010. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol 83:419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri BK, et al. 2012. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol 85:E270–E273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, et al. 2013. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War illness. PLoS One 8:e58493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC, et al. 2003. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, et al. 2005. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15:1332–1342 [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, et al. 2014. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin 6:252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. 1995. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39:315–325 [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. 2013. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25:74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. 2010. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett 485:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, et al. 2006. Reduced responsiveness is an essential feature of chronic fatigue syndrome: a fMRI study. BMC Neurol 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli U, et al. 1998. Brain positron emission tomography (PET) in chronic fatigue syndrome: preliminary data. Am J Med 105:54S–58S [DOI] [PubMed] [Google Scholar]
- Uddin LQ. 2015. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier CF, Milham MP. 2009. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 30:625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi A, Arbula S, Capizzi M, Causin F, d'Avella D. 2015. Domain-independent neural underpinning of task-switching: an fMRI investigation. Cortex 65C:173–183 [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, 2008. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 26:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiuchi K, Farkas J, Natelson BH. 2006. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging 26:83–86 [DOI] [PubMed] [Google Scholar]