Abstract
The performance of an assay to detect antibodies to a norovirus nonstructural fusion protein, designated VPR and consisting of three proteins (GI.1 virus protein genome-linked [VPg], a virus protease, and an RNA-dependent RNA polymerase), was evaluated. The assay sensitivity and specificity were 74.5% and >95%, respectively, for identifying GI.1 norovirus infection among persons who received either a monovalent GI.1 norovirus virus-like particle (VLP) vaccine or placebo by the intranasal route followed by an oral live GI.1 norovirus challenge.
TEXT
Noroviruses (NoVs) cause an estimated 20 million infections per year in the United States and are the leading cause of nonbacterial gastroenteritis worldwide (1, 2). The norovirus genome consists of 3 open reading frames (ORFs). ORF1 codes for several nonstructural proteins that are transcribed as a polyprotein before being cleaved into individual proteins by viral protease. ORF2 and ORF3 code for structural capsid proteins VP1 (the major capsid protein) and VP2 (a minor capsid protein), respectively (3).
A candidate vaccine that is based upon immunization with a norovirus VP1 protein has been developed (4). Norovirus infection can be detected by measuring seroresponses to the VP1 protein (5), but the sensitivity of serological detection targeting the VP1 protein and the diagnostic precision of this test are diminished by prior immunization (6). We previously developed a serological assay targeting a nonstructural protein, the norovirus protease, and the sensitivity of the assay was 53% when a homologous protease was used as the antigen (7). Preliminary studies indicated that although the viral polymerase induced seroresponses less frequently than the viral protease protein, a fusion protein (VPR) consisting of three nonstructural viral proteins (GI.1 virus protein genome-linked [VPg] plus protease plus RNA-dependent RNA polymerase) was able to detect seroresponses at a higher frequency than we reported for the viral protease alone. In the current study, we evaluated the performance of a serological assay using the VPR fusion protein to identify infection among persons who participated in a GI.1 norovirus candidate vaccine trial (LV01-103) that included a live oral challenge with a GI.1 norovirus (8).
Serum samples from persons in the LV01-103 study who gave permission for future use of collected samples and participated in the challenge portion of the study were included in the current study. Serum samples collected at prevaccination (day 0), post-vaccination 1 (day 21), post-vaccination 2 and prechallenge (day 42 or later), and postchallenge (day 30 postchallenge) were tested by enzyme-linked immunosorbent assay (ELISA) for the presence of antibody to the fusion protein, VPR. Full-length VPR was PCR-amplified from the Norwalk virus genome (GenBank accession no. NC_001959) and cloned into the pET46 Ek/LIC vector (EMD Millipore). Mutations E138A, C277A, and E319A were introduced with a site-directed mutagenesis kit (Stratagene) to completely inactivate the viral protease (C277A) and prevent autoproteolysis by removing the cleavage sites (E138A and E319A). The protein was then expressed in the BL21(DE3) strain of Escherichia coli. The integrity and purity of the polyprotein product were confirmed using a Coomassie-stained, SDS-PAGE gel, which demonstrated a single ∼100 kDa product, the expected size of the VPg (20 kDa), protease (18 kDa), and RNA polymerase (56 kDa) fusion protein. Purification was achieved with a combination of Ni affinity, SP Sepharose ion exchange, and S200 gel filtration chromatography into a final buffer containing 20 mM Tris (pH 8.0), 300 mM NaCl, 5 mM MgCl2, and 1 mM dithiothreitol (DTT). Endpoint dilutions were performed to determine antibody titers, and the relationship between serial titers in individual patients was evaluated. In brief, 100 ng of VPR protein diluted in 0.01 M phosphate-buffered saline (PBS) was coated onto 96-well polyvinyl chloride plates (Thermo Scientific) for 4 h at room temperature, and the plates were blocked overnight at 4°C with 10% blotto in 0.01 M PBS. After being washed with 0.01 M PBS plus 0.05% Tween 20 (PBS-Tween), sera diluted from 1:20 to 1:1,280 in PBS-5% blotto were added to duplicate wells and incubated for 1 h at 37°C. Anti-VPR antibodies were detected with a 1:5,000 dilution of peroxidase-labeled goat anti-human antibody (IgA, IgG, and IgM) (KPL) in PBS-5% blotto. The reaction was developed by addition of a mixture of TMB peroxidase solution (KPL) and stopped after 10 min by addition of 1 M H3PO4. The optical density (OD) was determined with a spectrophotometer at a wavelength of 450 nm, and the cutoff for a positive sample was an OD of >0.1. A positive-control serum sample from a person infected with Norwalk virus in a separate study (5) was run on each plate, and all samples from each individual were run in the same experiment.
Sera from 73 of the 84 persons who participated in the challenge study were available for study; 67 of these were in the per-protocol analysis, which is reported here since the intent-to-treat (ITT) and per-protocol analyses yielded similar results. All subjects had anti-VPR antibody detected at the time of study enrollment. The seroresponse frequency (a ≥4-fold increase in the antibody level) was assessed following vaccination and following challenge regardless of whether infection occurred. The gold standard for infection was the definition used in the LV01-103 study (8); all persons in the current analysis had fecal shedding of Norwalk virus as detected by reverse transcription (RT)-PCR and a ≥4-fold increase in the total serum ELISA antibody titer to Norwalk virus. Thirty-eight of 51 infected persons had a ≥4-fold increase in serum antibody to the VPR protein, with a geometric mean fold rise of 5.3 (Table 1). Anti-VPR seroresponse frequencies were similar among infected persons who did or did not meet the protocol-defined definition of gastroenteritis (76.3% and 69.2%, respectively). In contrast, none of the 16 persons who were not shown to be infected after challenge had a seroresponse to the VPR protein. The sensitivity and specificity of the VPR antibody assay following the Norwalk virus challenge were 74.5% and 100%, respectively.
TABLE 1.
Antibody responses to VPR fusion protein before and following Norwalk virus challenge
| Sample | No. of subjects | GMT (95% CI)a |
GMFRb (95% CI) from pre- to postchallenge | Postchallenge seroresponse frequency (% [95% CI])c | |
|---|---|---|---|---|---|
| Prechallenge | Postchallenge (day 30) | ||||
| Infected subjects | 51 | 84 (65–110) | 449 (339–596) | 5.3 (4.1–7.0) | 74.5 (60.4–85.7) |
| Gastroenteritis | 38 | 79 (59–105) | 461 (337–630) | 5.9 (4.3–8.0) | 76.3 (59.8–88.6) |
| No gastroenteritis | 13 | 104 (53–205) | 418 (204–855) | 4.0 (2.2–7.2) | 69.2 (38.6–90.9) |
| Subjects not infected | 16 | 135 (64–283) | 129 (63–262) | 1.0 (0.8–1.1) | 0 (0–20.6) |
| Infected vs. not infected P value | 0.134 | <0.001 | <0.001 | <0.001 | |
GMT, geometric mean titer; CI, confidence interval.
GMFR, geometric mean fold rise.
Four-fold or greater antibody titer rise to VPR protein from prechallenge.
We also looked at seroresponses after vaccination. Because the vaccine only contained the Norwalk virus VP1 protein, seroresponses to the VPR protein were not expected to occur. No seroresponses to the VPR protein were observed after the first inoculation of study product, while three occurred after the second (two in the vaccine recipients and one in a placebo recipient) (Fig. 1). Thus, the specificity of the assay following vaccination for the total study population was 95.5%.
FIG 1.
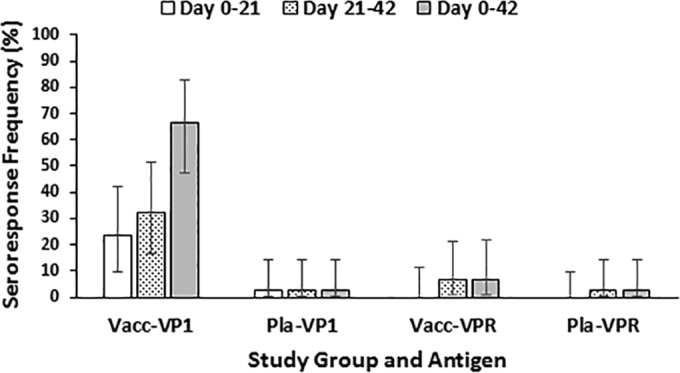
Seroresponse frequencies to capsid and VPR fusion proteins by vaccination group. The seroresponse frequencies to the capsid (VP1) antigen and to the fusion protein consisting of VPg, viral protease, and the viral RNA-dependent RNA polymerase (VPR) are shown for norovirus vaccine (Vacc) and placebo (Pla) recipients. Time intervals include before and after the first vaccination (days 0 to 21, white bar), before and after the second vaccination (days 21 to 42, stippled bar), and before the first vaccination to after the second vaccination (days 0 to 42, solid bar). The bars represent the 95% confidence intervals around the observed frequencies.
Each of the three persons who had a seroresponse to the VPR protein also had a seroresponse to the VP1 protein, raising the possibility that these subjects had a subclinical infection with another norovirus strain during the interval. Assay variability is another possibility; the three subjects were in both treatment groups. We have shown previously that serological responses to the viral protease are cross-reactive between genogroups (7), and a single study participant in the ITT group, a placebo recipient who was symptomatically infected with a GII.4 norovirus but not the GI.1 challenge strain during the challenge portion of the study, also had a seroresponse to the VPR protein. The latter subject developed gastroenteritis symptoms 19 min after receipt of the live GI.1 virus by oral challenge (8).
In summary, 74.5% of persons infected with a GI.1 norovirus had a seroresponse to the norovirus nonstructural fusion protein, VPR, while the diagnostic specificity of the assay was >95%. The sensitivity of the norovirus VPR antibody assay was higher than the 53% reported previously using the Norwalk viral protease alone as antigen (7), and the assay has potential utility for differentiating serological responses to inactivated and subunit norovirus vaccines from those induced by wild-type norovirus infection.
ACKNOWLEDGMENTS
This research was supported by Public Health Service grants NIH P01 AI057788 and NIH P30 DK56338, by Takeda Pharmaceuticals U.S.A. (TPUSA), and by the John S. Dunn Research Foundation.
REFERENCES
- 1.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, Parashar UD. 2013. Norovirus disease in the United States. Emerg Infect Dis 19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramani S, Atmar RL, Estes MK. 2014. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol 30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass RI, Parashar UD, Estes MK. 2009. Current concepts: norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson C, Bargatze RF, Goodwin R, Mendelman PM. 2013. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines 12:155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- 5.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinjé J, Gregoricus N, Frenck RW Jr, Moe CL, Al-Ibrahim MS, Barrett J, Ferreira J, Estes MK, Graham DY, Goodwin R, Borkowski A, Clemens R, Mendelman PM. 2015. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 211:870–878. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajami NJ, Barry MA, Carillo B, Muhaxhiri Z, Neill FH, Prasad BVV, Opekun A, Gilger MA, Graham DY, Atmar RL, Estes MK. 2012. Antibody responses to norovirus genogroup GI.1 and GII.4 proteases in volunteers administered Norwalk virus. Clin Vaccine Immunol 19:1980–1983. doi: 10.1128/CVI.00411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atmar RL, Bernstein DI, Harro CD, Al Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine protects against experimental human Norwalk virus illness. N Engl J Med 365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]


