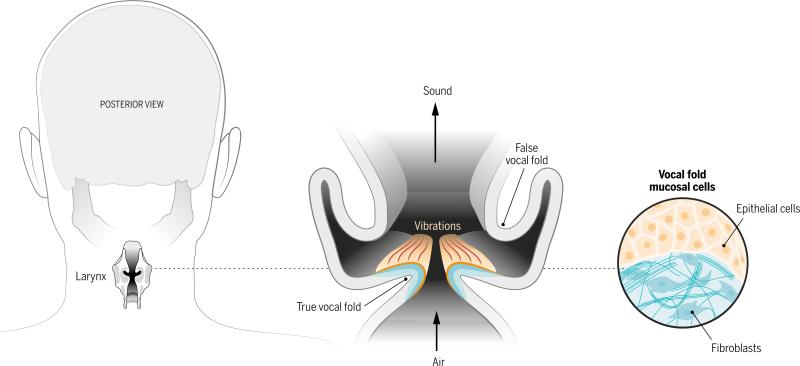
In most mammals, a vocal sound begins with the vibration of paired vocal cords within the larynx. These delicate structures--more accurately called vocal folds because they resemble folded layers of fabric--can stiffen after traumatic injury, cancer treatment, or unknown environmental or genetic insults. Current voice medicine has limited options to repair or replace damaged vocal folds when the voice disturbance is disabling (1). A recent study by Ling et al. (2) reports a tissue engineering approach that could lead to implants that replace the unique vocal fold mucosa and restore vocalization.
Vocal folds are made of specialized and complex layers of extracellular matrix constituents and cells, with a microstructure that promotes pliability and resilience. Human vocal folds undulate with wave-like regularity in response to air flow from the lungs and collide with each other hundreds of times per second during speech. Sound waves result from the rapidly repeating airflow interruptions. When stiffened, their functional extracellular matrix and epithelium are replaced by scar tissue that does not easily vibrate during voicing.
Ling et al. introduce an engineered tissue structure that they hope can replace the vocal fold mucosa as treatment for scarring. Primary human vocal fold cells are not widely available because of the irreparable voice injury caused by their excision. The authors isolated fibroblasts and epithelial cells from normal human vocal folds that were surgically removed for unrelated reasons. They expanded these cells and then assembled them into a three-dimensional collagen gel culture to mimic the vocal fold mucosa, which consists of epithelium overlying a “lamina propria” layer of fibroblasts in extracellular matrix. As the tissue-engineered construct developed in vitro, its profile of protein expression (proteome) took on new features relevant to tissue assembly and was different from either cell type cultured alone in collagen. Notably, the proteome contained numerous extracellular matrix proteins that are found in the vocal fold. Thus, the coculture approach with primary human vocal fold cells may provide a pathway to develop the sophisticated extracellular matrix organization that underpins vocal fold vibration. Although that degree of maturity was not yet achieved in vitro, the material's rheologic behavior was similar to that of excised native vocal fold mucosa.
The key specialized function of the vocal fold mucosa is periodic vibration powered by the aerodynamic energy of a translaryngeal pressure gradient. That vibration is due to the vocal folds’ unique pliability and is not satisfactorily achieved with other tissues such as skin or oral mucosa. Ling et al. demonstrated this function in vitro by applying their engineered tissue to excised canine larynges subjected to airflow (3). Quantitative analysis of digital images showed excellent tissue vibration similar to that of native vocal folds, at least for a short duration. Vibration occurred even though the immature extracellular matrix in the engineered tissue differed greatly from vocal folds. This supports the notion that the epithelium itself is critical for phonation, in addition to the underlying microstructure (4). The promising results should prompt longer-term vibration studies that better simulate actual voice use patterns.
A major issue that will face any nonautologous cell-based vocal fold implant is rejection by the immune system. Most solid organ transplants are performed for life-threatening diseases, for which the risk-benefit ratio of immunosuppressive medication is acceptable. Vocal fold mucosal disorders instead affect quality, not quantity, of life. Partly for that reason, only two whole-larynx transplants have been performed, and one of the patients was already immunosuppressed (5, 6). For widespread clinical application, a vocal fold graft cannot require host immune modulation. To assess immunogenicity, Ling et al. implanted their tissue grafts under the kidney capsules of mutant mice that were genetically engineered to replicate the human immune system. The grafts were not rejected, even when the immune cells and vocal fold cells came from different donors. This fortunate result may be attributed to the purity of the implanted cell populations, which are free of the antigen-presenting leukocytes present in whole-organ transplants. Ling et al.'s work lends hope that vocal fold cell transplants might be feasible without immunosuppression.
A hurdle yet to be addressed is the healing after implantation. Ling et al. placed their construct in the mouse kidney solely to assess immune response, not function after healing. The vocal folds are subject to unique stresses that may affect wound healing, including drying from constant airflow, exposure to bacterial flora and pathogens, and phonatory trauma. Scar formation within the construct would again impair the voice, and the implant would be futile. In that light, precisely replicating the vocal fold microstructure in vitro may be less important than controlling the environment for in vivo wound healing. Previous studies of vocal fold mucosa implantation in rabbits revealed some extracellular matrix alteration, although vibration was preserved (7). Identifying those features of an implant that minimize scarring during wound healing is critical for clinical application of this emerging technology.
The complete vocal fold mucosa replacement as proposed by Ling et al. is a radical approach for severe vocal fold scarring. A failed implant could worsen a person's voice, so it would initially be limited to the most refractory cases. For less severe scarring, a less risky approach such as cell injections is appropriate and is further along the clinical pipeline. Autologous fibroblasts and mesenchymal stem cells both have shown promise for improving function after injection into scarred vocal fold lamina propria (8, 9). But even if cell injections prove successful in clinical trials, there will undoubtedly still be patients whose extensive scarring cannot be reversed and who would benefit from complete mucosal replacement. Furthermore, if function and nontumorigenicity are demonstrated, a mucosal replacement such as that of Ling et al. could be considered at the time of laryngeal cancer resection. This single-surgery scheme would dramatically change the approach to vocal fold cancer treatment and voice rehabilitation.
The voice.
The microstructure of vocal folds (vocal cords), which includes complex layers of extracellular matrix constituents and cells, promotes the pliability needed to undulate in response to air flow. Damage and scarring stiffens this structure Vocal fold replacement through tissue engineering scheme would dramatically change the approach to voice rehabilitation.
ACKNOWLEDGMENTS
Supported by VA Career Development Award IK2BX001944 (J.L.L.) and NIH grant R01 DC011300 (D.K.C.).
REFERENCES AND NOTES
- 1.Welham NV, et al. Laryngoscope. 2011;121:1252. doi: 10.1002/lary.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling XX, et al. Sci. Transl. Med. 2015;7:314ra187. doi: 10.1126/scitranslmed.aab4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long J, et al. Otolaryngol. Head Neck Surg. 2010;142:438. doi: 10.1016/j.otohns.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Tse JR, Zhang Z, Long J. J. Acoust. Soc. Am. 2015;138:EL60. doi: 10.1121/1.4922765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farwell DG, et al. Laryngoscope. 2013;123:2502. doi: 10.1002/lary.24053. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz RR, Strome M. Otolaryngol. Head Neck Surg. 2014;150:509. doi: 10.1177/0194599813519748. [DOI] [PubMed] [Google Scholar]
- 7.Long J, et al. Laryngoscope. 2015;125:406. doi: 10.1002/lary.24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chhetri D, Berke G. Laryngoscope. 2011;121:785. doi: 10.1002/lary.21417. [DOI] [PubMed] [Google Scholar]
- 9.Svensson B, et al. Laryngoscope. 2011;121:2185. doi: 10.1002/lary.22143. [DOI] [PubMed] [Google Scholar]