Abstract
Studies have found that hypoxia is the most common feature in all of solid tumor progression, thus it has become a central issue in tumor physiology and cancer treatment. Hypoxia-inducible factor-1α (HIF-1α) could make the tumor produce adaptive biological response to hypoxia and become more aggressive. In this paper, we used enzyme linked immune sorbent assay to detect the plasma level of HIF-1α in patients with NSCLC and healthy volunteers. The results indicated that the 5-year survival rate of patients with squamous cell carcinomas is negatively correlated with the plasma level of HIF-1α and the 5-year survival rate of patients with low level of HIF-1α is higher than those with high level of HIF-1α. The plasma level of HIF-1α in patients with NSCLC is significantly higher than healthy volunteers. There is no significant correlation between the plasma level of HIF-1α and clinical features of NSCLC patients. In a word, there is no connection between the plasma level of HIF-1α and the clinical features of NSCLC patients as well as their prognosis. In stratified analysis, the plasma level of HIF-1α in patients with squamous cell carcinoma is associated with regional lymph node status.
At Present, lung cancer is the leading cause of cancer-related deaths worldwide1. It is the most commonly diagnosed cancers as well as the leading cause of cancer death in males in 2012 globally. The incidence rate and mortality of lung cancer are increasing in China and some other countries of Asia and Africa. In china, the incidence rate and mortality of lung cancer are highest in malignant tumors. NSCLC is the mainly pathological type of lung cancer, accounts for about 85% of all lung cancers2. Surgery is an important treatment for IA–IIIA patients with NSCLC, but the recurrence and metastasis are still the leading causes of death after the resection of primary tumors3. The clinician needs more effective predictors or biomarkers to predict the prognosis and curative effect, so as to gain a better understanding of the patient’s clinical condition. TNM staging of the tumor and the physical condition of patients themselves are the most reliable prognostic indicator for patients with NSCLC. But even in the same stage of patients with NSCLC, there are still great differences in their overall survival (OS). No reliable indicator could be used to evaluate the therapy effect and prognosis for patients with NSCLC in the same stage exactly. Many studies have begun to look for some new biological molecules by various methods.
HIF-1α was found in the 1990s during the studies of erythropoietin expression, hypoxia is high growth-promoting factor4. HIFs is a heterodimer comprising HIF-α and HIF-β subunits, and there are three kinds of HIF-α, HIF-1α, HIF-2α and HIF-3α. HIF-α is the functional subunit of HIFs, and it can determine the activity of HIFs. Hydroxylation was taken place on the HIF-α by the action of prolyl hydroxylases (PHD) in normoxia, and then ubiquitination, and degraded by the proteasome rapidly5. Its half-life is less than 1–2 minutes. In hypoxia condition, HIF-1α’s subunit expresses stably, the accumulation of undegradative HIF-1α makes itself reach a high level and transfer to the cell nuclear. HIF-1α and the structural subunit HIF-1β form the HIF-1 heterodimeric complex, thus becoming activated HIF-1α5. Activated HIF-1α can mediate the transcriptional activity of the target gene by binding to the hypoxia-response element (HRE) of the target gene6. The functions of target genes which mediated by HIF-1α mainly includes: 1, Participate in the process of promoting erythropoiesis, thus increasing the oxygen transport of organization. 2, Promote angiogenesis and increase local oxygen flow. 3, Regulate anaerobic glycolysis, thereby adapt to local hypoxia7.
Previous studies had found that hypoxia was the most common feature of tumor progress including NSCLC, hypoxic zones in tumors would subsequently appear in the process of tumor growth, the reason might be that some local blood vessels unmatured cannot provide sufficient oxygen needed for tumor growth8. Tumor cells through a series of changes in biological characteristics to adapt to the lack of oxygen to survive and proliferation, and these adaptive changes of hypoxia make the proliferation and invasive of tumor enhance significantly9. Several studies had found that the possible reasons for the adaptability response may as follows: In the process of tumor growth, when the blood supply could not meet the needs of the tumor growth, HIF-1α would be activated by the hypoxia within the tumor, a series of HIF-1α dependent genes activated, regulating the biological characteristics of tumor from multiple pathways, so that the adaptive changes happened10,11.
The relationship between HIF-1α and tumor is very complex. More than one hundred kinds of target genes associated with tumor growth and metastasis can be regulated and activated by HIF-1α, most of them were related to tumor progression and metastasis, such as gene involved in angiogenesis (VEGF, PDGF, PIGF), extracellular matrix degradation gene (MMPs), metastatic gene(SDF1, CXCR4, LOX), epithelial-mesenchymal transition gene (SNAIL, SIP) and so on12. All genes mentioned above have been known as target genes of HIF, by promoting the proliferation of tumor cell, tumor angiogenesis, and enhancing the athletic ability of tumor cell, and improving high reactivity of tumor cell to mitogenic signals and so on, hence conducive to the growth of tumor, to adapt to the lack of oxygen6,13,14.
In hypoxia environment, HIF-1α can activate the adaptive response of the tumor target genes. At present, the roles of HIF-1α in tumor related are major as follows: 1) It exists in many types of cancers, its level is related to tumor invasiveness and metastatic15,16. 2) HIF-1α expression is associated with the susceptibility to chemotherapy and radiotherapy of a variety of tumors, including NSCLC17,18. A high level of HIF-1α expression can decrease the susceptibility. In vitro studies have found that the therapeutic effect of paclitaxel is associated with HIF-1α levels. The resistance of tumer cells to paclitaxel significantly increased when the HIF-1α overespressed19. It is reported that endostatin combined with radiotherapy can significantly inhibit the activity of HIF-1α in tumor cells, thus increasing the activity of antitumor angiogenesis and metastasis20. 3) Some researches that HIF-1α inhibitor was used for anticancer therapy have been carried out: a. Research shows that the expression of HIF-1α will be inhibited after the application of nitric oxide donor drugs, thus the drug resistance of cancer cells to anticancer drugs can be significantly suppressed21. In addition, we have made it clear that nitroglycerin can inhibit the role of HIF-1α. In a randomized phase II clinical trial, the researchers compared the curative effect between two kinds of different treatment methods for NSCLC patients on stage III and IV. One therapeutic method is vinorelbine and cisplatin chemotherapy combined with nitroglycerin, and the other is only vinorelbine and cisplatin chemotherapy. The results showed that nitroglycerin could significantly reduce the side effects of chemotherapy, and could increase the curative effect of chemotherapy22,23. b. Currently, some HIF-1α inhibitors such as PX-478, TX-2098 and so on, are all in the preclinical or clinical trials phase24,25. c. In addition, studies have found that some drugs that have been applied in the clinical for many years also can inhibit the role of HIF-α. For example, topotecan is a known cytotoxic drug that can destroy DNA, a few studies have found that it can directly inhibit the HIF-1α transcription significantly26,27. There are studies showed that if the patients with lung cancer recieved a low dose of topoisomerase inhibitor every day, the expression of HIF-1α in lung cancer cells can be inhibit significantly33. In another research in vitro, researchers compared the influence on tumor cells in two different therapeutic methods: bevacizumab combined with topotecan and bevacizumab received alone. The results showed that the antitumor effect of beacizumab could significantly enhance by adding the HIF-1α inhibitors topotecan28. d. Moreover, the efficacy of radiotherapy will be obviously improved by inhibiting the HIF-1α level in the process of radiation therapy29.
Currently, HIF-1α has been widely used in the evaluation of tumor in anaerobic conditions. It has been shown to be a major regulator of cell adaptation to hypoxic stress and play a critical role in oncogenesis and angiogenesis30. Several studies have shown that high expression of HIF-1α makes the sensitivety of many tumors to chemotherapy and radiotherapy decrease, including NSCLC31,32. In addition, it was reported that the expression of HIF-1α could predict the prognosis and therapy effect of patients with NSCLC33. The method used in studies of HIF-1α is mainly immunohistochemistry. The tumor samples have to be obtained from patients, however, limited the application of this method. As for the plasma level of HIF-1α and its clinical significance in NSCLC, they are rarely reported.
The above researches have demonstrated that the role of HIF-1α in tumor pathological environmental factors and a certain value of HIF-1α as a therapeutic target. Therefore, we should choose suitable patient group to apply HIF-1α inhibitors. Through testing the plasma level of HIF-1α in patients with NSCLC, maybe we can evaluate the treatment efficacy of some drugs as well as the clinical condition of patients.
Results
Clinical characteristics of patients
The clinical characteristics of the 100 patients and 60 cases of healthy volunteers have been presented in Table 1. There was no statistical difference in age and gender between patients and volunteers (P = 0.432, P = 0.665).
Table 1. The clinical characteristics of the 100 patients and 60 cases of healthy volunteers.
| clinical characteristics | Case of patients(%) | healthy volunteer | X2 | P |
|---|---|---|---|---|
| Age | ||||
| Median | 59 | 57 | 0.432 | |
| Range | 36–84 | 31–82 | ||
| Gender | ||||
| Male | 80(80%) | 44(73%) | 0.361 | 0.665 |
| Female | 20(20%) | 16(27%) | ||
| Pathological type | ||||
| Adenocarcinoma | 40(40%) | |||
| Squamous cell carcinomas | 50(50%) | |||
| Others | 10(10%) | |||
| Tumor differentiation grade | ||||
| Poorly differentiated | 44(44%) | |||
| Middle differentiated | 55(55%) | |||
| High differentiated | 1(1%) | |||
| pTNM stage | ||||
| Ia stage | 20(20%) | |||
| Ib stage | 23(23%) | |||
| IIa stage | 19(19%) | |||
| IIb stage | 8(8%) | |||
| IIIa stage | 30(30%) | |||
| N stage | ||||
| N0 | 64(64%) | |||
| N1 | 9(9%) | |||
| N2 | 27(27%) | |||
| Smoking | ||||
| Yes | 59(59%) | |||
| No | 41(41%) | |||
Correlation analysis between the clinical features of NSCLC patients
The correlations between the clinical features of NSCLC patients are presented in Table 2. As can be seen clearly from the table, different histological types of patients with NSCLC have significant relationship with gender, smoking status, tumor size. There is a significant relationship between smoking status and tumor size.
Table 2. Correlation analysis between the clinical features of NSCLC patients.
| Pathological type | X2 | P | Tumor Size | X2 | P | Smoking | X2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma (n, %) | Squamous cell carcinomas (n, %) | Small (n, %) | Big (n, %) | Yes (n, %) | No (n, %) | |||||||
| Gender | ||||||||||||
| Male | 25(34.2) | 48(65.8) | 16.278 | <0.001 | 43(53.8) | 37(46.3) | 4.558 | 0.33 | 59(73.8) | 21(26.3) | 35.976 | <0.001 |
| Female | 15(88.2) | 2(11.8) | 16(80.0) | 4(20.0) | 0(0) | 20(100) | ||||||
| Smoking | ||||||||||||
| Yes | 16(29.1) | 39(70.9) | 13.502 | <0.001 | 30(50.8) | 29(49.2) | 3.954 | 0.047 | ||||
| No | 24(68.6) | 11(31.4) | 29(70.7) | 12(29.3) | ||||||||
| Diameter of tumor | ||||||||||||
| ≤4cm | 31(56.4) | 24(43.6) | 8.137 | 0.004 | ||||||||
| >4cm | 9(25.7) | 26(74.3) | ||||||||||
The Plasma level of HIF-1α in NSCLC patients
The plasma level of HIF-1α of 100 cases of patients with NSCLC and 60 healthy volunteers were tested. The median levels of HIF-1α in plasma of patients with NSCLC and healthy volunteers were 297.70 pg/ml and 274.92 pg/ml respectively. The plasma level of HIF-1α from patients was higher than healthy volunteers’, there was significant difference between two groups (P = 0.026)(Fig. 1).
Figure 1. The plasma level of HIF-1α in NSCLC patients and normals.
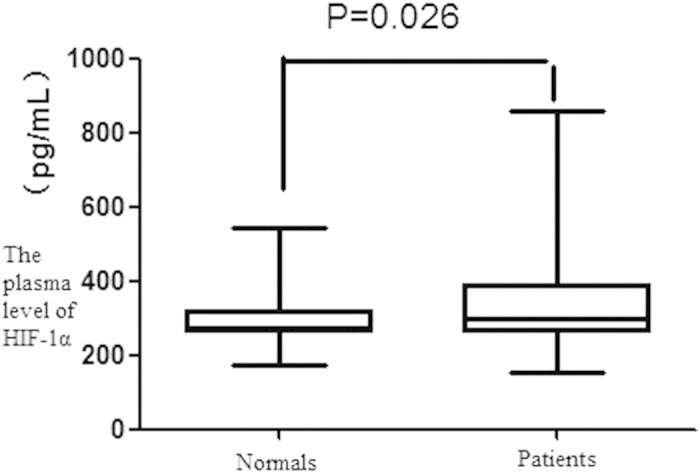
The plasma level of HIF-1α from NSCLC patients was higher than healthy volunteers, there was significant difference between two groups (P = 0.026).
The relationship between the plasma level of HIF-1α and the clinical features (age, sex, histological type, tumor differentiation grade, T stage, local lymph node status, pTNM stage, tumor size and smoking condition) of patient with NSCLC was analyzed respectively. Statistically significant correlation was not found. The detailed data was shown in Table 3.
Table 3. The relationship between the plasma level of HIF-1α and the clinical features.
| Cases | the plasma level of HIF-1α(pg/ml) | P | |
|---|---|---|---|
| Median(Range) | |||
| Age | |||
| ≤60y | 56 | 286.43(242.34–857.89) | 0.268 |
| >60y | 44 | 322.06(153.04–754.30) | |
| Gender | |||
| Male | 80 | 297.70(153.04–857.89) | 0.670 |
| Female | 20 | 288.14(243.62–754.30) | |
| Pathological type | |||
| Adenocarcinoma | 40 | 305.27(153.04–754.30) | 0.573 |
| Squamous cell carcinomas | 50 | 286.02(219.84–857.89) | |
| Others | 10 | 272.09(242.34–635.60) | |
| Tumor differentiation grade | |||
| Poorly differentiated | 44 | 302.25(242.34–754.30) | 0.397 |
| Middle-, high-differentiated | 56 | 286.43(153.04–857.89) | |
| T stage | |||
| T1 | 30 | 320.55(257.46–754.30) | 0.495 |
| T2 | 59 | 286.94(153.04–857.89) | |
| T3-4 | 11 | 307.70(250.81–558.71) | |
| N stage | |||
| N0 | 64 | 285.51(153.04–797.68) | 0.462 |
| N1-2 | 36 | 330.74(242.34–857.89) | |
| pTNM stage | |||
| I | 43 | 285.92(153.04–797.68) | 0.467 |
| II | 27 | 287.68(250.81–731.88) | |
| III | 31 | 335.92(242.34–857.89) | |
| Diameter of tumor | |||
| ≤4cm | 59 | 302.77(153.04–857.89) | 0.516 |
| >4cm | 42 | 285.92(219.84–757.68) | |
| Smoking | |||
| Yes | 59 | 302.76(219.84–857.89) | 0.343 |
| No | 41 | 285.92(153.04–754.30) | |
In this study, the plasma level of HIF-1α ≤ 297.70 was defined as a low level, the opposite as high level. The plasma levels of HIF-1α of patients with squamous carcinoma in different N stages was shown in Table 4. There was a significant difference between the two groups (X2 = 4.539, P = 0.033).
Table 4. The plasma level of HIF-1α of patients with squamous cell carcinoma in different N stages.
| Low level (n, %) | High level (n, %) | Total | |
|---|---|---|---|
| N0 | 24(64.9%) | 13(35.1%) | 37 |
| N1-2 | 4(30.8%) | 9(69.2%) | 13 |
| Total | 28 | 22 | 50 |
X2 = 4.539, P = 0.033.
The correlation between postoperative survival rate, clinicopathological features and the plasma level of HIF-1α of patients with NSCLC
By the end of the last follow-up time of this study (2014.11.30), follow-up has been available in all 100 cases. The median follow-up was 69.1 months (range: 3.4~79.7months). The 1-, 3- and 5-year postoperative survival rate of patients with NSCLC were 91%, 69%, 59% respectively, and not correlated with the plasma level of HIF-1α, age, gender, tumor differentiation grade, diameter of tumor and smoking status (P > 0.05). There was a significant relationship between the 3- and 5-year postoperative survival rate and pTNM stage (P < 0.05) (Table 5).
Table 5. Mono-factor analysis of postoperative survival rate of patients with NSCLC.
| 1-year postoperative survival rate(%) | P | 3-year postoperative survival rate(%) | P | 5-year postoperative survival rate(%) | P | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤60y | 91.1% | 0.978 | 67.9% | 0.780 | 64.3% | 0.225 |
| >60y | 90.9% | 70.5% | 52.3% | |||
| Gender | ||||||
| Male | 93.8% | 0.055 | 73.8% | 0.040 | 62.5% | 0.155 |
| Female | 80.0% | 50.0% | 45.0% | |||
| Pathological type | ||||||
| Adenocarcinoma | 92.5% | 0.777 | 72.5% | 0.873 | 57.5% | 0.409 |
| Squamous cell carcinomas | 94.0% | 74.0% | 66.0% | |||
| Tumor differentiation grade | ||||||
| Poorly differentiated | 88.6% | 0.464 | 63.6% | 0.304 | 54.5% | 0.422 |
| Middle-, high-differentiated | 92.9% | 73.2% | 62.5% | |||
| Diameter of tumor | ||||||
| ≤4cm | 93.2% | 0.352 | 67.8% | 0.755 | 61.0% | 0.623 |
| >4cm | 87.8% | 70.7% | 56.1% | |||
| pTNM stage | ||||||
| I stage | 97.7% | 0.126 | 79.1% | <0.001 | 69.8% | 0.001 |
| II stage | 85.2% | 85.2% | 74.1% | |||
| III stage | 86.7% | 40.0% | 30.0% | |||
| Smoking status | ||||||
| Yes | 93.2% | 0.352 | 74.6% | 0.148 | 62.7% | 0.365 |
| No | 87.8% | 61.0% | 53.7% | |||
| The plasma level of HIF-1α | ||||||
| ≤297.70 pg/ml | 96.2% | 0.061 | 76.9% | 0.075 | 63.5% | 0.345 |
| >297.70 pg/ml | 85.4% | 60.4% | 54.2% | |||
There was a significant relationship between 5-year postoperative survival rate of patients with squamous cell carcinomas and the plasma level of HIF-1α. The cut-off value of the plasma level of HIF-1α was set at the median (297.70 pg/ml). Patients with squamous cell carcinomas were divided into two groups (High Level > 297.70 pg/ml, Low level ≤ 297.70 pg/ml). The 5-year postoperative survival rate of low level group and high level was 78.6% (22/28), 50.0% (11/22) respectively. The difference between the two groups was statistical significantly (P = 0.034). There was no significant relationship between the 5-year postoperative survival rate and the plasma level of HIF-1α in patients with adenocarcinoma (Table 6).
Table 6. Mono-factor analysis of 5-year postoperative survival rate of patients with different pathological type.
| 5-year postoperative survival rate of Squamous cell carcinomas | P | 5-year postoperative survival rate of Adenocarcinoma | P | |
|---|---|---|---|---|
| Age | ||||
| ≤60y | 68.8% | 0.584 | 70.6% | 0.150 |
| >60y | 61.1% | 47.8% | ||
| Gender | ||||
| Male | 66.7% | 0.626 | 60.0% | 0.680 |
| Female | 50.0% | 53.3% | ||
| Tumor differentiation grade | ||||
| Poorly differentiated | 60.0% | 0.091 | 46.2% | 0.314 |
| Middle-, high-differentiated | 72.0% | 63.0% | ||
| Diameter of tumor | ||||
| ≤4cm | 66.7% | 0.778 | 61.3% | 0.368 |
| >4cm | 65.4% | 44.4% | ||
| pTNM stage | ||||
| I stage | 75.0% | 0.001 | 68.8% | 0.342 |
| II stage | 82.4% | 62.5% | ||
| III stage | 11.1% | 43.8% | ||
| Smoking status | ||||
| Yes | 66.7% | 0.851 | 56.3% | 0.896 |
| No | 63.6% | 58.3% | ||
| The plasma level of HIF-1α | ||||
| ≤297.70 pg/ml | 78.6% | 0.034 | 47.1% | 0.251 |
| >297.70 pg/ml | 50.0% | 65.2% | ||
The overall survival curves analysis
A retrospective chart review was performed and the overall survival rate was estimated by using the Kaplan-Meier method and compared by Log-rank test. The results showed that: until 2014.11.30, the overall survival rate of patients with stage I, II, III was 69.8%, 74.1%, 30.0% respectively. The differences in three groups, significantly, were statistical (P < 0.001) (Fig. 2). The overall survival rate of patients without regional lymph node metastasis was 71.9%, and significantly higher than patients had regional lymph node metastasis (P < 0.001) (Fig. 3).
Figure 2. The differences between stage I, II, III.
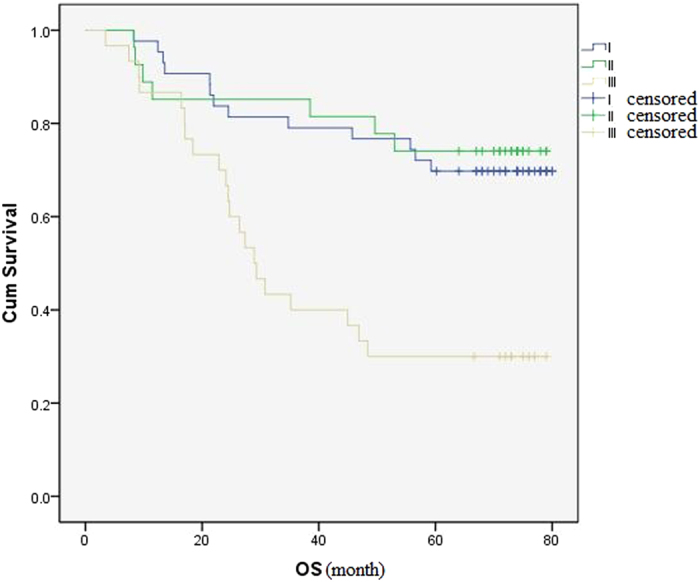
The overall survival rate of patients with stage I, II, III was 69.8%, 74.1%, 30.0% respectively. The differences between three groups were statistical significantly (P < 0.001).
Figure 3. The overall survival rate of patients with/without regional lymph node metastasis.
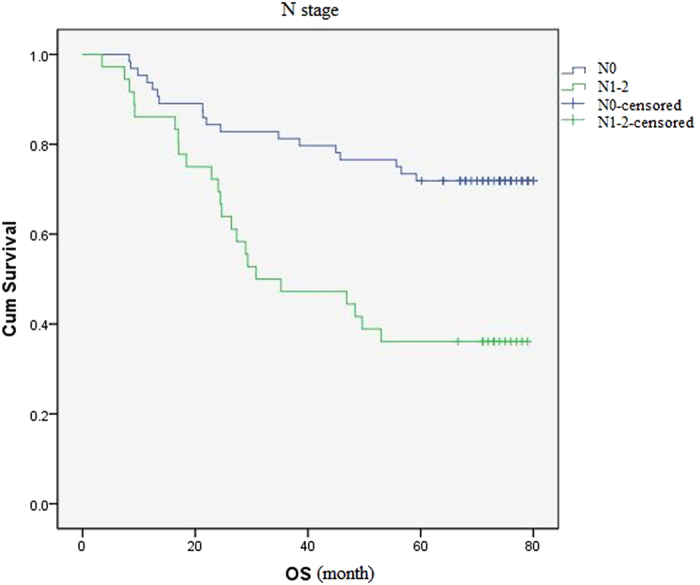
The overall survival rate of patients without regional lymph node metastasis was (71.9%), and significantly higher than patients had regional lymph node metastasis (P < 0.001).
Patients were divided into two groups according to the plasma level of HIF-1α: the cut-off value was set at the median (297.70 pg/ml), in group I the plasma level of HIF-1α was ≤297.70/ml (Low group), and in group II was >297.70/ml (High group). The overall survival rate of patients with squamous cell carcinomas in low group and high group was 78.6%, 50% respectively. The difference among the two groups was statistical significantly (P = 0.028) (Fig. 4).
Figure 4. The overall survival rate of patients with squamous cell carcinomas with different plasma levels of HIF-1α.
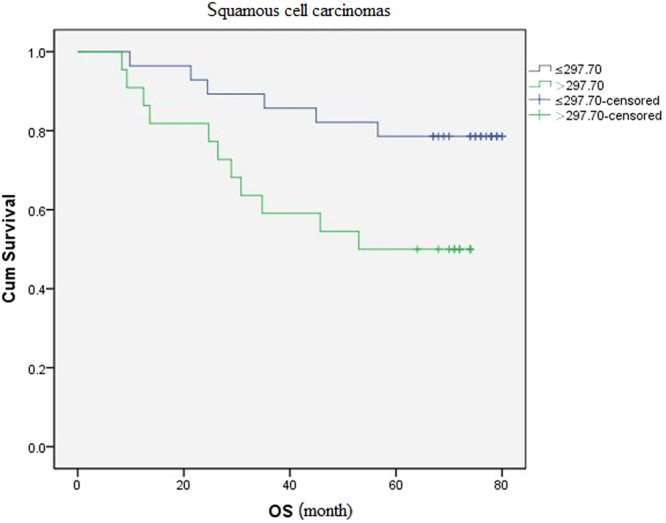
The difference between the two groups was statistical significantly (P = 0.028).
We further analyzed the relationship between the plasma HIF-1α and OS of patients in the same pTNM stage. Results showed that there was no statistical significance between both of them. To make the results more intuitive, we presented the results by a dot in a scatter plot (Figs 5, 6, 7). As we can see in the Figures, there was no statistical significance between both of them, but there were some trends in the mass. Patients with longer survival period were enriched in the lower half of the scatter plots. Therefore, if the sample size could be expanded in further studies, we may get results with statistical significance.
Figure 5. The scatter plot of OS and the plasma level of HIF-1α in patients (stage I).
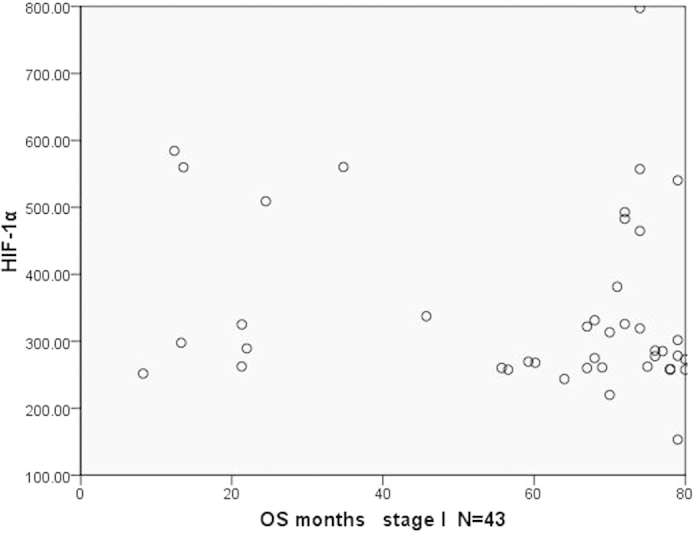
Figure 6. The scatter plot of OS and the plasma level of HIF-1α in patients (stage II).
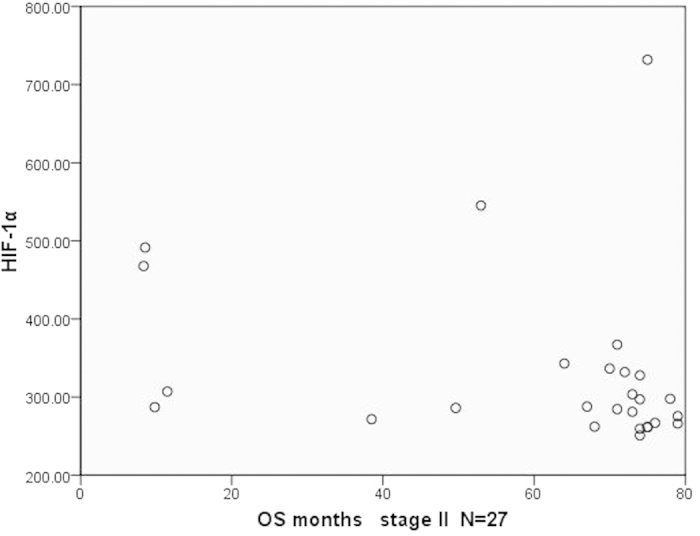
Figure 7. The scatter plot of OS and the plasma levels of HIF-1α in patients (stage III).
Analysis of Prognostic Factors in NSCLC patients
By the end of this study, in 100 cases patients, 59% patients were survival. The median survival time of dead patients was 69.1 months (Range: 3.4~79.7months). The multivariate analysis of survival related factors was statistically analyzed by using Cox proportional hazard model. The results demonstrated that: a) Analysis of all patients’ independent prognostic factors. We showed that pTNM stage was a influential prognostic factor for overall survival of patients with NSCLC (HR: 1.895, 95% CI: 1.296–2.771, P = 0.001). The rest of clinical and pathological features were all not (P > 0.05). b) Analysis of independent prognostic factors in all patients with squamous cell carcinomas. pTNM stage (P = 0.002) and the plasma level of HIF-1α (P = 0.028) were independent prognostic factors of patients with squamous cell carcinomas. c) Analysis of independent prognostic factors in all patients with adenocarcinoma. The results had no statistical significance.
Discussion
Because of the HIF-1α is related with hypoxia, this index has been widely used in the assessment of tumor hypoxia condition. But there is no unified and effective method to evaluate the activity level of HIF-1α within the tumor cells. Currently, immunohistochemistry (IHC) is the main research method whichwas used in the evaluation of HIF-1α levels in tumor patients. In addition, western bltting and reverse transcriptase polymerase chain reaction (RT-PCR) are also used in some researches. It has been reported that, for patients with psoriasis and require dialysis treatment, their angiogenesis and hypoxia condition can be evaluated by detecting the HIF-1α level within blood34,35. Additionally, there are limited prospective studies to evaluate the effect of breast cancer treatment by monitoring the HIF-1α level within the blood36. In China, some researches were reported that the treatment curative effect can be reflected by detecting the dynamic change of the HIF-1α level in patients with NSCLC and liver cancer before and after the interventional therapy38,40. But at present, reports about monitoring the level of HIF-1α within blood in patients with cancer are still rare.
Due to the imbalance of oxygen supply and oxygen consumption in the process of tumor growth, some hypoxic regions will subsequently appear, and further promote the rapid growth of cancer cells and the abnormal angiogenesis within tumors. Hypoxia is a common characteristic of solid tumors including NSCLC. Even in some tumor cells of early stage cancers, the condition of hypoxia is still evident. Many studies have since shown that the degree of hypoxia is related to insensitivity to chemotherapy and radiotherapy for patients with NSCLC, hihger invasive ability and a poor prognosis.
HIF-1α is the most crucial transcriptive factor that regulates diverse biological functions of a series of target genes such as proliferation, metastasis, invasion, apoptosis, and angiogenesis. Under the hypoxia environment, HIF-1α is activated in tumor cell, thus regulating the expression of hundreds of downstream target genes, thus promoting the activation of a large amount of protein factors, which participate in both tumor neovascularization and metastasis. It has been reported to be an important predictor of tumor progression for some types of solid tumors.
The aim of this study was to detect and analyse the plasma level of HIF-1α in patients with NSCLC, thus finding the relationships between the plasma level of HIF-1α and clinicopathological features. The survival data of postoperative patients with NSCLC was follow-up and collected. Correlation analysis of survival data, the plasma level of HIF-1α and clinicopathological features were analysed, and the results showed that the plasma level of HIF-1α was not an independent prognostic factor for patients with NSCLC. The pTNM stage was an independent prognostic factor for patients with NSCLC.
Currently, research on the level of HIF-1α of tumor patients is reported less. Some researches demonstrated that the plasma level of HIF-1α in patients with breast and liver cancer was significantly higher than normal person37,38. Dongping Xia et al. reported that the plasma level of HIF-1α in patients with NSCLC was significantly higher than healthy controls39. In this study, the plasma level of HIF-1α in patients with NSCLC was significantly higher than healthy volunteers’ (P = 0.026), and was consistent with the results of previous researches. But the results about the relationship between the plasma level of HIF-1α and tumor patients have not been reported yet. This research also didn’t find a significant relationship between the plasma level of HIF-1α and clinicopathological features of patients with NSCLC. The current researches and this study all found that the plasma level of HIF-1α of patients with NSCLC was higher than normal people’s. The reason might be tumor tissues with HIF-1α protein high expression appeared tissue necrosis, which resulted in a huge amount of HIF-1α entering the bloodstream, or there was a special regulation mechanism in hematological system itself of patients with NSCLC.
There are no reports about the plasma level of HIF-1α related to prognosis of patients with tumors at present. The correlation between both was first reported in this study. Although in the analysis of prognostic factors, the plasma level of HIF-1α was not a prognostic factor of patients with NSCLC, and was associated with the 5-year survival rate of patients with squamous cell carcinomas. In this study, the 5-year survival rate of patients with squamous cell carcinomas which their plasma level of HIF-1α lower than median was 78.6%, and much greater than the 5-year survival rate of those which the plasma level of HIF-1α higher than median (50.0%) (P = 0.034). Therefore, the plasma level of HIF-1α still can predict the prognosis of patients with NSCLC to some extent. In stratified analysis, we found that the plasma level of HIF-1α of patients with squamous cell carcinomas was associated with regional lymph node status.
In addition, there was a significant difference between different genders in the 3-year survival rate of patients with NSCLC and no statistically significant difference in 1-year survival rate of patients with different pTNM stages in this research. The reason may be related to the less number of cases and shorter follow-up time.
The results of this study demonstrated that HIF-1α may play crucial roles in some important processes of tumors, for example the growth progress and metastasis of the squamous cell carcinomas, or tumor angiogenesis, the plasma level of HIF-1α has crucial significance for the prediction of the prognosis of patients with NSCLC, especially in squamous cell carcinomas. However, it is still unclear that if the plasma level of HIF-1α was tested by dynamic monitoring, whether the results can reflect the curative effect of NSCLC related treatment, whether the plasma level of HIF-1α can reflect the condition of the patients’ disease. Previous studies had shown that many existing drugs, such as topoisomerase inhibitors-topotecan and etoposide had inhibition against HIF-1α40. It is still not clear that whether there will be a better curative effect when these drugs used in the patients of HIF-1α high expression. In addition, curative effects still need to be wait for the evaluation of the testing stage of new HIF-1α inhibitors25,26. Carry out more and further research on HIF-1α related maybe can find better HIF-1α target inhibitors and more appropriate patient group.
This study was designed to detect the plasma level of HIF-1α of 100 cases patients with NSCLC and the survival data was analyzed through follow-up. The results showed that the plasma level of HIF-1α of patients with NSCLC was significantly increased more than healthy people’s. The influencing factor of 3- and 5-year postoperative survival rate of patients with NSCLC was pTNM stage. No associations were observed between the plasma level of HIF-1α and the postoperative survival rate of patients, but the plasma level of HIF-1α was associated with the postoperative survival rate of patients with squamous cell carcinomas. The results of this study suggested that the plasma level of HIF-1α maybe provide a possible direction for further research and test, such as research on new therapeutic target of drugs based on HIF and the determination of drug-sensitive tumor types for HIF-1α inhibitors.
Methods
In this study, in order to provide valuable reference for the research in the future, we detected the plasma level of HIF-1α and analyzed the relationship between the clinical pathological features of patients with NSCLC and the plasma level of HIF-1α.
Ethics Statement
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute. Written informed consent was obtained from each patient and healthy volunteer, and the acquisition of blood samples was carried out as prescribed by the institutional guidelines. The method described in this study was carried out in accordance with the approved guidelines and regulations.
Patients and Healthy volunteers
In this study, patients who underwent pulmonary neoplasms resection (lobectomy or penumonecromy) in the thoracic surgery of Beijing Chest Hospital, Capital Medical University were recruited from May 2007 to August 2009. At last, a total of 100 patients were included in the study, the results of histopathological examination and different differentiation grades were based on the classification system of the World Health Organization revised in 2004 and the TNM staging system of UICC 2009 (Version 7). At the same time, 60 cases of healthy volunteers were recruited. The clinical characteristics of the 100 patients and 60 cases of healthy volunteers were presented in Table 1. The enter criterion of this study was presented in Table 7.
Table 7. The enter criterion of this study.
| Research object | Healthy volunteer |
|---|---|
| 1. Stage Ia-IIIa NSCLC patients diagnosed by cytology or pathology preoperatively; or confirmed by surgery pathology | 1. Over the age of 18 |
| 2. Preoperative routine examination and functional evaluation are in line with the operation indication | 2. No diseases of heart, liver, lungs and kidneys and other vital organs. |
| 3. The surgery method is radical resection of the primary lung tumor | 3. The collection time of female can’t in menstrual period and pregnancy period. |
| 4. Patients did not receive preoperative chemotherapy, radiotherapy, tumor targeted therapy, biological treatment and any other tumor-related treatment | |
| 5. No injuries and other surgery within the last two months | |
| 6. No history of other cancers | |
| 7. No diseases of heart, liver, lungs and kidneys and other vital organs |
The preoperative blood samples were collected from all the patients enrolled in this study, for the detection of plasma level of HIF-1α. At the same time, the blood samples of 60 cases of healthy volunteers were collected and detected too.
Collecting and dealing with the samples: 5 ml of fasting blood sample were drawn from antecubital vein of every patient with NSCLC in the morning 1–2 days before operation. The blood samples were put into the EDTA anticoagulative tube, then centrifuging ten minutes (1000 r/min) 2 hours after drawing blood, separated plasma, stored in −80 °C to be determined. Vein blood samples were collected with limosis from healthy volunteers at the same period, the same process method.
HIF-1α Enzyme-Linked Immunosorbent Assay (ELISA)
The concentration of HIF-1α in the NSCLC patients and healthy volunteers were determined using ELISA regent kits (SINO-AMERICAN BIOTECHNOLOGY CO. LTD. Luoyang, China) according to the manufacturer’s instructions and analyzed using a Labsytems Multiscan reader. The experiment was repeated twice with triplicate measurements in each experiment (measuring the optical density of each well at 450 nm wavelength within 5 minutes at the end of reaction).
Follow-up
All patients were followed-up by phone call consultation every 3 to 6 months after they were discharged from the hospital. Follow-up was completed in all patients until 2014.11.30 or the patient dead, and the median follow-up period was 68.5 months (range: 3.4~79.7months). During the period of follow-up, 1 case of patients was excluded because of dead within 3 months. In the rest of the 100 patients, all cases completed 5 years of follow-up. The follow-up time was censored if the patient was lost or dead during follow-up.
Statistical Analyses
All statistical analyses were examined using SPSS19.0 statistical software. Non-normal distribution was represented by the median (M). X2 test was used to analyze the relationship between two categorical variables. In this study, comparison between patients and healthy volunteers; correlation between the clinical characteristics of NSCLC patients; comparison between the plasma level of HIF-1α related to the N stage of patients with squamous cancer; the single-factor analysis of the 1-, 3- and 5-year postoperative survival rate for patients; the univariate analyses of 5-year survival rate of adenocarcinoma and squamous carcinoma patients were all analysed by using X2 test. When the sample size is less than 5, Fisher’s exact test was used.
The differences between two measuring parameters were analyzed by Mann-Whitney U test. In this study, the relationship between the clinical features and plasma level of HIF-1α, age of patients, comparison of the plasma level of HIF-1α between patients and healthy volunteers were analyzed by Mann-Whitney U test. Kruskal-Wallis H test was used between three or more measuring parameters.
Overall survival was assessed by the Kaplan–Meier method, while log rank test was used for comparison. Prognostic factors of OS were analysed by univariate and multivariate analysis. Variables with P < 0.05 and potential clinical confounding effects (e.g. age, sex and stage) were added to the final multivariate Cox proportional hazard model. Survival curves were drawn using the Kaplan-Meier method and compared by the log-rank test.
The comparison diagram of the plasma level of HIF-1α of NSCLC patients and healthy volunteers; comparison charts of the plasma level of HIF-1α associated with squamous carcinoma N stage were drawn by Graphpad Prism made 5.
Additional Information
How to cite this article: He, J. et al. The relationship between the preoperative plasma level of HIF-1α and clinic pathological features, prognosis in non-small cell lung cancer. Sci. Rep. 6, 20586; doi: 10.1038/srep20586 (2016).
Acknowledgments
The authors take full responsibility for the contents and expertise of this article. We thank Prof Baolan Li from Beijing Chest Hospital for discussions and editorial support.
Footnotes
Author Contributions J.H., Y.H., M.H., S.Z. and B.L. experimental design, experimental manipulation, data collection, statistical analysis and manuscript preparation. J.H., M.H. and S.Z. manuscript editing. B.L. approval of the final version of the manuscript.
References
- Torre L. A. et al. Global cancer statistics. CA: a cancer journal for clinicians 65, 87–108 (2015). [DOI] [PubMed]
- Chen W. et al. Lung cancer incidence and mortality in China. Thoracic Cancer 4, 102–108 (2013). [DOI] [PubMed] [Google Scholar]
- Williams B. A. et al. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann Thorac Surg 81, 1021–1027 (2006). [DOI] [PubMed] [Google Scholar]
- Semenza G. L. & Wang G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12, 5447–5454 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragonés J., Fraisl P., Baes M. & Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab 9, 11–22 (2009). [DOI] [PubMed] [Google Scholar]
- Kaelin W. G. & Ratcliffe P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30, 393–402 (2008). [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C., Nizet V. & Johnson R. S. Role of the hypoxia inducible factors HIF in iron metabolism. Cell cycle 7, 28–32 (2008). [DOI] [PubMed] [Google Scholar]
- Scagliotti G. V. et al. The role of histology with common first-line regimens for advanced non-small cell lung cancer: a brief report of the retrospective analysis of a three-arm randomized trial. J Thorac Oncol 4, 1568–1571 (2009). [DOI] [PubMed] [Google Scholar]
- Thomas S. et al. CD24 Is an Effector of HIF-1–Driven Primary Tumor Growth and Metastasis. Cancer Research 72, 5600–5612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren I. K. & Tavassoli A. Targeting tumour angiogenesis with small molecule inhibitors of hypoxia inducible factor. Chem Soc Rev 40, 4307–4317 (2011). [DOI] [PubMed] [Google Scholar]
- Harpole D. H., Richards W. G., Herndon J. E. & Suqarbaker D. J. Angiogenesis and molecular biologic substaging in patients with stage I non—small cell lung cancer. Ann Thorac Surg 61, 1470–1476 (1996). [DOI] [PubMed] [Google Scholar]
- Lu X. & Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16, 5928–5935 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike T. et al. Suppression of HIF-1α expression and radiation resistance in acute hypoxic conditions. Exp Ther Med 3, 141–145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell P. H. The HIF pathway in cancer. Semin cell dev biol. Academic Press, 16, 523–530 (2005). [DOI] [PubMed] [Google Scholar]
- Harris A. L. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2, 38–47 (2002). [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell 5, 405–406 (2004). [DOI] [PubMed] [Google Scholar]
- Semenza G. L. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20, 51–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin E. B. & Giaccia A. J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15, 678–685 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. et al. Hypoxia inducible factor-1 influences sensitivity to paclitaxel of human lung cancer cell lines under normoxic conditions. Cancer Sci 98, 1394–1401 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie F. F. et al. Effect of Recombinant Endostatin in Combination with Radiotherapy on the Expression of TGF-β1 and HIF-1α in Lung Adenocarcinoma Cell A549*. Asian Case Reports in Oncology 1, 5–10 (2012). [Google Scholar]
- Matthews N. E., Adams M. A., Maxwell L. R., Gofton T. E. & Graham C. H. Nitric oxide-mediated regulation of chemosensitivity in cancer cells. J Natl Cancer Inst 93, 1879–1885 (2001). [DOI] [PubMed] [Google Scholar]
- Reinmuth N. et al. Randomized, double-blind phase II study to compare nitroglycerin plus oral vinorelbine plus cisplatin with oral vinorelbine plus cisplatin alone in patients with stage IIIB/IV non-small cell lung cancer (NSCLC). Lung Cancer 83, 363–368 (2014). [DOI] [PubMed] [Google Scholar]
- Yasuda H. et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non–small-cell lung cancer. J Clin Oncol 24, 688–694 (2006). [DOI] [PubMed] [Google Scholar]
- Miyake K. et al. The novel hypoxic cytotoxin, TX-2098 has antitumor effect in pancreatic cancer; possible mechanism through inhibiting VEGF and hypoxia inducible factor-1α targeted gene expression. Exp Cell Res 318, 1554–1563 (2012). [DOI] [PubMed] [Google Scholar]
- Lee K. & Kim H. M. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharm Res 34, 1583–1585 (2011). [DOI] [PubMed] [Google Scholar]
- Rapisarda A. & Melillo G. UVC inhibits HIF-1α protein translation by a DNA damage-and topoisomerase I-independent pathway. Oncogene 26, 6875–6884 (2007). [DOI] [PubMed] [Google Scholar]
- Choi Y. J. et al. HIF-1α modulation by topoisomerase inhibitors in non-small cell lung cancer cell lines. J Cancer Res Clin 135, 1047–1053 (2009). [DOI] [PubMed] [Google Scholar]
- Rapisarda A. et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther 8, 1867–1877 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. et al. Endostar down-regulates HIF-1 and VEGF expression and enhances the radioresponse to human lung adenocarcinoma cancer cells. Mol Biol Rep 39, 89–95 (2012). [DOI] [PubMed] [Google Scholar]
- Lu X. & Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16, 5928–5935 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci 33, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29, 625–634 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J. J., Yang M. H., Hsu H. S., Hsu W. H., Liu J. S. & Wu K. J. Prognostic significance of hypoxia-inducible factor-1α, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 64, 1082–1089 (2009). [DOI] [PubMed] [Google Scholar]
- Hamed E. A., El-Abaseri T. B., Mohamed A. O., Ahmed A. R. & EI-Metwally T. H. Hypoxia and oxidative stress markers in pediatric patients undergoing hemodialysis: cross section study. BMC nephrology 13, 136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilopoulos Y. et al. High serum levels of HIF-1α in psoriatic patients correlate with an over-expression of IL-6. Cytokine 62, 38–39 (2013). [DOI] [PubMed] [Google Scholar]
- Ece H., Cigdem E., Yuksel K., Ahmet D., Hakan E. & Oktay T. M. Use of oral antidiabetic drugs (metformin and pioglitazone) in diabetic patients with breast cancer: how does it effect serum Hif-1 alpha and 8Ohdg levels? Asian Pac J Cancer Prev 13, 5143–5148 (2012). [DOI] [PubMed] [Google Scholar]
- Jia Z. Z., Jiang G. & Feng Y. Serum HIF-1α and VEGF levels pre-and post-TACE in patients with primary liver cancer. Chinese Med J-Peking 26, 158–162 (2011). [DOI] [PubMed] [Google Scholar]
- Ece H., Cigdem E., Yuksel K., Ahmet D., Hakan E. & Oktay T. M. Use of oral antidiabetic drugs (metformin and pioglitazone) in diabetic patients with breast cancer: how does it effect serum Hif-1 alpha and 8Ohdg levels? Asian Pac J Cancer Prev 13, 5143–5148 (2012). [DOI] [PubMed] [Google Scholar]
- Xia D. P., Zeng Y. L. & Song S. L. Clinical study of serum HIF-1α and VEGF dynamic and correlation before and after interventional therapy for patients with non-small cell lung cancer. Modern Oncology 20, 1201–1203 (2012). [Google Scholar]
- Choi Y. J. et al. HIF-1α modulation by topoisomerase inhibitors in non-small cell lung cancer cell lines. J Cancer Res Clin 135, 1047–1053 (2009). [DOI] [PubMed] [Google Scholar]


