Abstract
Background Information
Dysregulated micro‐RNAs have been reported in many human cancers, including renal cell carcinoma. Recent studies indicated that miR‐490 is involved in tumour development and progression. However, the expression profile and function in renal cell carcinoma remains unknown.
Results
Herein, we showed that miR‐490‐5p was down‐regulated in renal cell carcinoma tissues and cells compared with the adjacent normal tissues and normal cells. We also provided evidence that miR‐490‐5p acts as a tumour suppressor in renal carcinoma in a variety of in vitro and in vivo assays. Mechanistically, miR‐490‐5p was verified to directly bind to 3′ UTR of the PIK3CA mRNA and reduce the expression of PIK3CA at both mRNA and protein levels, which further inhibits phosphatidylinositol 3‐kinase/Akt signalling pathway. We further showed that knockdown of PIK3CA can block the growth inhibitory effect of miR‐490‐5p, and over‐expression of PIK3CA can reverse the inhibitory effect of miR‐490‐5p on renal cancer cell tumourigenicity.
Conclusions
Taken together, our results indicated for the first time that miR‐490‐5p functions as a tumour suppressor in renal carcinoma by targeting PIK3CA.
Significance
Our findings suggest that miR‐490‐5p may be a potential gene therapy target for the treatment of renal cell carcinoma.
Keywords: miR‐490‐5p, PIK3CA, Renal cell carcinoma, Tumour suppressor
Abbreviations used
- ccRCCs
clear cell renal cell carcinomas
- HRP
horseradish peroxidase
- miRNAs
micro‐RNAs
- PI3K
phosphatidylinositol 3‐kinase
- RCC
renal cell carcinoma
- WT
wild‐type
Introduction
Renal cell carcinoma (RCC) is a common urologic tumour and accounts for about 143,000 deaths in 2012 (Frew and Moch, 2014). The most common RCC is clear cell renal cell carcinomas (ccRCCs) which accounts for about 75–80% of RCC (Dey et al., 2012; Sato et al., 2013). The initiation and progression of ccRCC is a complicated process involving many gene alterations, such as loss of chromosome 3p (harbours VHL, PBRM1, BAP1 and SETD2) (Young and Simon, 2012; Sato et al., 2013), the chromosomal amplification of p62 (Li et al., 2013a), the deletion of HIF1α (Shen et al., 2011) and other forms of gene dysregulation (Purdue et al., 2011; Sato et al., 2013; Gerlinger et al., 2014). Therefore, understanding the precise molecular mechanisms involved in the pathogenesis and the identification of new biomarkers of ccRCC will be critical to explore new potential strategies for early diagnosis and therapy. Currently, increasing evidence indicates that deregulation of micro‐RNAs (miRNAs) affects cell growth and development of cancer.
miRNAs are small (∼22 nt) non‐coding RNAs and involved in the regulation of gene expression primarily by promoting mRNA degradation and/or translational inhibition (Bartel, 2004; Zhang et al., 2013). Each miRNA has tens or hundreds of target mRNAs which in turn mediate miRNA's function (He and Hannon, 2004). Therefore, miRNAs can act as oncogenes or as tumour suppressor genes during tumour development and progression. The deregulation of miRNA has frequently been found in many cancers, and multiple miRNAs expression panels can classify tumour type and stage. Mounting studies have demonstrated that altered patterns of miRNA expression are involved in ccRCC (Jung et al., 2009; Petillo et al., 2009; Juan et al., 2010; Weng et al., 2010; Zhou et al., 2010; White et al., 2011; Youssef et al., 2011; Osanto et al., 2012; Wach et al., 2013). Furthermore, many dysregulated miRNAs were reported to be functionally involved in ccRCC (Chow et al., 2010; Liu et al., 2010; Wu et al., 2012; Khella et al., 2013). For instance, our previous studies found that miR‐34a suppresses renal cancer cell proliferation and metastasis by targeting CD44 (Yu et al., 2014). Ma et al. (2015) found that miR‐185 inhibits renal cancer cell proliferation by targeting VEGFA directly.
In the present study, we found that miR‐490‐5p expression was significantly down‐regulated in ccRCC tissues and cell lines. Cell proliferation, colony formation and cell migration of ACHN and 786‐O cells are inhibited by over‐expression of miR‐490‐5p in vitro. Mechanistically, we pinpointed miR‐490‐5p's role as a tumour suppressor by inhibiting the phosphatidylinositol 3‐kinase (PI3K)/Akt signalling pathway. To our knowledge, our study is the first to document the role of miR‐490‐5p in ccRCC.
Results
miR‐490‐5p is down‐regulated in ccRCC tissues and cells
Several reports indicated that miR‐490‐5p and ‐3p is de‐regulated in many human cancers. However, the expression and function of miR‐490 in ccRCCs are still unclear. To this end, miR‐490‐5p and ‐3p expression levels were first evaluated in 27 pairs of ccRCC tissues and adjacent non‐tumour tissues. The real‐time PCR analysis showed that miR‐490‐5p was significantly down‐regulated in 96.3% (26/27) ccRCCs (Figure 1A), whereas expression of miR‐490‐3p has no significant difference between tumour and adjacent non‐tumour tissues (P = 0.26) (Figure 1B), which suggested a possible anti‐tumourigenic role of miR‐490‐5p in ccRCC. We next examined miR‐490‐5p expression in five human renal cancer cell lines (ACHN, 786‐O, OS‐RC‐2, Caki‐1 and SN12PM6) and control cell line HK2 (human kidney proximal tubular epithelial cell) by quantitative PCR. It revealed that all of the five renal cancer cell lines manifest as noticeably downregulation of miR‐490‐5p as compared with HK‐2 cells (Figure 1C).
Figure 1.
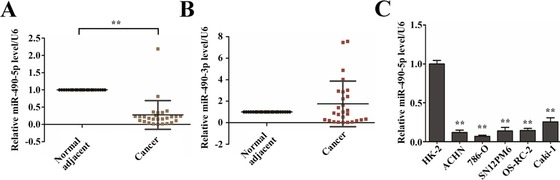
Downregulation of miR‐490‐5p expression in human renal cancer cell lines and tissues (A) Relative miR‐490‐5p expression in 27 ccRCCs and adjacent non‐tumour tissues. Each dot represents one sample. Total RNAs were extracted from ccRCCs and adjacent non‐tumour tissues, respectively, followed by quantitative PCR using stemloop RT methods, and miR‐490‐5p expression values were expressed as ratios with U6 snRNA (internal control). Statistical significance was evaluated by paired sample t test. (B) Relative miR‐490‐3p expression in 27 ccRCCs and adjacent non‐tumour tissues. miR‐490‐3p expression values were expressed as ratios with U6 snRNA. ** indicates significant differences, P < 0.01. (C) miR‐490‐5p expression was analysed in HK‐2, ACHN, 786‐O, SN12PM6, OS‐RC‐2 and Caki‐1 cells. Expression was normalised to HK‐2 cells (control). Data are plotted as the mean ± SD of three independent experiments. **P < 0.01 versus Ctrl.
miR‐490‐5p directly targets 3′ UTR of PIK3CA
To explore the molecular mechanism responsible for the function of miR‐490‐5p in ccRCC, we used TargetScan and miRanda to predict the targets of miR‐490‐5p. Among all these predicted targets, PIK3CA as a previously reported protein playing critical roles in cell growth, cell apoptosis and tumourigenicity was chosen as one of our candidates (Figure 2A). Next, we cloned the wild‐type (WT) or mutant 3′ UTR into the psiCheck‐2 plasmid (a luciferase reporter vector) and performed luciferase reporter assays (Figure 2B). The luciferase reporter assays unveiled that the overexpression of miR‐490‐5p decreased the luciferase reporter activity controlled by the PIK3CA WT 3′ UTR. However, this inhibitory effect can be abrogated as proved in the luciferase reporter vector containing PIK3CA mutant 3′ UTR (Figure 2C). Consistently, real‐time PCR assays and western blot showed that both the mRNA and protein levels of PIK3CA strikingly decreased by the over‐expression of miR‐490‐5p or miR‐490‐5p mimics in ACHN cells (Figures 2D and 2E). Together, these results indicated that PIK3CA was a direct target of miR‐490‐5p in ACHN cells.
Figure 2.
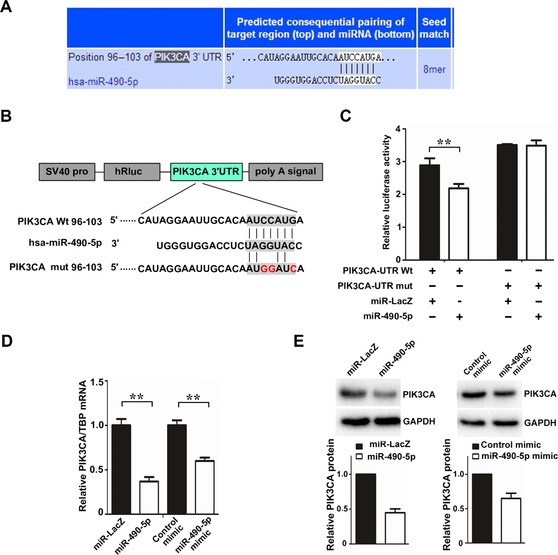
miR‐490‐5p down‐regulates the expression of PIK3CA (A) TargetScan was performed to predict the potential binding sites of miR‐490‐5p in 3′ UTRs of PIK3CA gene. (B) The binding sites for miR‐490‐5p in the PIK3CA 3′ UTR and several mutated nucleotides within the binding sites are shown. The WT and mutated 3′ UTR sequences (338 bp) were cloned into the psiCHECK2 vector. (C) Relative luciferase activity of ACHN cells transfected with plasmids carrying WT or mutated 3′ UTR of PIK3CA gene and miR‐490‐5p. After transfection for 36 h, luciferase activities were measured and normalised with internal control. **P < 0.01 versus Ctrl. (D, E) The mRNA (D) and protein (E) levels of PIK3CA were examined by quantitative real‐time PCR and WB in ACHN cells stably over‐expressing miR‐490‐5p/miR‐LacZ control and transiently transfected with miR‐490‐5p mimic/mimic control, respectively.
miR‐490‐5p inhibits cell growth, migration and invasion in renal cancer
To further investigate the roles of miR‐490‐5p in the development of renal cancer, we overexpressed miR‐490‐5p in 786‐O and ACHN cells by lentiviral vector. Quantitative PCR analysis showed that the level of miR‐490‐5p increased by about 56‐fold and 47‐fold following infection by lentivirus in 786‐O and ACHN cells, respectively (Figure 3A). Next, cell viability and colony formation ability were evaluated. We found that over‐expression of miR‐490‐5p in 786‐O and ACHN cells significantly decreased cell viability and colony formation ability in vitro (Figures 3B and 3C). Significantly, over‐expression of flag‐PIK3CA in ccRCC cells increased cell growth and colony formation ability (Figures 3B and 3C), which is in contrary to the phenotypic alterations upon miR‐490‐5p over‐expression, suggesting that PIK3CA may be a downstream effector of miR‐490‐5p.
Figure 3.
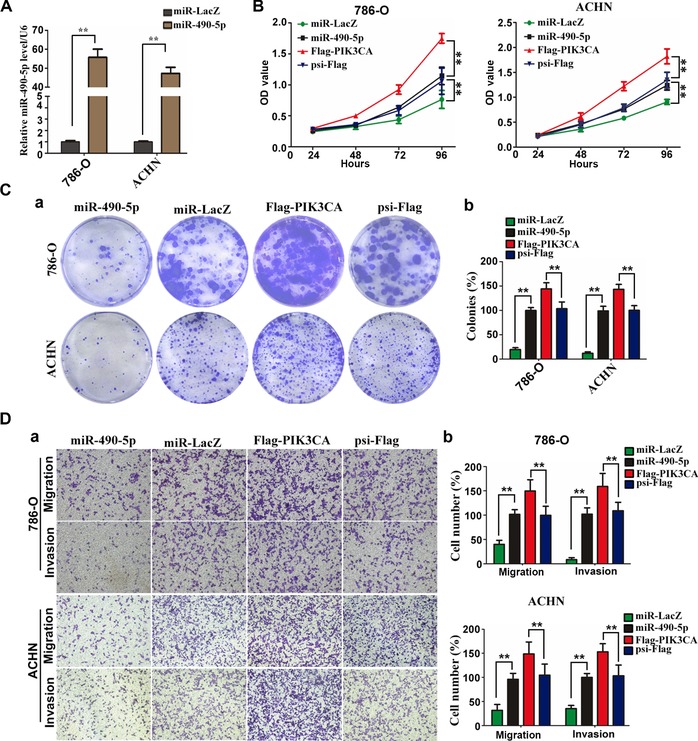
miR‐490‐5p inhibits cell growth, migration and invasion in renal cancer cells (A) miR‐490‐5p expression was analysed by real‐time PCR in 786‐O and ACHN cells transfected with miR‐490‐5p. (B) CCK‐8 kit was utilised to quantify cell viability after infection with lentivirus expressing miR‐490‐5p, miR‐LacZ, Flag‐PIK3CA or psi‐Flag at each time point. Data are plotted as the mean ± SD of three independent experiments. ** indicates significant differences, P < 0.01. (C) Cells were plated in 6‐well plate at a density of 1000 cells/well. The number of colonies was measured after 14 days. a Representative photographs of cell culture plates following staining for colony formation of ACHN and 786‐O cell. b Number of colonies was quantified (n = 3, **P < 0.01 vs. Ctrl.). (D) Trans‐well assays were utilised to analyse the migration and invasion of miR‐490‐5p, miR‐LacZ, psi‐Flag or Flag‐PIK3CA‐transfected stable 786‐O and ACHN cells. a Representative photographs were taken at ×200 magnification. b Number of migrated and invaded cells was quantified in four random images from each treatment group. Results are mean ± SD from three independent experiments plotted as percent (%) migrating and invading cells relative to miR‐LacZ expressing cells. ** indicates significant differences, P < 0.01.
Metastasis is one of the most dangerous properties of most cancers, and migration and invasion are two essential factors for metastasis (Zhang et al., 2013). Consistent with previous reports (Vivanco and Sawyers, 2002), over‐expression of PIK3CA in 786‐O and ACHN cells promoted cell migration and invasion capability in our study (Figure 3D). We therefore explored whether miR‐490‐5p regulates the migration and invasion of ccRCC lines. Also, as 786‐O and ACHN cells were infected with miR‐490‐5p or miR‐LacZ and allowed to migrate through a trans‐well membrane into complete media, we found that miR‐490‐5p can lead to 60 and 68.4% reduction in migratory potential for 786‐O and ACHN, respectively (Figure 3D). Similarly, miR‐490‐5p overexpression also significantly reduced invasion capability by 91.6 and 65% in 786‐O and ACHN cells, respectively (Figure 3D). Taken together, these results suggest that miR‐490‐5p may act as a tumour suppressor and play an important role in ccRCC metastasis.
PI3K/Akt pathway is involved in the anti‐tumourigenic roles of miR‐490‐5p in ccRCC
Given that PIK3CA can positively regulate PI3K/Akt/mTORC1 activity, which can enhance cell viability and tumour formation, we sought to determine whether miR‐490‐5p regulates renal cancer cell proliferation and tumour formation via PI3K/Akt pathway. As expected, phosphorylation of AKT and p70S6K (an indicator of mTORC1 activity) was reduced in cells stably expressing sh‐PIK3CA or miR‐490‐5p, whereas the total AKT and p70S6K had no change (Figure 4A). In contrast, there was no significant change on the total and phosphorylation ERK in ACHN cells stably expressing sh‐PIK3CA or miR‐490‐5p, when compared with the corresponding control group (Figure 4A). On the basis of these data, we reasoned that miR‐490‐5p may regulate renal cancer cell viability and proliferation via PI3K/Akt pathway. We evaluated this possibility by co‐transfecting PIK3CA short hairpin RNA together with or without miR‐490‐5p into ACHN cells. Our data showed that PIK3CA knockdown and miR‐490‐5p over‐expression all significantly decreased cell viability in ACHN cells (Figure 4B). Simultaneous knockdown of PIK3CA and over‐expression of miR‐490‐5p also result in decreased cell viability, but the inhibitory effect is not additive (Figure 4B). Moreover, by infection of lentivirus expressing the PIK3CA gene, the PIK3CA expression was rescued in the stable miR‐490‐5p cell line; thus, the cell viability was partially reversed (Figure 4C). Compared with flag‐PIK3CA/miR‐490‐5p‐expressing cells, over‐expression of PIK3CA alone resulted in an obvious increase in cell viability (Figure 4C). These results suggest that PIK3CA is one of the important mediators for miR‐490‐5p's role in renal cancer cell viability and proliferation. To further characterise the in vivo effects of miR‐490‐5p on tumourigenicity, a BALB/c nude mouse orthotopic xenograft model was performed. We found that over‐expression of miR‐490‐5p inhibited in vivo tumour growth with statistical significance (Figure 4D). Furthermore, the inhibitory effect of miR‐490‐5p on renal cancer cell tumour formation was reversed by concomitant PIK3CA over‐expression (Figure 4D), suggesting that the effect of miR‐490‐5p on in vivo tumour formation is mediated, at least in part, through PIK3CA. Together, miR‐490‐5p inhibits ccRCC cell proliferation and tumour formation by inhibiting PI3K/Akt pathway.
Figure 4.
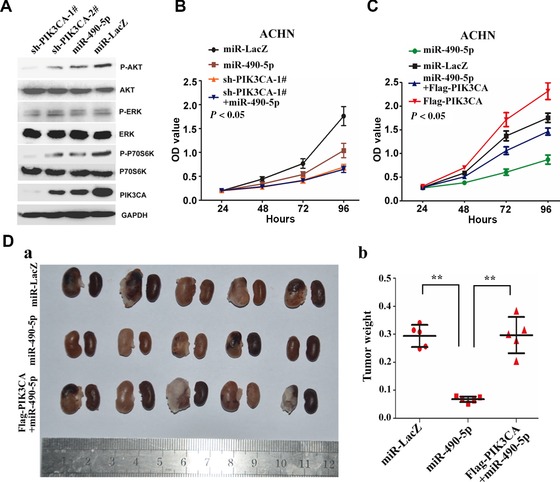
PI3K/Akt pathway is crucial for miR‐490‐5p‐inhibited cell growth and in vivo tumour formation (A) miR‐490‐5p expression inhibits PI3K‐Akt signalling. ACHN cells were infected with lentivirus expressing sh‐PIK3CA‐1#, sh‐PIK3CA‐1#, miR‐490‐5′ or miR‐LacZ. Immunoblot on lysates from cells expressing sh‐PIK3CA‐1#, sh‐PIK3CA‐1#, miR‐490‐5′ or miR‐LacZ was performed to evaluate the expression of PIK3CA, total and phosphorylated Akt, ERK and p70S6K protein levels. GAPDH is an internal control. (B, C) The cell viability of ACHN cells was determined by CCK8 assays after transfection of miR‐LacZ, miR‐490‐5p or/and sh‐PIK3CA‐1# (B), or/and Flag‐PIK3CA (C) by lentiviral vectors, at each time point. (D) ACHN cells were infected with lentivirus expressing miR‐LacZ, miR‐490‐5p or/and Flag‐PIK3CA to establish a stably expressing cell line. After infection for 96 h, the cells subjected to orthotopic xenograft assay according to Materials and Methods. a Representative images with primary tumours in the left and right kidney of nude mice after orthotopic injections of stably transfected cells. b Tumour weight is calculated according to the formula: weight of tumour = weight of kidney with tumour − weight of kidney without tumour (n = 5–8, **P < 0.01 vs. Ctrl.). The data represent means ± SDs.
Discussion
Previous reports have shown that miR‐490‐5p and ‐3p are deregulated in various cancers. miR‐490‐3p has shown to be down‐regulated in gastric cancer (Shen et al., 2015), lung cancer (Gu et al., 2014), ovarian epithelial carcinoma (Chen et al., 2015b), osteosarcoma cell lines (Liu et al., 2015), colon cancer and Ewing's sarcoma (Zhang et al., 2013), suggesting miR‐490‐3p could be a potential tumour suppressor. Zhang et al. (2013) found that miR‐490‐3p is up‐regulated in hepatocellular carcinoma tissues and cells and functions as an oncomiR in HCC. These suggest that miR‐490‐3p exerts either pro‐ or anti‐tumourigenic effects in a cell‐type and context‐dependent manner (Zhang et al., 2015). While in urological cancers, it is reported that the aberrant expression of miR‐490‐5p is related to cancer initiation, development and prognosis (Ambs et al., 2008). Also, miR‐490‐5p was found to be down‐regulated in bladder cancer tissue and cell lines, and its over‐expression in bladder cancer cells significantly inhibited the cell proliferation by targeting c‐Fos (Li et al., 2013b; Lan et al., 2015). Nevertheless, the expression and function of miR‐490‐5p and ‐3p in ccRCC remain elusive. In our research, the results proved that down‐regulation of miR‐490‐5p was a frequent event in ccRCCs, and this down‐regulation increased ccRCC cell viability, colony formation and migration and invasion abilities. However, expression of miR‐490‐3p was not consistently down‐regulated (P > 0.05). The inconsistency of miR‐490‐5p and ‐3p expression levels in renal cancer tissues and cells may be due to their different stability. Although miRNAs are globally stable, the post‐transcriptional modification of pre‐miRNAs or mature miRNAs affects miRNA stability (Kai and Pasquinelli, 2010; Ruegger and Grosshans, 2012). Recent studies have shown that target mRNA and Argonaute proteins can inhibit or promote miRNAs degradation. In addition, methylation, uridylation and adenylation of miRNAs may also regulate miRNA stability. Given that the expression of miR‐490‐5p and ‐3p in renal cancer tissue is not consistent, it is very interesting to examine the molecular mechanism of miR‐490‐5p and ‐3p modification and degradation.
Notably, we provide evidence that PIK3CA might act as a target of miR‐490‐5p. Over‐expression of miR‐490‐5p led to a significant reduction in PIK3CA mRNA and protein level, and further down‐regulated the phosphorylation of AKT and p70S6K. This process activates PI3K/Akt which is involved in many pathways, especially in tumourogenesis, such as cell proliferation, apoptosis, glucose metabolism and energy metabolism (Vivanco and Sawyers, 2002; Fresno Vara et al., 2004). PIK3CA, located on chromosome 3, encodes the p110α catalytic subunit of PI3K (Wang et al., 2014). Amplification and gain‐of‐function mutations in PIK3CA occur frequently in many human cancers, including ccRCC (Samuels et al., 2005; Wu et al., 2005; Sato et al., 2013). As a key regulator of PI3K signalling pathway, PIK3CA regulates the generation of phosphatidylinositol triphosphate which further activates Akt/PKB kinase (Lang and Ling, 2012). AKT kinase is the centre of several major cell signalling pathways, and its activation can trigger a cascade of responses including cell proliferation and survival by phosphorylating a variety of substrates (Vivanco and Sawyers, 2002). Consistently, our data demonstrated that miR‐490‐5p can inhibit PI3K/Akt pathway, and over‐expression of miR‐490‐5p inhibits cancer cell growth, migration and invasion in vitro and tumourigenicity in vivo. Furthermore, knockdown of PIK3CA can block the growth inhibitory effect of miR‐490‐5p, and over‐expression of PIK3CA can reverse the inhibitory effect of miR‐490‐5p on renal cancer cell proliferation and tumour formation in vivo, indicating that miR‐490‐5p will suppress tumour growth in RCC, at least partially, by targeting PIK3CA.
In summary, we have described a novel miR‐490‐5p/PIK3CA/Akt pathway, which may be potentially involved in tumourigenesis and progression of many cancers. Identification of the relationship between miR‐490‐5p and PI3K/Akt pathway would help us to have a better understanding of molecular mechanisms of cancerogenesis.
Materials and methods
Human samples
Tissue samples from clear cell RCC and the adjacent normal tissues were obtained from patients receiving radical nephrectomy in the Department of Urology at Tongji Hospital of Huazhong University of science and technology in China. The samples were obtained from the period of 2012–2014 with informed consent and Ethics Committee's approval. All the samples were kept in liquid nitrogen before RNA extraction.
Antibodies
Following antibodies were used in the experiments: anti‐p70S6K (#9202), antiphospho‐p70S6K (Thr389) (#9205), anti‐AKT (#9272), anti‐phospho‐AKT (Ser473) (#4058), anti‐ERK (#4695), anti‐phospho‐ERK (Thr202/Tyr204) (#9101), anti‐PIK3CA (#4254) from Cell Signaling Technology; anti‐GAPDH (CW0100) purchased from Beijing CWBio; goat anti‐mouse IgG horseradish peroxidase (HRP)‐linked whole antibody (SA1‐74039) and goat anti‐rabbit IgG HRP‐linked whole antibody (SA1‐9510) purchased from Pierce Company.
Plasmid constructs
miR‐LacZ was obtained from Invitrogen. miR‐490‐5p was synthesised and cloned into pLenti6.4‐MSGW/EmGFP‐miR containing EGFP (Invitrogen). miR‐490‐F and miR‐490‐R were mixed, annealed and then ligated into pLenti6.4‐MSGW/EmGFP‐miR. Flag‐PIK3CA recombinant was constructed by cloning the coding region of PIK3CA (3207 bp) into BamHI and XhoI sites of psi‐Flag‐C1 (from Rongjia zhou's laboratory). The PIK3CA cDNA was PCR amplified using primers PIK3CA‐F and PIK3CA‐R, digested by BamHI and XhoI and ligated into psi‐Flag‐C1 cut with BamHI and XhoI to create the Flag‐PIK3CA. The PIK3CA 3’UTR (338 bp) was amplified from human genomic DNA and cloned into the psiCHECK2 vector (Promega). Human genomic DNA was PCR amplified using primers PIK3CA‐UTR‐F and PIK3CA‐UTR‐R, digested by XhoI and NotI and ligated into psiCHECK2 cut with XhoI and NotI to create the psiCHECK2‐PIK3CA‐WT. The psiCHECK2‐PIK3CA‐Mut construct, which the miR‐490‐5p seed sequence in 3′ UTR of PIK3CA mRNA was partially substituted (5′‐ATGGATCA‐3′), was generated by a two‐step PCR‐based mutagenesis procedure using psiCHECK2‐PIK3CA‐WT as the template. First‐step PCR products were used to amplify two partially overlapping fragments (120 and 260 bp) by using primers PIK3CA‐UTR‐F plus PIK3CA‐mut‐R and PIK3CA‐mut‐F plus PIK3CA‐UTR‐R. Both fragments, after annealed, played the role of template for second‐step PCR. PIK3CA‐UTR‐F and PIK3CA‐UTR‐R, as the second‐step PCR primers, were used to amplify the mutant 3′ UTR of PIK3CA. The resulting mutant second‐step PCR product was cut by both XhoI and NotI and ligated into to psiCHECK2 to obtain psiCHECK2‐PIK3CA‐mut. The primers for making these constructs were as follows: miR‐490‐F, TGCTGCCATGGATCTCCAGGTGGGTGTTTTGGCCACTGACTGACACCCACCTAGATCCATGG; miR‐490‐R, CCTGCCATGGATCTAGGTGGGTGTCAGTCAGTGGCCAAAACACCCACCTGGAGATCCATGGC; PIK3CA‐F, CGCGGATCCATGCCTCCACGACCATCATC; PIK3CA‐R, CCGCTCGAGTCAGTTCAATGCATGCTGTTTAAT; PIK3CA‐UTR‐F, CCGCTCGAGAAAGATAACTGAGAAAATGAAAGCTCA; PIK3CA‐UTR‐R, AAAAGCGGCCGCTCCATCATTTCTATATATTTTGGGGAT; PIK3CA‐mut‐F, TGCATAGGAATTGCACAATGGATCAACAGCATTA; PIK3CA‐mut‐R, GTAAATTCTAATGCTGTTGATCCATTGTGCAATT.
RNA interference and mimics
Hsa‐miR‐490‐5p mimic and the corresponding mimic control oligo were purchased from RiboBio. Oligos corresponding to the target sequences were annealed and cloned into the HpaI and XhoI sites of the pSicoR‐Puro plasmid. The following target regions were chosen: PIK3CA‐1#, GCATTGACTAATCAAAGGA; PIK3CA‐2#, GACAAGAGAATTTGAGAGG.
RNA extraction and real‐time PCR
Total RNAs were extracted by trizol (Invitrogen) and cDNAs were synthetised using Rever Ace qPCR RT Kit (TOYOBO). Real‐time PCR was performed using SYBR Green Realtime PCR Master Mix (Roche) and the ABI ViiA7 QPCR System (Applied Biosystems). Amplification conditions were as follows: 95°C for 15 s, 60°C for 15 s, 72°C for 45 s for 40 cycles in a 25‐μl reaction mix containing 1 × SYBR green. The expression levels of miR‐490‐3p and miR‐490‐5p were quantified using stemloop RT according to the manufacturer's protocol (Roche). All reagents for stemloop RT were obtained from Roche and RiboBio. The U6 snRNA was used as an internal control. For miRNA and mRNA PCR, the reactions were incubated in a 96‐well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 20 s, 60°C for 20 s and 72°C for 1 min. The following primers were used for qPCR: PIK3CA‐5′, TGCTAAAGAGGAACACTGTCCA; PIK3CA‐3′, GGTACTGGCCAAAGATTCAAAG; TBP‐5′, TGCACAGGAGCCAAGAGTGAA; TBP‐3′, CACATCACAGCTCCCCACCA.
Cell culture and transfection
ACHN, 786‐O, SN12PM6, OS‐RC‐2, Caki‐1, HK‐2 and HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) added with 10% foetal bovine serum (HyClone) and incubated in a humidified incubator (Thermo Fisher Scientific) set as 5% CO2 and 37°C. All plasmids transfections were operated with LipofectamineTM2000 (Invitrogen).
Virus generation and infection, luciferase activity assays, colony formation and cell proliferation, cell migration and invasion assays
These experiments were performed as described in Chen et al. (2015a).
Xenograft orthotopic implantations
For in vivo studies, 1 × 106 ACHN cells stably expressing miR‐LacZ, miR‐490‐5p or/and flag‐PIK3CA were injected into the left kidney of male BALB/c nude mice at 4–5 weeks of age. Eight weeks after the implantation of the xenografts, animals were euthanised and xenografts were harvested, and assessed for tumour weight. The tumour weight was monitored by measuring the weight of left kidney (W L) and right kidney (W R) of the tumour by an analytical balance and was calculated using the formula W t = W L−W R. All experiments were approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
Statistical analysis
The data are presented as the mean ± SD. Differences among groups were determined by a two‐way ANOVA followed by a post hoc Tukey test. Comparisons between two groups were performed using an unpaired Student's t‐test. A value of P less than 0.05 was considered significant.
Conflict of interest
The authors have declared no conflict of interest.
Funding
This work was supported by National Natural Science Foundation of China (31072238, 31172441, 31372562, 81170650, 81402105, 81402087); National Major Scientific and Technological Special Project for Significant New Drugs Development (2012ZX09303018); China Postdoctoral Science Foundation (2014M552048); Natural Science Foundation of Hubei Province of China (2012FFB02412 and 2013CFB174). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Ambs, S. , Prueitt, R.L. , Yi, M. , Hudson, R.S. , Howe, T.M. , Petrocca, F. , Wallace, T.A. , Liu, C.‐G. , Volinia, S. , Calin, G.A. , Yfantis, H.G. , Stephens, R.M. and Croce, C.M. (2008) Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 68, 6162–6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- Chen, K. , Yu, G. , Gumireddy, K. , Li, A. , Yao, W. , Gao, L. , Chen, S. , Hao, J. , Wang, J. , Huang, Q. , Xu, H. and Ye, Z. (2015a) ZBRK1, a novel tumor suppressor, activates VHL gene transcription through formation of a complex with VHL and p300 in renal cancer. Oncotarget 6, 6959–6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Chen, X. , Xiu, Y.L. , Sun, K.X. and Zhao, Y. (2015b) MicroRNA‐490‐3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer Lett. 362, 122–130 [DOI] [PubMed] [Google Scholar]
- Chow, T.‐f.F. , Mankaruos, M. , Scorilas, A. , Youssef, Y. , Girgis, A. , Mossad, S. , Metias, S. , Rofael, Y. , Honey, R.J. , Stewart, R. , Pace, K.T. and Yousef, G.M. (2010) The miR‐17‐92 cluster is over expressed in and has an oncogenic effect on renal cell carcinoma. J. Urol. 183, 743–751 [DOI] [PubMed] [Google Scholar]
- Dey, A. , Seshasayee, D. , Noubade, R. , French, D.M. , Liu, J. , Chaurushiya, M.S. , Kirkpatrick, D.S. , Pham, V.C. , Lill, J.R. , Bakalarski, C.E. , Wu, J. , Phu, L. , Katavolos, P. , LaFave, L.M. , Abdel‐Wahab, O. , Modrusan, Z. , Seshagiri, S. , Dong, K. , Lin, Z. , Balazs, M. , Suriben, R. , Newton, K. , Hymowitz, S. , Garcia‐Manero, G. , Martin, F. , Levine, R.L. and Dixit, V.M. (2012) Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337, 1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno Vara, J.A. , Casado, E. , de Castro, J. , Cejas, P. , Belda‐Iniesta, C. and Gonzalez‐Baron, M. (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 30, 193–204. [DOI] [PubMed] [Google Scholar]
- Frew, I.J. and Moch, H. (2014) A clearer view of the molecular complexity of clear cell renal cell carcinoma. Annu. Rev. Pathol. 10, 263–289 [DOI] [PubMed] [Google Scholar]
- Gerlinger, M. , Horswell, S. , Larkin, J. , Rowan, A.J. , Salm, M.P. , Varela, I. , Fisher, R. , McGranahan, N. , Matthews, N. , Santos, C.R. , Martinez, P. , Phillimore, B. , Begum, S. , Rabinowitz, A. , Spencer‐Dene, B. , Gulati, S. , Bates, P.A. , Stamp, G. , Pickering, L. , Gore, M. , Nicol, D.L. , Hazell, S. , Futreal, P.A. , Stewart, A. and Swanton, C. (2014) Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 46, 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H. , Yang, T. , Fu, S. , Chen, X. , Guo, L. and Ni, Y. (2014) MicroRNA‐490‐3p inhibits proliferation of A549 lung cancer cells by targeting CCND1. Biochem. Biophys. Res. Commun. 444, 104–108 [DOI] [PubMed] [Google Scholar]
- He, L. and Hannon, G.J. (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 [DOI] [PubMed] [Google Scholar]
- Juan, D. , Alexe, G. , Antes, T. , Liu, H. , Madabhushi, A. , Delisi, C. , Ganesan, S. , Bhanot, G. and Liou, L.S. (2010) Identification of a microRNA panel for clear‐cell kidney cancer. Urology 75, 835–841 [DOI] [PubMed] [Google Scholar]
- Jung, M. , Mollenkopf, H.J. , Grimm, C. , Wagner, I. , Albrecht, M. , Waller, T. , Pilarsky, C. , Johannsen, M. , Stephan, C. , Lehrach, H. , Nietfeld, W. , Rudel, T. , Jung, K. and Kristiansen, G. (2009) MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J. Cell. Mol. Med. 13, 3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, Z.S. and Pasquinelli, A.E. (2010) MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat. Struct. Mol. Biol. 17, 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khella, H.W.Z. , Bakhet, M. , Allo, G. , Jewett, M.A.S. , Girgis, A.H. , Latif, A. , Girgis, H. , Von Both, I. , Bjarnason, G.A. and Yousef, G.M. (2013) miR‐192, miR‐194 and miR‐215: a convergent microRNA network suppressing tumor progression in renal cell carcinoma. Carcinogenesis 34, 2231–2239 [DOI] [PubMed] [Google Scholar]
- Lan, G. , Yang, L. , Xie, X. , Peng, L. and Wang, Y. (2015) MicroRNA‐490‐5p is a novel tumor suppressor targeting c‐FOS in human bladder cancer. Arch. Med. Sci. 11, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, Q. and Ling, C. (2012) MiR‐124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochem. Biophys. Res. Commun. 426, 247–252 [DOI] [PubMed] [Google Scholar]
- Li, L. , Shen, C. , Nakamura, E. , Ando, K. , Signoretti, S. , Beroukhim, R. , Cowley, G.S. , Lizotte, P. , Liberzon, E. , Bair, S. , Root, D.E. , Tamayo, P. , Tsherniak, A. , Cheng, S.‐C. , Tabak, B. , Jacobsen, A. , Hakimi, A.A. , Schultz, N. , Ciriello, G. , Sander, C. , Hsieh, J.J. and Kaelin, W.G., Jr. (2013a) SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell 24, 738–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Xu, X. , Xu, X. , Hu, Z. , Wu, J. , Zhu, Y. , Chen, H. , Mao, Y. , Lin, Y. , Luo, J. , Zheng, X. and Xie, L. (2013b) MicroRNA‐490‐5p inhibits proliferation of bladder cancer by targeting c‐Fos. Biochem. Biophys. Res. Commun. 441, 976–981 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Brannon, A.R. , Reddy, A.R. , Alexe, G. , Seiler, M.W. , Arreola, A. , Oza, J.H. , Yao, M. , Juan, D. , Liou, L.S. , Ganesan, S. , Levine, A.J. , Rathmell, W.K. and Bhanot, G.V. (2010) Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell renal cell carcinoma. BMC Syst. Biol. 4, 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Xu, G. , Liu, H. and Li, T. (2015) MicroRNA‐490‐3p regulates cell proliferation and apoptosis by targeting HMGA2 in osteosarcoma. FEBS Lett. 589, 3148–3153 [DOI] [PubMed] [Google Scholar]
- Ma, X. , Shen, D. , Li, H. , Zhang, Y. , Lv, X. , Huang, Q. , Gao, Y. , Li, X. , Gu, L. , Xiu, S. , Bao, X. , Duan, J. and Zhang, X. (2015) MicroRNA‐185 inhibits cell proliferation and induces cell apoptosis by targeting VEGFA directly in von Hippel–Lindau‐inactivated clear cell renal cell carcinoma. Urol. Oncol. 22, 169.e1–169.e11 [DOI] [PubMed] [Google Scholar]
- Osanto, S. , Qin, Y. , Buermans, H.P. , Berkers, J. , Lerut, E. , Goeman, J.J. and van Poppel, H. (2012) Genome‐wide microRNA expression analysis of clear cell renal cell carcinoma by next generation deep sequencing. PLoS One 7, e38298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petillo, D. , Kort, E.J. , Anema, J. , Furge, K.A. , Yang, X.J. and Teh, B.T. (2009) MicroRNA profiling of human kidney cancer subtypes. Int. J. Oncol. 35, 109–114 [DOI] [PubMed] [Google Scholar]
- Purdue, M.P. , Johansson, M. , Zelenika, D. , Toro, J.R. , Scelo, G. , Moore, L.E. , Prokhortchouk, E. , Wu, X. , Kiemeney, L.A. , Gaborieau, V. , Jacobs, K.B. , Chow, W.‐H. , Zaridze, D. , Matveev, V. , Lubinski, J. , Trubicka, J. , Szeszenia‐Dabrowska, N. , Lissowska, J. , Rudnai, P. , Fabianova, E. , Bucur, A. , Bencko, V. , Foretova, L. , Janout, V. , Boffetta, P. , Colt, J.S. , Davis, F.G. , Schwartz, K.L. , Banks, R.E. , Selby, P.J. , Harnden, P. , Berg, C.D. , Hsing, A.W. , Grubb, R.L. , Boeing, H. , Vineis, P. , Clavel‐Chapelon, F. , Palli, D. , Tumino, R. , Krogh, V. , Panico, S. , Duell, E.J. , Quiros, J.R. , Sanchez, M.‐J. , Navarro, C. , Ardanaz, E. , Dorronsoro, M. , Khaw, K.‐T. , Allen, N.E. , Bueno‐de‐Mesquita, H.B. , Peeters, P.H.M. , Trichopoulos, D. , Linseisen, J. , Ljungberg, B. , Overvad, K. , Tjonneland, A. , Romieu, I. , Riboli, E. , Mukeria, A. , Shangina, O. , Stevens, V.L. , Thun, M.J. , Diver, W.R. , Gapstur, S.M. , Pharoah, P.D. , Easton, D.F. , Albanes, D. , Weinstein, S.J. , Virtamo, J. , Vatten, L. , Hveem, K. , Njolstad, I. , Tell, G.S. , Stoltenberg, C. , Kumar, R. , Koppova, K. , Cussenot, O. , Benhamou, S. , Oosterwijk, E. , Vermeulen, S.H. , Aben, K.K.H. , van der Marel, S.L. , Ye, Y. , Wood, C.G. , Pu, X. , Mazur, A.M. , Boulygina, E.S. , Chekanov, N.N. , Foglio, M. , Lechner, D. , Gut, I. , Heath, S. , Blanche, H. , Hutchinson, A. , Thomas, G. , Wang, Z. , Yeager, M. , Fraumeni, J.F. , Skryabin, K.G. , McKay, J.D. , Rothman, N. , Chanock, S.J. , Lathrop, M. and Brennan, P. (2011) Genome‐wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat. Genet. 43, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger, S. and Grosshans, H. (2012) MicroRNA turnover: when, how, and why. Trends Biochem. Sci. 37, 436–446 [DOI] [PubMed] [Google Scholar]
- Samuels, Y. , Diaz Jr, L.A. , Schmidt‐Kittler, O. , Cummins, J.M. , DeLong, L. , Cheong, I. , Rago, C. , Huso, D.L. , Lengauer, C. , Kinzler, K.W. , Vogelstein, B. and Velculescu, V.E. (2005) Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Yoshizato, T. , Shiraishi, Y. , Maekawa, S. , Okuno, Y. , Kamura, T. , Shimamura, T. , Sato‐Otsubo, A. , Nagae, G. , Suzuki, H. , Nagata, Y. , Yoshida, K. , Kon, A. , Suzuki, Y. , Chiba, K. , Tanaka, H. , Niida, A. , Fujimoto, A. , Tsunoda, T. , Morikawa, T. , Maeda, D. , Kume, H. , Sugano, S. , Fukayama, M. , Aburatani, H. , Sanada, M. , Miyano, S. , Homma, Y. and Ogawa, S. (2013) Integrated molecular analysis of clear‐cell renal cell carcinoma. Nat. Genet. 45, 860–867 [DOI] [PubMed] [Google Scholar]
- Shen, C. , Beroukhim, R. , Schumacher, S.E. , Zhou, J. , Chang, M. , Signoretti, S. and Kaelin, W.G., Jr. (2011) Genetic and functional studies implicate HIF1alpha as a 14q kidney cancer suppressor gene. Cancer Discov. 1, 222–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Xiao, Z. , Wu, W.K. , Wang, M.H. , To, K.F. , Chen, Y. , Yang, W. , Li, M.S. , Shin, V.Y. , Tong, J.H. , Kang, W. , Zhang, L. , Li, M. , Wang, L. , Lu, L. , Chan, R.L. , Wong, S.H. , Yu, J. , Chan, M.T. , Chan, F.K. , Sung, J.J. , Cheng, A.S. and Cho, C.H. (2015) Epigenetic silencing of miR‐490‐3p reactivates the chromatin remodeler SMARCD1 to promote Helicobacter pylori‐induced gastric carcinogenesis. Cancer Res. 75, 754–765 [DOI] [PubMed] [Google Scholar]
- Vivanco, I. and Sawyers, C.L. (2002) The phosphatidylinositol 3‐Kinase‐AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- Wach, S. , Nolte, E. , Theil, A. , Stohr, C. , T Rau, T. , Hartmann, A. , Ekici, A. , Keck, B. , Taubert, H. and Wullich, B. (2013) MicroRNA profiles classify papillary renal cell carcinoma subtypes. Br. J. Cancer 109, 714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Tang, Q. , Li, M. , Jiang, S. and Wang, X. (2014) MicroRNA‐375 inhibits colorectal cancer growth by targeting PIK3CA. Biochem. Biophys. Res. Commun. 444, 199–204 [DOI] [PubMed] [Google Scholar]
- Weng, L. , Wu, X. , Gao, H. , Mu, B. , Li, X. , Wang, J.H. , Guo, C. , Jin, J.M. , Chen, Z. , Covarrubias, M. , Yuan, Y.C. , Weiss, L.M. and Wu, H. (2010) MicroRNA profiling of clear cell renal cell carcinoma by whole‐genome small RNA deep sequencing of paired frozen and formalin‐fixed, paraffin‐embedded tissue specimens. J. Pathol. 222, 41–51 [DOI] [PubMed] [Google Scholar]
- White, N.M.A. , Bao, T.T. , Grigull, J. , Youssef, Y.M. , Girgis, A. , Diamandis, M. , Fatoohi, E. , Metias, M. , Honey, R.J. , Stewart, R. , Pace, K.T. , Bjarnason, G.A. and Yousef, G.M. (2011) miRNA profiling for clear cell renal cell carcinoma: biomarker discovery and identification of potential controls and consequences of miRNA dysregulation. J. Urol. 186, 1077–1083 [DOI] [PubMed] [Google Scholar]
- Wu, G. , Xing, M. , Mambo, E. , Huang, X. , Liu, J. , Guo, Z. , Chatterjee, A. , Goldenberg, D. , Gollin, S.M. , Sukumar, S. , Trink, B. and Sidransky, D. (2005) Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 7, R609–R616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Weng, L. , Li, X. , Guo, C. , Pal, S.K. , Jin, J.M. , Li, Y. , Nelson, R.A. , Mu, B. , Onami, S.H. , Wu, J.J. , Ruel, N.H. , Wilczynski, S.P. , Gao, H. , Covarrubias, M. , Figlin, R.A. , Weiss, L.M. and Wu, H. (2012) Identification of a 4‐microRNA signature for clear cell renal cell carcinoma metastasis and prognosis. PLoS One 7, e35661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R.M. and Simon, M.C. (2012) Untuning the tumor metabolic machine: HIF‐alpha: pro‐ and antitumorigenic? Nat. Med. 18, 1024–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, Y.M. , White, N.M.A. , Grigull, J. , Krizova, A. , Samy, C. , Mejia‐Guerrero, S. , Evans, A. and Yousef, G.M. (2011) Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur. Urol. 59, 721–730 [DOI] [PubMed] [Google Scholar]
- Yu, G. , Li, H. , Wang, J. , Gumireddy, K. , Li, A. , Yao, W. , Tang, K. , Xiao, W. , Hu, J. , Xiao, H. , Lang, B. , Ye, Z. , Huang, Q. and Xu, H. (2014) miRNA‐34a suppresses cell proliferation and metastasis by targeting CD44 in human renal carcinoma cells. J. Urol. 192, 1229–1237 [DOI] [PubMed] [Google Scholar]
- Zhang, L.Y. , Liu, M. , Li, X. and Tang, H. (2013) miR‐490‐3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum–Golgi intermediate compartment protein 3 (ERGIC3). J. Biol. Chem. 288, 4035–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.F. , Li, K.K. , Gao, L. , Li, S.Z. , Chen, K. , Zhang, J.B. , Wang, D. , Tu, R.F. , Zhang, J.X. , Tao, K.X. , Wang, G. and Zhang, X.D. (2015) miR‐191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPbeta. Oncotarget 6, 4144–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Chen, J. , Li, Z. , Li, X. , Hu, X. , Huang, Y. , Zhao, X. , Liang, C. , Wang, Y. , Sun, L. , Shi, M. , Xu, X. , Shen, F. , Chen, M. , Han, Z. , Peng, Z. , Zhai, Q. , Chen, J. , Zhang, Z. , Yang, R. , Ye, J. , Guan, Z. , Yang, H. , Gui, Y. , Wang, J. , Cai, Z. and Zhang, X. (2010) Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS One 5, e15224 [DOI] [PMC free article] [PubMed] [Google Scholar]


