Abstract
Background
Hyponatraemia is a very common medical condition that is associated with multiple poor clinical outcomes and is often managed suboptimally because of inadequate assessment and investigation. Previously published guidelines for its management are often complex and impractical to follow in a hospital environment, where patients may present to divergent specialists, as well as to generalists.
Design
A group of senior, experienced UK clinicians, met to develop a practical algorithm for the assessment and management of hyponatraemia in a hospital setting. The latest evidence was discussed and reviewed in the light of current clinical practicalities to ensure an up‐to‐date perspective. An algorithm was largely developed following consensus opinion, followed up with subsequent additions and amendments that were agreed by all authors during several rounds of review.
Results
We present a practical algorithm which includes a breakdown of the best methods to evaluate volume status, simple assessments for the diagnosis of the various causes and a straightforward approach to treatment to minimise complexity and maximise patient safety.
Conclusion
The algorithm we have developed reflects the best available evidence and extensive clinical experience and provides practical, useable guidance to improve patient care.
Keywords: ADH antagonists, Hyponatraemia, SIADH
Introduction
Hyponatraemia is the most commonly seen electrolyte disturbance both in hospital inpatients and in the community 1. There are numerous recognised causes of hyponatraemia, and extremes of volume status – such as dehydration or fluid overload – are common precipitants. The syndrome of inappropriate antidiuretic hormone secretion (SIADH), the oft‐quoted cause of hyponatraemia by medical students, is a diagnosis of exclusion that, by definition, is associated with a normal volume status.
The majority of cases of hyponatraemia are mild, and it is generally thought of as an asymptomatic condition, although even mild hyponatraemia may have detrimental effects on patients 2, 3. The symptoms of hyponatraemia are nonspecific in the majority, and hyponatraemia is often discovered coincidentally. Rapid changes in sodium levels or acute profound hyponatraemia can be associated with neurological features and are medical emergencies that require urgent intervention and close supervision in a monitored environment.
The two main problems encountered in routine clinical practice relate to the appropriate assessment and initial investigations required to identify the cause of the hyponatraemia in any given patient, and the therapeutic manoeuvres necessary to ameliorate the condition. Several guidelines for treating hyponatraemia have been proposed 4, 5; however, they often contain algorithms that are complex and impractical for use by nonspecialists. As a group of clinicians from varied specialties, but with a particular interest in hyponatraemia, we have therefore created a simple clinical algorithm that aims to maximise utility and patient safety, whilst being easy to use.
Methodology
A group of senior, experienced UK clinicians, many of whom have published in the field of inpatient hyponatraemia and SIADH, met under the direction of Professor Grossman to develop a practical algorithm for the assessment and management of hyponatraemia. The latest evidence was discussed and reviewed in the light of clinical practicalities to ensure an up‐to‐date perspective. The algorithm was largely developed during the meeting following consensus opinion, followed up with subsequent additions and amendments that were agreed by all authors during several rounds of review.
Given the dearth of evidence in many aspects of the management of hyponatraemia, we have based the algorithm (Fig. 1) on widely accepted recommendations, expert opinion and published consensus guidelines.
Figure 1.
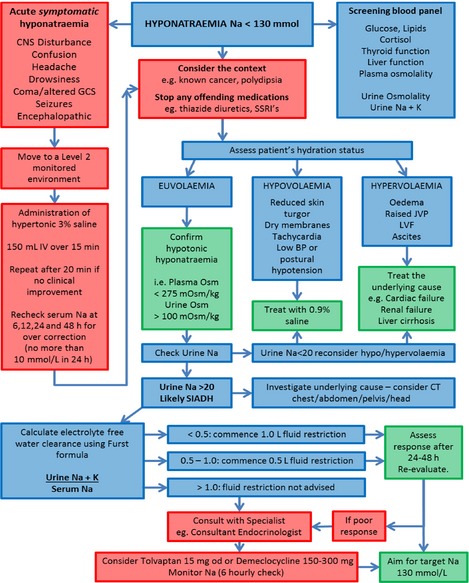
UK algorithm for management of inpatients with hyponatraemia. CNS, central nervous system; CT, computed tomography; GCS, Glasgow Coma Score; IV, intravenous; JVP, jugular venous pressure; K, potassium; LVF, left ventricular fibrillation; Na, sodium; od, once daily; Osm, osmolality; SSRI, selective serotonin reuptake inhibitor.
Making the right diagnosis
The management of patients with hyponatraemia begins with a good clinical history and examination, an understanding of the timeline of the change in serum sodium values and some basic tests to rule out obvious or worrying causes.
Baseline investigations
It is important to consider common causes of hyponatraemia, such as hyperglycaemia, as well as pseudo‐hyponatraemia, whereby unmeasured factors, such as alcohol or triglycerides, can produce a spuriously low sodium result. A screening investigation panel must therefore be undertaken as soon as the problem is suspected and should include blood glucose, lipid profile, random (ideally at 09:00) cortisol (unless the patient is taking synthetic glucocorticoids), thyroid function tests, liver function tests and plasma osmolality. A urine sample should be taken to check urinary osmolality, sodium and potassium.
Consider the context
Known conditions may inform the diagnosis. Taking a good history will reveal the likelihood, for example, of primary polydipsia or recent medication changes that may have an influence on serum sodium levels. Other medical conditions, such as an underlying malignancy and its treatment, may provide a clear explanation for the revealed electrolyte imbalance – certain types of lung cancer are well recognised to precipitate SIADH. Such knowledge, along with appropriate initial investigations and clinical judgement, may thereby allow one to circumvent the full algorithm and proceed rapidly with the right therapy. These principles should be considered at all points of the algorithm.
Assessment of volume status
Diagnosis and management of hyponatraemia depends on assessment of whether the patient is hypovolaemic, hypervolaemic or euvolaemic. However, this is frequently difficult and often suboptimal, even when performed by experienced clinicians 6. If the volume status is unclear, an infusion of normal saline (e.g. 1 L over 12 h) as a therapeutic trial will often reveal the true situation. Hypovolaemic patients will respond well (typically serum sodium will rise > 5 mmol/L), whereas patients with SIADH will often not improve and may experience a worsening of hyponatraemia. It is imperative that serum sodium is rechecked 6 h after the infusion is started.
Acute severe hyponatraemia
If the patient has significant neurological symptoms (see Fig. 1 ‘acute symptomatic hyponatraemia’ for examples), this is a medical emergency and should be treated immediately, without waiting for the diagnosis of the cause of hyponatraemia. See Box 1 for the management of acute severe hyponatraemia.
Box 1. Management of acute symptomatic hyponatraemia.
Acute symptomatic hyponatraemia is a medical emergency, and patients should be moved to a Level 2 monitored environment. A consultant endocrinologist or nephrologist should be consulted as soon as possible.
Treatment involves the use of hypertonic saline to gradually correct the hyponatraemia, with the goal of ensuring that the sodium level does not rise by more than 6 mmol/L in the first 6 h or 10 mmol/L in the first 24 h. Rapid overcorrection leads to a risk of osmotic demyelination syndrome.
We suggest starting with 150 mL of 3% saline IV over 15 min. If there is no clinical improvement, repeat the dose after 20 min. Check serum sodium at 6, 12, 24 and 48 h to ensure that overcorrection (serum sodium rise of 10 mmol/L or more in 24 h or less) has not occurred. If the sodium does rise excessively, then intravenous dextrose or desmopressin (e.g. DDAVP) may be required. The serum sodium does not need to be normalised with hypertonic saline; an increase of 4–6 mmol/L often leads to major clinical improvements.
Treatment of hyponatraemia
Rates of correction
The treatment of this condition needs to take into account the duration of the hyponatraemia and the degree of symptoms relating to it. The acute treatment of symptomatic hypotonic hyponatraemia requires an understanding of its targets and risks, as well as continuous monitoring of the patient's clinical status and relevant serum biochemical values.
Patients who have hyponatraemia for more than 48 h are at risk of neurological sequelae if the correction of serum sodium occurs too rapidly 7 due to the development of osmotic demyelination (central pontine demyelination). A safe limit for the treatment of hyponatraemia is a rise of no more than 10 mmol/L in the first 24 h and 8 mmol/L in the subsequent 24 h (18 mmol/L in 48 h).
Certain medical disorders are known to place patients at an increased risk of complications from rapid correction of serum sodium concentration. Individuals most at risk for developing osmotic demyelination syndrome are elderly patients, children under 16 years of age, malnourished patients, patients with alcoholism, patients with central nervous system disease or hypoxaemia and patients in the post‐operative setting. A tighter safety limit for correction of 8 mmol/L in 24 h and 14 mmol/L in 48 h should be considered in such patients.
Large‐volume polyuria in this context is an ominous sign. For these patients, early identification of risk factors, close monitoring of serum sodium correction and the use of 5% dextrose with or without desmopressin to prevent or reverse overcorrection are important components of treatment 8.
Stop any offending medications
Where practical, discontinue the use of medications that may be causing or exacerbating hyponatraemia (Table 1). The most common are thiazide diuretics, selective serotonin reuptake inhibitors, proton pump inhibitors, angiotensin‐converting enzyme inhibitors and loop diuretics.
Table 1.
Causes of drug‐induced hyponatraemia 23
| Anticancer agents |
Vinca alkaloids (vincristine, vinblastine) Platinum compounds (cisplatin, carboplatin) Alkylating agents (intravenous cyclophosphamide, melphalan and ifosfamide) |
| Antidepressants |
Tricyclic antidepressants Selective serotonin reuptake inhibitors Monoamine oxidase inhibitors |
| Anti‐epileptic drugs |
Carbamazepine Oxcarbazepine Sodium valproate |
| Antihypertensive agents |
Angiotensin‐converting enzyme inhibitors Amlodipine |
| Antipsychotic drugs |
Phenothiazines Butyrophenones |
| Diuretics |
Thiazides Indapamide Amiloride Loop diuretics |
| Proton pump inhibitors | Omeprazole |
Treatment of hypovolaemic patients
Hypovolaemic hyponatraemia is common and is usually caused by sodium loss through the gastrointestinal or renal tract. Characteristic symptoms are reduced skin turgor, dry mucous membranes, tachycardia, low blood pressure or postural hypotension. Patients should be treated with 0·9% saline.
Treatment of hypervolaemic patients
Hypervolaemic hyponatraemia may be caused by cardiac failure, renal failure or liver cirrhosis. Characteristic symptoms are oedema, raised jugular venous pressure, left ventricular failure and/or ascites. Management should focus on treating the underlying cause to improve hyponatraemia. In these circumstances, loop diuretics will produce a diuresis that exceeds the increased 24‐h urine sodium losses they produce and so can be used safely.
Diagnosis of euvolaemic patients
If a patient is euvolaemic, it is important to confirm that the patient has hypotonic hyponatraemia. Check plasma osmolality and urine osmolality. If plasma osmolality is > 275 mOsm/kg, consider causes of hypertonic hyponatraemia, such as hyperglycaemia or mannitol infusions.
If urinary osmolality is < 100 mOsm/kg, consider primary polydipsia or ‘beer drinker's potomania’ (low‐solute diet associated with reduced capacity to excrete free water).
If plasma osmolality is < 275 mOsm/kg, and urine osmolality is > 100 mOsm/kg, check urinary sodium concentration. SIADH is the likely diagnosis if urinary sodium is > 20 mmol/L. If urinary sodium is < 20 mmol/L, reconsider the volume status of the patient, as this usually reflects intravascular volume depletion.
Treatment of patients with SIADH
SIADH is characterised by the presence of hypotonic hyponatraemia in a context of inadequately diluted urine given the hypo‐osmolality in plasma, in the absence of a low effective circulating volume (either with hypovolaemia or hypervolaemia) 5. In most, if not all, cases, the most common cause of hyponatraemia is the nonosmotic release of arginine vasopressin (AVP) 9.
Exclude the following conditions for definitive diagnosis: renal failure, adrenal insufficiency, severe hypothyroidism, as well as nonosmotic physiological stimuli of AVP secretion (e.g. volume depletion, pain, stress and nausea 10). Causes of SIADH are multiple and varied and range from medication side effects to underlying malignancy. If there is no clear cause following initial investigations, or if there is a repeat admission with hyponatraemia, consider systematic radiological investigations, such as computed tomography of the chest/abdomen/pelvis and magnetic resonance imaging of the head.
Fluid restriction is the mainstay of treatment for SIADH; however, the degree of restriction necessary will vary, depending on the patient's ability to excrete electrolyte‐free water.
Once SIADH has been definitively diagnosed, use the Furst formula (Box 2) to estimate electrolyte‐free water clearance through the urine/plasma electrolyte ratio (U/P). If U/P is 0·5–1·0, then commence fluid restriction of 500 mL/day. If U/P is < 0·5, then commence fluid restriction of 1000 mL/day. If U/P > 1·0, then there is no excretion of electrolyte‐free water and fluid restriction is unlikely to be beneficial.
Box 2. The Furst formula.
Dilute urine may be considered to have an isotonic portion and an electrolyte‐free water portion. The proportions of electrolyte‐free water and isotonic urine can be measured by the ratio of effective solutes (principally sodium and potassium, with associated anions) between the plasma and the urine 21.
Restriction of water intake to less than the amount of electrolyte‐free water excreted will cause plasma tonicity, and hence serum sodium, to rise. The amount of electrolyte‐free water that can be cleared from the kidneys will therefore affect the patient's response to fluid restriction. A clinically useful equation simplified by Furst et al. for estimating free‐water clearance is the urine/plasma electrolyte ratio measured in a urine sample 22:
The principle of fluid restriction for the management of SIADH should be clearly understood and followed. The degree of fluid restriction can be difficult to calculate, initiate and maintain for patients in hospital and is also an added burden to the patients themselves as well as the nursing staff. When the urine to plasma electrolyte ratio is > 1·0, almost no amount of water restriction will result in a rise in serum sodium because free water is being retained, a process that will promote hyponatraemia.
Undertaking effective fluid restriction
Important features in the practice of fluid restriction include the use of fluid balance sheets, bedside notices and removal of excess bedside fluids. Paramedical staff, such as volunteers with tea rounds, must be made aware of the importance of the practice. Daily assessment of urea and electrolytes and clinical review must be made on all patients who are fluid restricted.
Specialist review
Patients in whom fluid restriction is not advised, or patients who have a poor response to fluid restriction after 24–48 h, should be reviewed by a consultant with experience of treating hyponatraemia – such as a consultant endocrinologist – who may consider use of pharmacological treatments, such as tolvaptan or demeclocycline. However, the latter treatment may cause problems with subsequent chemotherapy, for example candidiasis or impairment of renal function.
Treatment with tolvaptan
By binding to and blocking the V2 receptor, vaptan drugs can be used to correct hyponatraemia in SIADH. This is an attractive therapeutic avenue because it tackles the cause of the underlying fluid retention 11.
The SALT‐1 and SALT‐2 trials have shown that serum sodium can be safely improved in patients with hyponatraemia through removal of excess free water, producing additional benefits in patients’ quality of life, as measured by 12‐item Short Form General Health Survey scores 11. The prompt correction of hyponatraemia in patients with SIADH may be associated with a reduction in hospital stay 12.
Before initiating tolvaptan, it is essential to remove any fluid restriction and ensure that patients can drink in response to thirst, as correction of hyponatraemia can occur too rapidly if they are combined. Tolvaptan should be initiated at a dose of 15 mg, orally, once daily, and serum sodium should be monitored 6 h after starting treatment to exclude overly rapid correction 13. Clinical experience is limited and its use should be restricted to consultant endocrinologists, oncologists, nephrologists or other appropriate specialists with significant experience of using this medication. We suggest that it should be prescribed as single doses and not as a repeated prescription: in many cases, just two or possibly three doses are required.
Treatment with demeclocycline
In the United Kingdom, demeclocycline is licensed to treat hyponatraemia associated with SIADH secondary to malignant disease, where fluid restriction is ineffective, and the patient does not have liver cirrhosis 13. Demeclocycline should be started at a dose of 150 mg three times daily, assessed after 3 days and increased if necessary. However, the patient's response to demeclocycline can be unpredictable, and it has a slow onset of action 5. The evidence base is sparse, and the frequency of its known hepatotoxic and nephrotoxic side effects 14, 15, 16, 17 is unclear.
Considerations regarding hyponatraemia and SIADH management
There remains controversy in the literature regarding the importance of prompt intervention in the setting of hyponatraemia and its implications for morbidity and inpatient hospital length of stay. Critics suggest that there is a lack of evidence regarding the link between hyponatraemia and worsening patient outcomes, and indeed the majority of the work that has been carried out in this regard has been through retrospective studies rather than looking at prospective work with interventions to correct hyponatraemia 18. Hyponatraemia is certainly associated with a number of disease states and may be a poor prognostic marker, but is not a discrete disease state in itself. It is usually a consequence of an inability of the kidneys to excrete a free water load, but given the complex aetiology of many medical conditions and their comorbidities, it can be difficult to ascertain to what degree the hyponatraemia is independently contributing to morbidity and mortality.
Whether hyponatraemia in a patient with cancer is merely a marker of poor prognosis or whether its presence may alter the patient's quality of life has not been definitively answered, but there is increasing evidence that hyponatraemia can no longer be considered just a biochemical ‘bystander’ in the ill patient 19. A systematic diagnostic approach is necessary to determine the specific aetiology of a patient's hyponatraemia. Therapy must then be dictated not only by recognised reversible causes, such as advanced hypothyroidism, adrenal insufficiency, diuretics or other medicines, but also by whether the hyponatraemia occurs acutely or chronically and the degree of symptoms related to it 5.
Critically, most evidence suggests that overenthusiastic treatment of SIADH is considerably more dangerous than treatment that is slow or relatively ineffective. It is vital that all doctors should be aware of the need for a slow pace of normalisation of serum sodium except in the most extreme circumstances: primum non nocere.
Summary
Hyponatraemia is under‐recognised, incorrectly investigated and suboptimally managed, leading to poor patient outcomes. There is a well‐recognised association with inpatient morbidity and mortality. Frequently, insufficient diagnostic assessment and investigations take place, and this can affect both patient management and outcomes 20. Failure to treat hyponatraemia correctly may impede both patient outcomes and other factors, such as hospital length of stay. With regard to medical treatments, AVP antagonists offer a novel therapeutic approach. Currently, clinical experience is limited with these agents and randomised controlled trials to compare vaptans with the current standard of care are needed to demonstrate a clear benefit to patients with SIADH‐related hyponatraemia to add to the evidence already available. We feel that the algorithm we have developed (Fig. 1) reflects the best available evidence and extensive clinical experience and provides practical, useable guidance to improve patient care.
Author contributions
All authors contributed equally to the development and design of the algorithm. Dr Grant and Professor Grossman were lead authors in drafting the submitted manuscript, to which all authors contributed.
Address
Department of Diabetes, Royal Sussex County Hospital, Eastern Road, Brighton BN2 5BE, UK (P. Grant); Department of Endocrinology, University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Queen Elizabeth Medical Centre, Edgbaston, Birmingham B15 2TH, UK (J. Ayuk); Diabetes and Endocrinology, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK (P.‐M. Bouloux, M. Cohen); Diabetes and Endocrinology, Portsmouth Hospitals NHS Trust, Level C, Queen Alexandra Hospital, Portsmouth PO6 3LY, Hampshire, UK (I. Cranston); Department of Diabetes and Endocrinology, Leeds Teaching Hospitals NHS Trust, St James's University Hospital, Beckett Street, Leeds, West Yorkshire LS9 7TF, UK (R. D. Murray); Department of Endocrinology and Diabetes, Cardiff University School of Medicine, Sir Geraint Evans Building, Heath Park, Cardiff CF14 4XN, UK (A. Rees); Department of Medical Oncology, Christie Hospital, NHS Trust, Wilmslow Road, Manchester M20 4BX, UK (N. Thatcher); Department of Endocrinology, Oxford Centre for Diabetes, Endocrinology and Metabolism, Radcliffe Department of Medicine, University of Oxford, Churchill Hospital, Headington, Oxford OX3 7LE, UK (A. Grossman).
Acknowledgements
The authors were members of the recent UK hyponatraemia management consensus guideline committee, which was convened by Otsuka Pharmaceuticals UK Ltd., the manufacturers of tolvaptan. Venue and attendee travel costs relating to this meeting were supported by Otsuka Pharmaceuticals UK Ltd., and Professor Grossman and Dr Cranston were paid speaker fees to present data at the meeting.
The authors have not received any honoraria in relation to this manuscript. Paul Grant has no competing interests; Ashley Grossman received lecture fees and support to attend educational meetings from Otsuka Pharmaceuticals UK Ltd.; John Ayuk received lecture fees from Otsuka Pharmaceuticals UK Ltd., outside the submitted work; Pierre‐Marc Bouloux received personal fees from Otsuka Pharmaceuticals UK Ltd., outside the submitted work; Mark Cohen received nonfinancial support from Otsuka Pharmaceuticals UK Ltd., during the conduct of the study; grants received from Otsuka Pharmaceuticals UK Ltd and personal fees from Otsuka Pharmaceuticals UK Ltd., outside the submitted work; Iain Cranston received a speaker honorarium from Otsuka Pharmaceuticals UK Ltd., for presenting at BES The Annual Society for Endocrinology Conference in 2014; Robert D Murray received lecture fees and support to attend educational meetings from Otsuka Pharmaceuticals UK Ltd.; Aled Rees received nonfinancial support from Otsuka Pharmaceuticals UK Ltd., during the conduct of the study; Nicholas Thatcher received personal fees from Otsuka Pharmaceuticals UK Ltd., during the conduct of the study and outside the submitted work.
Editorial assistance in the preparation of this article was provided by apothecom and funded by Otsuka Pharmaceuticals UK Ltd.
Eur J Clin Invest 2015; 45 (8):888–894
References
- 1. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–72. [DOI] [PubMed] [Google Scholar]
- 2. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006;119:71 e1–8. [DOI] [PubMed] [Google Scholar]
- 3. Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G et al Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta‐analysis. PLoS One 2013;8:e80451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D et al Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014;170:G1–47. [DOI] [PubMed] [Google Scholar]
- 5. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH et al Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013;126(10 Suppl 1):S1–42. [DOI] [PubMed] [Google Scholar]
- 6. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 1987;83:905–8. [DOI] [PubMed] [Google Scholar]
- 7. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000;342:1581–9. [DOI] [PubMed] [Google Scholar]
- 8. Gharaibeh KA, Brewer JM, Agarwal M, Fülöp T. Risk factors, complication and measures to prevent or reverse catastrophic sodium overcorrection in chronic hyponatremia. Am J Med Sci 2014;349:170–5. [DOI] [PubMed] [Google Scholar]
- 9. Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med 2010;123:652–7. [DOI] [PubMed] [Google Scholar]
- 10. Hoorn EJ, Zieste R. Hyponatremia revisited: translating physiology to practice. Nephron Physiol 2008;108:46–59. [DOI] [PubMed] [Google Scholar]
- 11. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS et al Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099–112. [DOI] [PubMed] [Google Scholar]
- 12. Grant P. New drugs for hyponatraemia. Cost effectiveness of tolvaptan. BMJ 2011;342:d1947. [DOI] [PubMed] [Google Scholar]
- 13. British National Formulary (BNF 68). Joint Formulary Committee. London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society; 2014. [Google Scholar]
- 14. De Troyer A. Demeclocycline. Treatment for syndrome of inappropriate antidiuretic hormone secretion. JAMA 1977;237:2723–6. [DOI] [PubMed] [Google Scholar]
- 15. Forrest JN Jr, Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med 1978;298:173–7. [DOI] [PubMed] [Google Scholar]
- 16. Perks WH, Walters EH, Tams IP, Prowse K. Demeclocycline in the treatment of the syndrome of inappropriate secretion of antidiuretic hormone. Thorax 1979;34:324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trump DL. Serious hyponatremia in patients with cancer: management with demeclocycline. Cancer 1981;47:2908–12. [DOI] [PubMed] [Google Scholar]
- 18. Hoorn EJ, Lindemans J, Zietse R. Acute and concomitant deterioration of hyponatremia and renal dysfunction associated with heart and liver failure. Clin Nephrol 2006;65:248–55. [DOI] [PubMed] [Google Scholar]
- 19. Schrier RW, Sharma S, Shchekochikhin D. Hyponatraemia: more than just a marker of disease severity? Nat Rev Nephrol 2013;9:37–50. [DOI] [PubMed] [Google Scholar]
- 20. Huda MS, Boyd A, Skagen K, Wile D, van Heyningen C, Watson I et al Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J 2006;82:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldberg M. Hyponatremia. Med Clin North Am 1981;65:251–69. [DOI] [PubMed] [Google Scholar]
- 22. Furst H, Hallows KR, Post J, Chen S, Kotzker W, Goldfarb S et al The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci 2000;319:240–4. [DOI] [PubMed] [Google Scholar]
- 23. Liamis G, Milionis H, Elisaf M. A review of drug‐induced hyponatremia. Am J Kidney Dis 2008;52:144–53. [DOI] [PubMed] [Google Scholar]


