Abstract
Objective
To evaluate the effects of water versus beverages sweetened with non‐nutritive sweeteners (NNS) on body weight in subjects enrolled in a year‐long behavioral weight loss treatment program.
Methods
The study used a randomized equivalence design with NNS or water beverages as the main factor in a trial among 303 weight‐stable people with overweight and obesity. All participants participated in a weight loss program plus assignment to consume 24 ounces (710 ml) of water or NNS beverages daily for 1 year.
Results
NNS and water treatments were non‐equivalent, with NNS treatment showing greater weight loss at the end of 1 year. At 1 year subjects receiving water had maintained a 2.45 ± 5.59 kg weight loss while those receiving NNS beverages maintained a loss of 6.21 ± 7.65 kg (P < 0.001 for difference).
Conclusions
Water and NNS beverages were not equivalent for weight loss and maintenance during a 1‐year behavioral treatment program. NNS beverages were superior for weight loss and weight maintenance in a population consisting of regular users of NNS beverages who either maintained or discontinued consumption of these beverages and consumed water during a structured weight loss program. These results suggest that NNS beverages can be an effective tool for weight loss and maintenance within the context of a weight management program.
Introduction
There is continued controversy regarding the potential benefit of non‐nutritive sweeteners (NNS) for body weight management 1, 2, 3, 4, 5, 6, 7. Some observational studies have reported a positive association between NNS consumption and BMI and weight gain over time 1, 2, 8, questioning the benefit of NNS for weight management. Observational studies, however, are unable to establish cause and effect. Randomized trials comparing foods and beverages sweetened with NNS versus caloric sweeteners have generally found that risk of weight gain is reduced among subjects consuming NNS 9, 10, 11. There have been relatively few long‐term (≥1 year) randomized trials of NNS for weight loss and maintenance 12, 13, 14. In particular, there have been few studies comparing beverages sweetened with NNS to water 13, 15, 16, which is the recommended beverage for maintaining good health 17, 18.
Because of the relative paucity of data examining the long‐term effects of NNS consumption on body weight management, the 2015 Dietary Guidelines Advisory Committee recently concluded that there is insufficient evidence to recommend the use of low‐calorie sweeteners as a strategy for long‐term weight loss and weight maintenance 17. The Committee recommended that further prospective research was needed to establish the effects of low‐calorie sweeteners on body weight and other health outcomes.
This article reports data from a year‐long trial comparing beverages sweetened with NNS to water as part of a behavioral weight management program consisting of 12 weeks of active weight loss and 40 weeks of weight maintenance. Results from the 12‐week weight loss phase of this trial were published previously and showed non‐equivalence, with the NNS group showing greater weight loss at 12 weeks than the water group 19.
Methods
Participants
Blue Chip Recruiting (www.bluechiprecruiting.ca) recruited participants from the general population through the use of flyers, e‐mails, and other advertisements (e.g., radio). Of the 506 applicants screened, 308 subjects were enrolled in the trial between October 2012 and April 2013 at The University of Colorado Denver (n = 151, in four cohorts) and Temple University (n = 157, in five cohorts), see Figure 1. Participants were male and female, ages 21 to 65, BMI 27 to 40 kg/m2, representing a range of races and ethnicities (Table 1). Of the 308 participants enrolled, five withdrew from the trial prior to the start of the study; 303 participants began treatment. There were no significant differences in attrition by site.
Figure 1.
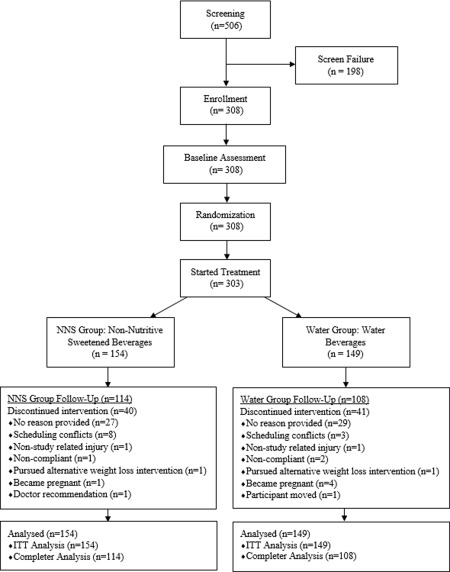
Consort diagram. Screening, enrollment, randomization, and follow‐up of study participants.
Table 1.
Baseline subject characteristics by groupa
| NNS group (n = 158) | Water group (n = 150) | |
|---|---|---|
| Age (years) b | 48.3 ± 10.4 | 47.3 ± 10.6 |
| Gender [n, (%)] | ||
| Male | 28 (18%) | 25 (17%) |
| Female | 130 (82%) | 125 (83%) |
| Ethnicity [n, (%)] c | ||
| Hispanic/Latino | 23 (15%) | 12 (8%) |
| Not Hispanic/Latino | 133 (85%) | 138 (92%) |
| Race [n, (%)] | ||
| White | 107 (58%) | 101 (67%) |
| Black/African American | 42 (27%) | 43 (29%) |
| Asian or Pacific Islander | 1 (0.6%) | 4 (3%) |
| American Indian or Alaskan Native | 1 (0.6%) | 0 (0%) |
| Multiracial origin/other | 7 (4.6%) | 2 (1.2%) |
| Baseline weight (kg) | 93.92 ± 13.29 | 93.03 ± 12.99 |
| BMI (kg m−2) d | 33.92 ± 4.25 | 33.30 ± 3.98 |
| Systolic BP (mm Hg) | 118.79 ± 12.08 | 117.87 ± 12.58 |
| Diastolic BP (mm Hg) | 76.70 ± 7.56 | 76.21 ± 7.29 |
χ 2 analyses completed for gender, ethnicity, and race. χ 2 analysis showed no between‐group differences.
Two‐sample t‐test statistics showed no between‐group differences. Two‐sample t‐test analyses completed for age, BMI, weight, systolic BP, diastolic BP. Mean ± SD (all such values).
n = 156 in NNS group.
n = 156 in NNS group and n = 147 in water group.
There were no significant differences between the two groups in demographic profile.
NNS: non‐nutritive sweeteners; BP: blood pressure.
Individuals were initially screened over the phone or through an online application and potentially eligible subjects were then screened in person. Eligibility requirements included being weight stable within 10 pounds during the past 6 months, participating in no more than 300 min of physical activity weekly, drinking NNS beverages at least three times per week, and be willing to discontinue NNS beverages if randomized to the water group. Regular NNS users were chosen to ensure exposure to NNS during the trial as many people do not like the taste of NNS and selecting non‐users could limit intake reducing the ability to detect effects of NNS beverages on body weight regulation. Women who were lactating or pregnant during the previous 6 months or who were planning on becoming pregnant were excluded. Individuals with diabetes, CVD, and uncontrolled hypertension (as well as other diseases potentially interfering with weight loss, e.g., gastrointestinal or thyroid disease) or who used medications affecting weight and metabolism were excluded. Eligible participants required physician approval stating that the nutrition and exercise requirements were not contraindicated and that they were in good general health.
The study was approved by the Temple University IRB and the Western IRB at the University of Colorado site. All participants provided informed consent.
Study design
This was a 1‐year equivalence trial comparing beverages sweetened with NNS to water as part of a behavioral weight management program that included 12 weeks of weight loss followed by 40 weeks of weight maintenance. A computer‐generated randomization schedule assigned participants, within each site, to either the NNS beverage or water treatment arms stratified by sex, to ensure an equal distribution of women and men in each treatment group.
The study protocol specified preplanned data analyses on the primary outcome of weight loss at 12 weeks (weight loss period) and at the end of 1 year (weight loss maintenance period).
Intervention
Weight loss
For the 12‐week weight loss intervention, all participants received a comprehensive cognitive‐behavioral weight loss intervention called The Colorado Weigh 20 involving weekly, 60‐min instructional classes. Additional details regarding The Colorado Weigh classes and the weight loss intervention are described elsewhere 19.
Weight loss maintenance
All participants attended nine monthly, 60‐min group meetings led by registered dieticians or clinical psychologists as part of The Colorado Weigh 20. Participants attended group meetings stratified by treatment (NNS or water) group. Participants were weighed at each monthly meeting. Participants were advised to consume 25% to 35% of calories from fat (using a fat gram counter and total calorie guidebook) and to include 6 days of unsupervised exercise per week in order to meet the weight loss maintenance recommendation of 60 min of moderate activity daily 21. Participants in both treatment groups received the same curriculum, with the only difference being discussion regarding the type of beverages they were instructed to consume. Adherence to the treatment was assessed by study dietitians based on food, beverage, and physical activity logs as well as monitoring weight loss.
Daily energy intake targets were individualized and calculated as each participant's estimated resting metabolic rate (RMR) × 1.6 physical activity level (PAL). Energy intake targets were adjusted to maintain weight based on each individual's PAL. Physical activity was assessed using a Body Media armband activity tracking device (Manufacturer: Body Media, Model AB155) for 1 week at baseline and during weeks 4, 12, 24, 36, and 52.
NNS beverage group
Participants randomized to the NNS beverage group were asked to consume at least 24 fluid ounces (710 ml) of NNS beverages per day during the entire year‐long trial, with unrestricted water consumption. Premixed beverages containing <5 kcal per 8 ounce serving (237 ml) and containing NNS qualified as NNS beverages.
Water group
Participants randomized to the water group were asked to consume at least 24 fluid ounces (710 ml) of water per day during the entire year‐long trial and to refrain from NNS beverage consumption. They were instructed to not add NNS (e.g., aspartame—NutraSweet® or Equal®; sucralose—Splenda®; or stevia—Truvia®; as well as diet creamers) to beverages such as coffee or tea. However, they were permitted to consume foods containing NNS (e.g., artificially sweetened gum, candies, cookies, gelatin, pudding, ice cream, yogurt), although they were not instructed to do so as part of the weight loss program.
Participants were given four manufacturer coupons monthly (from The Coca‐Cola Company, PepsiCo and Dr. Pepper Snapple Group), redeemable for a monthly supply of NNS beverages or bottled water. Participants were asked to record their daily beverage intake using paper logs. This information was used to assess treatment adherence. While all participants were encouraged to consume only non‐caloric beverages as part of the behavioral treatment program, they were allowed to consume any beverages as long as they remained compliant with their required intake of either 24 ounces of NNS beverages or 24 ounces of water daily.
Measurements
All assessments, except for height, were conducted at baseline, 12 weeks (post‐weight loss phase) and 52 weeks (post‐weight loss maintenance phase). Height was measured to the nearest 0.1 cm at the screening visit prior to baseline and at 52 weeks with a wall‐mounted stadiometer. Body weight was measured to the nearest 0.1 kg on a digital scale. Waist circumference, measured at the top of the iliac crest, was determined based on two consecutive measures within 0.5 cm. Blood pressure (while seated) was recorded as the average of two measures. Standard venipuncture method was used to collect fasting blood samples for lipid and glucose measurements. Urine was provided for measurement of urine osmolality. Additional methodological details are described elsewhere 19.
Participants completed questionnaires at baseline and at 12, 24, 36, and 52 weeks to assess changes in perceived hunger (using a 100 mm visual analog scale anchored at “not at all hungry” and “extremely hungry”). Beverage treatment adherence was determined from daily beverage logs collected monthly. Participants were compensated at intervals for meeting assessment requirements at weeks 12, 24, 36, and 52 (total compensation = $340).
Power of the study
The primary outcome addressed in this report is the change in body weight during the 1‐year trial. The study was designed as an equivalence trial with the hypothesis that there would be no clinically meaningful difference in weight between those consuming NNS beverages or water. The bounds of equivalence for between‐group differences at 1 year of weight loss were pre‐specified at ±2.2 kg. Assuming the true difference was 0.73 kg (1/3 of the equivalence margin) and common SD of 4.2 kg, a sample size of 63 per arm was required using two, one‐sided t‐tests to ensure at least 80% power with an alpha level of P < 0.05 to establish equivalence.
Statistical analysis
Intention‐to‐treat (baseline observation carried forward (BOCF)) was the primary analysis used for assessing weight loss using monthly body weights as the dependent variable. In a secondary analysis we only examined participants who completed the entire 1‐year trial. The primary outcome measure was change in body weight over the 1‐year period.
The primary hypothesis was that NNS beverage and water treatments would be equivalent with upper and lower bounds of equivalence set at ±2.2 kg. The value chosen for body weight difference would not be clinically meaningful. The mean and the upper and lower 90% confidence limits for the difference in weight loss maintenance between the treatment groups would have to be within ±2.2 kg in order to be considered equivalent. Other weight‐related outcomes analyzed included weight change from the point of maximum weight loss until the end of 1 year and the percentage of participants who lost at least 5% of total body weight after 1 year. A sensitivity analysis for weight loss differences between treatment groups was conducted using several methods: a linear mixed model, multiple imputation, ANCOVA and two independent t‐tests (or χ 2 when appropriate). All methods showed consistent results. Reported here are the t‐test results (two one‐sided t‐tests; the standard approach for evaluating equivalence 22) and 90% confidence intervals. This model yielded the most conservative result (i.e., least likely to show a difference) among those tested in the sensitivity analysis. Linear mixed effects models were used to analyze secondary outcomes (waist circumference, systolic blood pressure, blood measures, urine osmolality, hunger, and physical activity) which consisted of classification variables of time (baseline, 52 weeks), group (NNS or water) and their interaction term as fixed effects and compound symmetry covariance. Within‐ and between‐group contrasts were tested under this model. Between‐site outcomes were also tested.
Results
Of the 303 subjects who began treatment, 222 or 73% completed the 1‐year trial. Compliance with the beverage consumption prescription at 52 weeks (≥24 ounces/day of NNS beverages or water) was high based on beverage logs: 98.1% and 97.8% in the NNS and water groups, respectively. Partial compliance (consumption of >0 and <24 ounces/day of water or NNS beverages) was 0.7% and 0.9% for the NNS and water groups, respectively, and non‐compliance (consumption of 0 ounces/day) was 1.2% and 1.3% for the NNS and water groups, respectively. These values represent the mean percentage of total study days subjects met the different compliance criteria. Participants in the water and NNS groups attended 84.5 ± 23.7% (SD) and 85.1 ± 23.1% of the instructional sessions, respectively. The means were not different (P = 0.8262).
Water and NNS treatments were non‐equivalent in this trial. Results using the Sattherwaite two‐sample t‐test showed the NNS treatment was superior to water for weight loss at both 12 weeks 19 and 1 year. Subjects in both treatment groups achieved and maintained significant weight loss over the 1‐year trial (Table 2 shows BOCF and completer analysis). Maximum mean weight loss occurred at week 20 in the water group (5.5 kg) and at week 28 in the NNS group (8.6 kg) (Figure 2a). Both groups regained weight after reaching the maximum weight loss although the rate of gain was significantly less (P < 0.001) for the NNS group (Figure 2b), which also met the weight maintenance definition of less than 3% weight regain 23. Nearly 19% more subjects in the NNS group lost at least 5% of their body weight from baseline to week 52 compared to the water group (Figure 3). There were no significant between‐site differences in weight loss or weight maintenance outcomes (P = 0.4452).
Table 2.
Absolute weight loss (kg) at 1 year for all participants and for completers only
| Group | Baseline weight (kg) | Week 52 clinic weight (kg) | Change | 90% CL mean change | P value for change |
|---|---|---|---|---|---|
| Absolute weight loss (kg) for all participants using a baseline observation carried forward analysis | |||||
| NNS (n = 154) | 93.91 ± 13.46 | 87.70 ± 14.79 | −6.21 ± 7.65* | −5.19 to −7.23 | <0.001 |
| Water (n = 149) | 93.15 ± 12.94 | 90.70 ± 13.70 | −2.45 ± 5.59* | −1.70 to −3.21 | <0.001 |
| NNS–water | −0.76 ± 13.21 | −3.00 ± 14.26 | −3.76 ± 6.72* | −2.49 to −5.03 | <0.001 |
| Absolute weight loss (kg) for completers | |||||
| NNS (n = 114) | 93.20 ± 13.02 | 84.81 ± 13.78 | −8.39 ± 7.79* | −7.18 to −9.60 | <0.001 |
| Water (n = 108) | 93.64 ± 13.53 | 90.25 ± 14.53 | −3.39 ± 6.33* | −2.38 to −4.40 | <0.001 |
| NNS–water | −0.43 ± 13.27 | −5.44 ± 14.15 | −5.01 ± 7.12* | −3.43 to −6.59 | <0.001 |
Baseline observation carried forward analysis includes all those participants who dropped out of the study in the analysis. This analysis mimics the clinical setting. Completer analysis includes only participants who completed 52 weeks of the trial. Although equivalence cannot be established, participants lost more weight in the non‐nutritive sweetener (NNS) group as compared to the water group. All analyses were completed using a Sattherwaite two‐sample t‐test. All values are mean ± SD unless otherwise noted. Statistically significant values (P < 0.05) are shown by an asterisk (*) and statistically significant P values are shown in bold.
Figure 2.
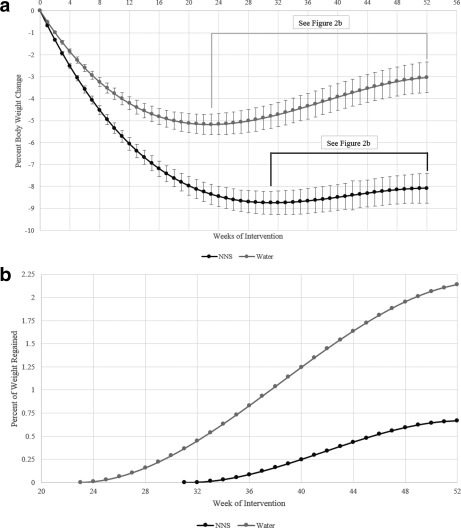
(a) Fitted model for group mean percent body weight change over time using a polynomial mixed effects model, which was fit to the weekly weight data expressed as percent change from the individual baseline weight, and an intention‐to‐treat analysis. Errors bars represent the standard error of the mean. P < 0.001 for between‐group comparisons at all time points. (b) Fitted model for group mean percent body weight regained from point of maximum weight loss using an intention‐to‐treat analysis. P < 0.001 for difference in velocity of weight regain between groups.
Figure 3.
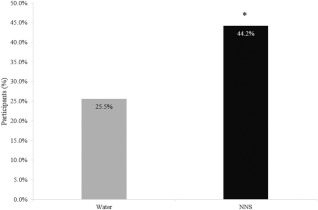
Percentage of participants who achieved at least 5% weight loss. Results based on χ 2 analysis. Analysis includes those participants who dropped out of the study, using the baseline observation carried forward. This analysis mimics the clinical setting. Difference = 0.1865 or 18.65% difference between groups with 90% CI (0.1065‐0.2735), n = 154 for NNS, n = 149 for water. *P < 0.001.
Treatment related effects on blood chemistries, subjective hunger, and physical activity time are shown in Table 3. Waist circumference decreased in both groups with the NNS group losing significantly more girth compared to the water group. Systolic blood pressure was reduced at 52 weeks in the NNS group and was significantly different than in the water group which saw no change from baseline. Both groups experienced significant declines in total cholesterol, LDL cholesterol and triglycerides and increases in HDL cholesterol as a function of weight loss although the difference between groups was only significant for triglycerides (P < 0.001). Urinary osmolality did not change with treatment and was not different between groups. Subjects in the water group reported feeling significantly more hungry at 52 weeks compared to baseline which was different than the NNS group which reported no increase in subjective hunger. Total amount of time spent engaging in moderate and vigorous activity increased similarly for both treatments over the year.
Table 3.
Cardiometabolic, hunger, and physical activity changes from baseline to week 52 in the NNS and water groupsa
| Assessment periodb | ||||
|---|---|---|---|---|
| Outcome variable and group | Baseline | Week 52 | Change | P value for change |
| Waist circumference (cm) | ||||
| NNS | 108.00 ± 0.89 | 99.33 ± 0.97 | −8.67 ± 0.80* | <0.001 |
| Water | 107.10 ± 0.91 | 102.93 ± 1.00 | −4.17 ± 0.83* | <0.001 |
| NNS–water | 0.90 ± 1.27 | −3.60 ± 1.39 | −4.50 ± 1.16* | <0.001 |
| Systolic BP (mm Hg) | ||||
| NNS | 118.85 ± 1.01 | 116.36 ± 1.13 | −2.49 ± 1.14* | 0.030 |
| Water | 117.93 ± 1.03 | 119.67 ± 1.17 | 1.73 ± 1.18 | 0.143 |
| NNS–water | 0.92 ± 1.44 | −3.31 ± 1.63 | −4.23 ± 1.64* | 0.011 |
| Glucose (mg dl−1) | ||||
| NNS | 91.44 ± 1.37 | 92.28 ± 1.46 | 0.85 ± 1.03 | 0.411 |
| Water | 90.92 ± 1.39 | 92.55 ± 1.50 | 1.63 ± 1.06 | 0.127 |
| NNS–water | 0.52 ± 1.96 | −0.27 ± 2.09 | −0.78 ± 1.48 | 0.597 |
| Cholesterol (mg dl−1) | ||||
| NNS | 190.68 ± 2.77 | 182.39 ± 2.99 | −8.28 ± 2.38* | <0.001 |
| Water | 193.23 ± 2.82 | 190.99 ± 3.08 | −2.24 ± 2.46 | 0.363 |
| NNS–water | −2.56 ± 3.95 | −8.60 ± 4.29 | −6.04 ± 3.42 | 0.079 |
| HDL (mg dl−1) | ||||
| NNS | 52.95 ± 1.23 | 58.18 ± 1.37 | 5.23 ± 1.33* | <0.001 |
| Water | 53.50 ± 1.25 | 56.47 ± 1.42 | 2.97 ± 1.37* | 0.032 |
| NNS–water | −0.56 ± 1.75 | 1.71 ± 1.97 | 2.26 ± 1.91 | 0.237 |
| LDL (mg dl−1) | ||||
| NNS | 115.22 ± 2.40 | 106.46 ± 2.59 | −8.76 ± 2.11* | <0.001 |
| Water | 116.67 ± 2.43 | 112.02 ± 2.68 | −4.65 ± 2.19* | 0.032 |
| NNS–water | −1.45 ± 3.42 | −5.56 ± 3.73 | −4.11 ± 3.04 | 0.177 |
| Triglycerides (mg dl−1) | ||||
| NNS | 120.71 ± 5.79 | 92.68 ± 6.25 | −28.03 ± 4.92* | <0.001 |
| Water | 119.20 ± 5.89 | 115.29 ± 6.42 | −3.91 ± 5.09 | 0.443 |
| NNS–water | 1.51 ± 8.26 | −22.60 ± 8.96 | −24.12 ± 7.08* | <0.001 |
| Urine osmolality (mOsmol kg−1) | ||||
| NNS | 567.36 ± 21.70 | 568.22 ± 24.75 | 0.86 ± 27.71 | 0.975 |
| Water | 592.54 ± 22.06 | 609.21 ± 25.35 | 16.66 ± 28.50 | 0.559 |
| NNS–water | −25.18 ± 30.94 | −40.99 ± 35.56 | −15.81 ± 39.75 | 0.691 |
| How hungry did you feel over the past week (scale 1‐100?) | ||||
| NNS | 52.11 ± 1.55 | 50.83 ± 1.75 | −1.28 ± 1.94 | 0.509 |
| Water | 48.07 ± 1.58 | 53.23 ± 1.83 | 5.16 ± 2.02* | 0.011 |
| NNS–water | 4.04 ± 2.21 | −2.40 ± 2.53 | −6.44 ± 2.80* | 0.022 |
| Moderate/vigorous PA (min/d) | ||||
| NNS | 38.58 ± 3.19 | 75.66 ± 4.03 | 37.07 ± 4.92* | <0.001 |
| Water | 38.34 ± 3.25 | 69.48 ± 4.20 | 31.14 ± 5.09* | <0.001 |
| NNS–water | 0.25 ± 4.56 | 6.18 ± 5.83 | 5.93 ± 7.08 | 0.403 |
All analyses are from compound symmetry models. Statistically significant values (P < 0.05) are shown by an asterisk (*) and statistically significant P values are shown in bold. For waist circumference: n = 115 for NNS and 107 for water. For systolic BP: n = 115 for NNS and 108 for water. For glucose, cholesterol, HDL, and triglycerides: n = 114 for NNS and 107 for water. For LDL: n = 114 for NNS and 105 for water. For urine osmolality: n = 114 for NNS and 108 for water. For “How hungry did you feel over the past week”: n = 114 for NNS and 103 for water. For total moderate/vigorous PA: n = 95 for NNS and 87 for water.
All values are mean ± standard error.
NNS: non‐nutritive sweetener group; BP: blood pressure; PA: physical activity.
Mean caffeine consumption, assessed from the weekly beverage logs, was 173.5 ± 14.0 mg/d in the water group and 207.3 ± 13.7 mg/d in the NNS group. This difference was not statistically significant (P = 0.0856). Mean caffeine consumption among completers was 208.3 ± 16.6 mg/d in the NNS group and 185.6 ± 17.0 mg/day in the water group (P = 0.3473). Among completers, the average daily consumption of caffeine was not significantly correlated (P = 0.3397) with the absolute amount of weight change over 52 weeks in either group.
Discussion
This 1‐year randomized clinical trial provides evidence that NNS beverages may be an effective tool to aid in weight loss and maintenance, among regular users of NNS beverages, when used as part of a behavioral weight loss treatment program. In this equivalence trial design, when compared to the most commonly recommended beverage for good health, water 17, 18, NNS beverages were shown to be non‐equivalent and were superior for both weight loss 19 and maintenance. These findings are important as there continues to be uncertainty about the benefit of NNS for weight management based largely on observational studies showing associations between NNS consumption, obesity and weight gain 1, 2, 8. In addition, it has been suggested, based on some animal studies, that NNS promote obesity by interfering with normal mechanisms of energy balance through dissociating the link between sweet taste and metabolizable energy 3, 24. Results of the present trial are not consistent with the findings from observational studies in humans or studies in animals.
The present results are consistent with the few other published long‐term human trials that evaluated NNS for weight loss 12, 15. In a prospective randomized trial, Blackburn et al. found that people with obesity in a weight loss program using NNS food and beverage products lost more weight and maintained a greater weight loss over 2 years compared to subjects not using NNS 12. Tate et al. (2012) conducted a 6‐month randomized trial in people with obesity and found greater weight loss over 6 months and a greater likelihood of achieving a 5% weight loss in participants drinking beverages with NNS compared with participants in an attention control group. There was no difference in the likelihood of achieving a 5% weight loss between participants in the water group versus the control or between the water group versus the NNS group. Observational data from subjects in the National Weight Control Registry indicate that NNS beverages and foods are commonly used as tools to help maintain weight loss among individuals who maintained a weight loss of at least 30 pounds for at least 1 year 25, 26.
The reason for the difference between results from randomized trials and observational studies in humans cannot be determined from these data. It is possible that results from observational studies are due to reverse causality whereby overweight individuals may choose to consume NNS beverages to reduce their risk of weight gain 9, 27. Alternatively, residual confounding may be an issue in cases where insufficient factors about subject characteristics and behaviors were adjusted for in the data analysis 27, 28. Furthermore, there may be differences in cognitive behavior between subjects in our study and free living subjects not enrolled in a weight loss program regarding how they use NNS beverages in the context of their diet. In the present study, subjects were in a program designed to teach them healthy behaviors that promote weight loss and maintenance. The observational studies included all users of NNS regardless of participation in any formal weight loss program 10. It is plausible that the effects of NNS on weight loss might be greater when people are actively trying to lose weight in a formal behavior change program compared to when NNS are simply used as a dietary substitute for regular sugar. For example, some people (e.g., those not intentionally focused on losing weight) might cognitively compensate for the absence of energy in the NNS beverages by intentionally consuming more solid food 11 which would mitigate weight loss.
It is not possible from the present data to explain why the NNS group lost more weight than the water group despite receiving identical weight loss instruction and beverage interventions that both contained zero calories. Physical activity was measured objectively and was not different between treatments. Both groups increased moderate and vigorous physical activity to the same extent over the course of the study as measured by arm band accelerometers. Caffeine intake was not different between treatments such that potential effects on resting energy expenditure of caffeine withdrawal among participants in the water group cannot explain the smaller weight loss observed in that group. Subjects in the water group did, however, report increased hunger relative to the NNS group which reported no increase in hunger from baseline, consistent with previous data from short‐term studies 29. It is possible that, in the water group, limiting access to sweetness in beverages may have promoted a desire to seek sweetness from other aspects of the diet, perhaps to achieve some “reward homeostasis” 30, thereby leading them to consume more sweet foods 31 resulting in greater energy intake and less weight loss. In addition, subjects in the water group were asked to make two changes in their drinking habits, both cessation of NNS consumption and increasing water consumption, which potentially presented a larger behavioral challenge than the NNS group that only had to adhere to twice daily consumption of NNS beverages.
Greater weight loss among NNS users in the present study argues against effects of NNS to stimulate consumption of sweet foods or high‐energy foods (as has been suggested 1) compared to the effects of water. Other studies have also found no increase in consumption of sweet foods among consumers of NNS 32, 33.
Treatment effects on blood lipids were consistent with those expected based on the degree of weight loss for each group. A recent report has suggested that NNS may adversely affect gut microflora in a way that impairs glucose tolerance and promotes diabetes 34. In the present study there was no change in fasting blood glucose after 52 weeks of NNS consumption. There were also no between group differences in fasting blood glucose and values were in the clinically normal range for both treatment groups. These results do not support the notion that NNS hinders weight loss by disrupting normal appetite regulation and glucose homeostasis 3, 24, 34, at least within the context of a year‐long weight loss program.
This study has some limitations. First, only people with overweight and obesity who were regular NNS beverage users were studied and the effects in normal weight and NNS‐naïve subjects may be different. Second, subjects were actively trying to lose weight using a formal behavior management program and therefore may not represent effects on weight in people not enrolled in such a program.
Conclusion
In conclusion, among participants enrolled in a comprehensive weight loss program, regular users of NNS beverages who were asked to consume NNS beverages lost significantly more weight, and maintained significantly greater weight losses, over 1 year than subjects asked to stop NNS beverage consumption and consume water alone. These results provide support for the use of NNS beverages as a tool to help with weight loss and maintenance.
Acknowledgments
Authors thank University of Colorado study team (KB, GMC, KF, DO and RS) and Temple University study team (BB, KB, RC, PD, HL, ML, DM, and HP). Thanks to CT (Colleen Tewksbury) with Biofortis Clinical Research, Inc., for providing CRO support.
Funding agencies: The study was fully funded by The American Beverage Association. The American Beverage Association was not involved in the design, conduct, interpretation, or manuscript preparation of this study. Furthermore, a third‐party organization (Biofortis‐Provident) was hired at the PIs’ request. Biofortis‐Provident audited data at both clinical sites to check for the accuracy and integrity of the data.
Disclosure: J.C.P. and J.O.H. received consulting fees from The Coca‐Cola Company outside of the submitted work. The other authors declared no conflict of interest.
Author contributions: J.C.P., J.B., M.C., H.R.W., G.D.F., Z.P., and J.O.H. were in involved in study design, data analysis, and data interpretation; C.B., S.J.H., S.S.V., and A.C.W. were involved in data collection. All authors were involved in writing the manuscript and approved the final submission.
This article was published online on 26 December 2015. After online publication, updates were made to the text. This notice is included in the online and print versions to indicate that both have been corrected on 09 January 2016.
References
- 1. Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long‐term weight gain. Obesity (Silver Spring) 2008;16:1894‐1900. [DOI] [PubMed] [Google Scholar]
- 2. Fowler SP, Williams K, Hazuda HP. Diet soda intake is associated with long‐term increases in waist circumference in a biethnic cohort of older adults: The San Antonio longitudinal study of aging. J Am Geriatr Soc 2015;63:708‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swithers SE, Martin AA, Davidson TL. High‐intensity sweeteners and energy balance. Physiol Behav 2010;100:55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosbergen D. Diet soda health risks: study says artificial sweeteners may cause weight gain, deadly diseases. 2013; http://www.huffingtonpost.com/2013/07/11/diet-soda-health-risks_n_3581842.html. Accessed December 7, 2013.
- 5. Klatell J. Can diet soda make you gain weight? 2007; http://www.cbsnews.com/news/can‐diet‐soda‐make‐you‐gain‐weight/. Accessed December 7, 2013.
- 6. Patterson NAP. Diet drinks are not the sweet solution to fight obesity, health problems. Purdue Today 2013; http://www.purdue.edu/newsroom/releases/2013/Q3/prof‐diet‐drinks‐are‐not‐the‐sweet‐solution‐to‐fight‐obesity,‐health‐problems.html. Accessed December 7, 2013. [Google Scholar]
- 7. Ludwig DS. Artificially sweetened beverages: cause for concern. JAMA 2009;302:2477‐2478. [DOI] [PubMed] [Google Scholar]
- 8. Stellman SD, Garfinkel L. Artificial sweetener use and 1‐year weight change among women. Prev Med 1986;15:195‐202. [DOI] [PubMed] [Google Scholar]
- 9. Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433‐440. [DOI] [PubMed] [Google Scholar]
- 10. Miller PE, Perez V. Low‐calorie sweeteners and body weight and composition: a meta‐analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de la Hunty A, Gibson S, Ashwell M. A review of the effectiveness of aspartame in helping with weight control. Nutr Bull 2006;31:115‐128. [Google Scholar]
- 12. Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight‐control program on short‐ and long‐term control of body weight. Am J Clin Nutr 1997;65:409‐418. [DOI] [PubMed] [Google Scholar]
- 13. Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar‐sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar‐free or sugar‐sweetened beverages and body weight in children. N Engl J Med 2012;367:1397‐1406. [DOI] [PubMed] [Google Scholar]
- 15. Tate DF, Turner‐McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the choose healthy options consciously everyday (choice) randomized clinical trial. Am J Clin Nutr 2012;95:555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maersk M, Belza A, Stodkilde‐Jorgensen H, et al. Sucrose‐sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6‐mo randomized intervention study. Am J Clin Nutr 2012;95:283‐289. [DOI] [PubMed] [Google Scholar]
- 17. Millen BE. Scientific Report of the 2015 Dietary Guidelines Advisory Committee Department of Health and Human Services; Washington D.C: 2015. [Google Scholar]
- 18. Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the united states. Am J Clin Nutr 2006;83:529‐542. [DOI] [PubMed] [Google Scholar]
- 19. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non‐nutritive sweetened beverages on weight loss during a 12‐week weight loss treatment program. Obesity (Silver Spring) 2014;22:1415‐1421. [DOI] [PubMed] [Google Scholar]
- 20. Wyatt HR, Jortberg BT, Babbel C, et al. Weight loss in a community initiative that promotes decreased energy intake and increased physical activity and dairy consumption: calcium weighs‐in. J Phys Act Health 2008;5:28‐44. [DOI] [PubMed] [Google Scholar]
- 21. Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long‐term maintenance of substantial weight loss. Am J Clin Nutr 1997;66:239‐246. [DOI] [PubMed] [Google Scholar]
- 22. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med 2011;26:192‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond) 2006;30:391‐399. [DOI] [PubMed] [Google Scholar]
- 24. Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high‐intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64:1430‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phelan S, Lang W, Jordan D, Wing RR. Use of artificial sweeteners and fat‐modified foods in weight loss maintainers and always‐normal weight individuals. Int J Obes (Lond) 2009;33:1183‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Catenacci VA, Pan Z, Thomas JG, et al. Low/no calorie sweetened beverage consumption in the national weight control registry. Obesity (Silver Spring) 2014;22:2244‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar‐sweetened beverage consumption will reduce the prevalence of obesity and obesity‐related diseases. Obes Rev 2013;14:606‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar‐sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Black RM, Tanaka P, Leiter LA, Anderson GH. Soft drinks with aspartame: effect on subjective hunger, food selection, and food intake of young adult males. Physiol Behav 1991;49:803‐810. [DOI] [PubMed] [Google Scholar]
- 30. Bellisle F, Drewnowski A, Anderson GH, Westerterp‐Plantenga M, Martin CK. Sweetness, satiation, and satiety. J Nutr 2012;142:1149S‐1154S. [DOI] [PubMed] [Google Scholar]
- 31. Appleton KM, Blundell JE. Habitual high and low consumers of artificially‐sweetened beverages: Effects of sweet taste and energy on short‐term appetite. Physiol Behav 2007;92:479‐486. [DOI] [PubMed] [Google Scholar]
- 32. Binkley J, Golub A. Comparison of grocery purchase patterns of diet soda buyers to those of regular soda buyers. Appetite 2007;49:561‐571. [DOI] [PubMed] [Google Scholar]
- 33. Piernas C, Tate DF, Wang X, Popkin BM. Does diet‐beverage intake affect dietary consumption patterns? Results from the choose healthy options consciously everyday (choice) randomized clinical trial. Am J Clin Nutr 2013;97:604‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181‐186. [DOI] [PubMed] [Google Scholar]


