Abstract
Background
The benefits of endovascular repair of ruptured abdominal aortic aneurysm remain controversial, without any strong evidence about advantages in specific subgroups.
Methods
An individual‐patient data meta‐analysis of three recent randomized trials of endovascular versus open repair of abdominal aortic aneurysm was conducted according to a prespecified analysis plan, reporting on results to 90 days after the index event.
Results
The trials included a total of 836 patients. The mortality rate across the three trials was 31·3 per cent for patients randomized to endovascular repair/strategy and 34·0 per cent for those randomized to open repair at 30 days (pooled odds ratio 0·88, 95 per cent c.i. 0·66 to 1·18), and 34·3 and 38·0 per cent respectively at 90 days (pooled odds ratio 0·85, 0·64 to 1·13). There was no evidence of significant heterogeneity in the odds ratios between trials. Mean(s.d.) aneurysm diameter was 8·2(1·9) cm and the overall in‐hospital mortality rate was 34·8 per cent. There was no significant effect modification with age or Hardman index, but there was indication of an early benefit from an endovascular strategy for women. Discharge from the primary hospital was faster after endovascular repair (hazard ratio 1·24, 95 per cent c.i. 1·04 to 1·47). For open repair, 30‐day mortality diminished with increasing aneurysm neck length (adjusted odds ratio 0·69 (95 per cent c.i. 0·53 to 0·89) per 15 mm), but aortic diameter was not associated with mortality for either type of repair.
Conclusion
Survival to 90 days following an endovascular or open repair strategy is similar for all patients and for the restricted population anatomically suitable for endovascular repair. Women may benefit more from an endovascular strategy than men and patients are, on average, discharged sooner after endovascular repair.
Short abstract
Strong evidence of equivalence
Introduction
There have been four randomized trials of endovascular versus open repair of ruptured abdominal aortic aneurysm (AAA), all with the intention of showing the superiority of endovascular aneurysm repair (EVAR). The first trial was a single‐centre pilot trial of 32 patients conducted in Nottingham, UK, between 2002 and 2004; the 30‐day mortality rate was 53 per cent in each treatment group1. Following this, two further trials of similar design were developed in the Amsterdam area of the Netherlands (Amsterdam Acute Aneurysm, AJAX trial) and in France (Endovasculaire versus Chirurgie dans les Anévrysmes Rompus, ECAR trial); patients were considered for randomization only if their aortic anatomy was suitable for EVAR. The sample sizes of these trials were informed by systematic reviews2, 3 of observational series that showed a strong benefit in operative mortality for EVAR compared with open repair. The Amsterdam trial4 randomized 116 patients and failed to show any benefit for EVAR with respect to either the primary endpoint (combined 30‐day morbidity and mortality) or 30‐day mortality. The French trial5 randomized 107 patients and again failed to show any significant difference in 30‐day mortality between the randomized groups. The last trial to start (Immediate Management of the Patient with Ruptured Aneurysm: Open Versus Endovascular repair, IMPROVE trial), and the largest, was in the UK (with 1 Canadian centre), but was based on a different design, with patients being randomized at the point of clinical diagnosis of ruptured aneurysm to an endovascular strategy (EVAR if found anatomically suitable on subsequent CT, open repair if not) versus open repair; this trial was designed to have more than 90 per cent power to detect a 14 per cent difference in 30‐day mortality with recruitment of 600 patients. Even with 613 patients, IMPROVE6 failed to show a 30‐day mortality benefit for the endovascular strategy, partly owing to a lower mortality rate than anticipated in the open repair group. It was large enough to allow limited subgroup analysis6. An editorial commentary7 accompanying publication of this trial suggested that the sample size calculations might have been too optimistic and that, given the advances in intensive therapy, 90‐day survival should have been chosen as the primary outcome. For patients, and their families, the most important outcome is reaching home alive, not 30‐day mortality.
To counter some of these criticisms and permit more robust subgroup analyses, the ruptured aneurysm trialists agreed to collaborate to allow an individual‐patient meta‐analysis of outcomes to 90 days after rupture. Given the different trial designs, an individual‐patient approach has the added advantage of pooling data from similar groups of patients, for example by selecting out those anatomically suitable for EVAR from the IMPROVE trial for comparison with patients from the AJAX and ECAR trials.
Unfortunately, the data from the Nottingham trial could not be retrieved, so this meta‐analysis is based on the data from the AJAX4, ECAR5 and IMPROVE6 trials only. The purpose was to test the hypotheses that, particularly for patients anatomically suitable for EVAR, endovascular repair offers an improvement in 90‐day survival and earlier hospital discharge compared with open repair, as well as identifying whether specific subgroup analyses by age, sex and Hardman index (a validated morbidity score for ruptured aneurysm8, 9) had better 90‐day survival with EVAR. In addition, the meta‐analysis presents 30‐day outcomes, including whether morphological features affected 30‐day mortality and whether abdominal compartment syndrome was more common after EVAR than open repair.
Methods
The methods for the three trials included in this meta‐analysis have been published previously10, 11, 12. The AJAX trial randomized 116 patients, with CT showing probable rupture, and patients being eligible for both open and endovascular repair, in three centres between 2004 and 2011; sealed envelopes were used for randomization to either open or endovascular repair (aortouni‐iliac grafts for EVAR). The ECAR trial randomized 107 patients, with CT showing confirmed rupture and an aortic anatomy suitable for EVAR, and a systolic BP exceeding 80 mmHg; treatment allocation was by weekly rotation, in 14 centres between 2008 and 2012. The IMPROVE trial randomized 613 eligible patients with an in‐hospital clinical diagnosis of ruptured aneurysm in 29 centres between 2009 and 2013; an independent contractor provided telephone randomization, with computer‐generated assignation of patients in a 1 : 1 ratio, using variable block size and stratified by centre. For IMPROVE, patients were randomized before CT to either an endovascular strategy (with open repair if EVAR was not anatomically feasible) or open repair.
The three data sets were merged based on fields available in the case record forms of the largest trial (IMPROVE), range checks were conducted and queries resolved with the individual trial coordinating centres.
Statistical analysis
The primary analyses considered the groups as randomized within each trial, irrespective of the different trial designs. Mortality was assessed at both 30 and 90 days after randomization (for IMPROVE) and following admission (for AJAX and ECAR), with 90‐day survival being the primary outcome of interest. The odds ratio for both 30‐ and 90‐day mortality for the endovascular strategy or EVAR versus open repair was estimated using logistic regression, adjusting for trial to obtain a one‐stage fixed‐effect pooled estimate. A secondary analysis was conducted by estimating the odds ratio separately within each study and then pooling using random‐effects meta‐analysis, with between‐study heterogeneity estimated using a method of moments13. The proportion of between‐trial variability beyond that expected by chance was quantified using the I 2 statistic14. Analyses were then repeated for odds ratios estimated from logistic regression models adjusted for age, sex and Hardman index. Multiple imputation using chained equations was used to account for missing baseline co‐variables in adjusted analyses (Appendix S1, supporting information)15.
Secondary analyses were conducted with the purpose of making the groups in the different trials more homogeneous. Only those patients with a final diagnosis of ruptured AAA and considered suitable for EVAR were retained in the analyses. For AJAX and ECAR, suitability for EVAR was a prerequisite for inclusion in the trial. For the IMPROVE trial, suitability for EVAR was defined as either local CT assessment of suitability or, if not assessed locally, a ‘within liberal instructions for use’ definition from a core laboratory CT analysis was used.
Kaplan–Meier survival plots and cumulative incidence of time to primary hospital discharge were produced by randomized group within each trial separately and accompanied by a log rank test. A Cox proportional hazards model was used to assess whether the randomized groups differed in terms of the cause‐specific hazard of primary hospital discharge (competing in‐hospital mortality not withstanding).
The subgroups age, sex and Hardman index were assessed for differences in the effect of the endovascular and open strategies by including an interaction term between the subgroup and randomized group in a logistic regression model.
The reporting of complications and reinterventions was very different in the three trials, but they all reported on the use of occlusion balloons and the incidence of abdominal compartment syndrome and other non‐occlusive mesenteric hypoperfusion syndromes16; however, the abdominal compartment syndrome data for the AJAX trial were collected retrospectively.
The effect of aortic morphology on 30‐day mortality was also assessed within each trial for AAA diameter, aortic neck diameter, aortic neck length and proximal neck α angulation. Because these analyses are not a comparison between randomized groups, all analyses were adjusted for the following potential confounding factors: age, sex (AJAX and IMPROVE), Hardman index, admission mean arterial BP, treatment commenced and randomized group (IMPROVE only). Analysis of each morphological variable was conducted both adjusted and unadjusted for the other three morphological variables.
Results
The baseline characteristics of the 836 patients are shown in Table 1. Because the design of the IMPROVE trial was different from that of the other two trials, the number of patients with ruptured aortoiliac aneurysm (core laboratory diagnosis) who were anatomically suitable for EVAR is shown. The one patient in ECAR who did not have direct aneurysm repair underwent axillobifemoral bypass. The demographic details are shown for all 836 patients randomized. The patients in IMPROVE tended to be older, with more women included, and patients had larger aneurysms.
Table 1.
Descriptive statistics for the three trials
| AJAX (n = 116) | ECAR (n = 107) | IMPROVE (n = 613) | |
|---|---|---|---|
| Randomized group | |||
| EVAR/EVAR strategy | 57 (49·1) | 56 (52·3) | 316 (51·5) |
| Open repair | 59 (50·9) | 51 (47·7) | 297 (48·5) |
| rAAA suitable for EVAR‡ | n = 113 | n = 104 | n = 310 |
| EVAR/EVAR strategy | 57 (50·4) | 54 (51·9) | 168 (54·2) |
| Open repair | 56 (49·6) | 50 (48·1) | 142 (45·8) |
| Procedure commenced | |||
| EVAR | 57 (49·1) | 56 (52·3) | 192 (31·3) |
| Open repair | 59 (50·9) | 50 (46·7) | 331 (54·0) |
| No aneurysm repair | 0 (0) | 1 (0·9) | 90 (14·7) |
| Age (years)* | 74·2(9·4) | 74·4(10·6) | 76·7(7·6) |
| Sex ratio (M : F) | 99 : 17 | 97 : 10 | 480 : 133 |
| Admission mean arterial BP (mmHg)*, § | n = 113 | n = 104 | n = 601 |
| 87(27) | 108(30) | 81(24) | |
| Hardman index | n = 61 | n = 105 | n = 539 |
| 0 | 26 (43) | 41 (39·0) | 164 (30·4) |
| 1 | 19 (31) | 44 (41·9) | 254 (47·1) |
| 2 | 12 (20) | 11 (10·5) | 94 (17·4) |
| ≥ 3 | 4 (7) | 9 (8·6) | 27 (5·0) |
| Morphology | |||
| Maximum aortic diameter (mm)* |
n = 92 76(16) |
n = 106 77(20) |
n = 518 84(19) |
| Aneurysm neck diameter (mm)*, ¶ |
n = 92 26(4) |
n = 106 24(4) |
n = 430 26(4) |
| Neck length (mm)† |
n = 92 25 (19–34) |
n = 101 21 (15–30) |
n = 481 22 (10–34) |
| Proximal neck angle (°)* |
n = 92 39(21) |
n = 96 34(26) |
n = 478 33(20) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) and
median (i.q.r.).
Suitability for endovascular aneurysm repair (EVAR) in IMPROVE defined by local assessment of suitability if available; otherwise a core laboratory assessment of ‘within liberal instructions for use’ was used to define suitability. Suitability not assessed in 46 ruptured abdominal aortic aneurysms (rAAAs) in IMPROVE and one in ECAR.
Mean arterial BP recorded only in ECAR; for AJAX and IMPROVE it was approximated by 2/3 diastolic + 1/3 systolic BP.
IMPROVE and ECAR measured top neck diameter; AJAX measured maximum neck diameter.
The operative details and discharge data for the patients are shown in Table 2. Both AJAX and ECAR used predominantly aortouni‐iliac endografts, which restricted completion of the procedure, with femorofemoral cross‐over grafts, under local anaesthesia. The in‐hospital mortality rate was higher for IMPROVE (37·4 per cent) than for either AJAX (27·6 per cent) or ECAR (27·9 per cent) but, for those discharged alive, patients in IMPROVE were discharged sooner and more often directly to home. Overall survival to 90 days is shown in Fig. 1.
Table 2.
Operative data and outcomes
| AJAX (n = 116) | ECAR (n = 107) | IMPROVE (n = 613) | |
|---|---|---|---|
| Time to start of repair (h)*, † | |||
| Randomized to EVAR | 1·23 | 2·9 | 0·77 |
| Randomized to open repair | 0·75 | 1·3 | 0·61 |
| Operative data | |||
| Anaesthesia initiated in those who | n = 55 | n = 56 | n = 188 |
| commenced EVAR | |||
| General anaesthetic | 29 (53) | 48 (86) | 85 (45·2) |
| Local anaesthetic | 26 (47) | 8 (14) | 103 (54·8) |
| Graft type | |||
| EVAR commenced | n = 57 | n = 56 | n = 182 |
| Aortouni‐iliac | 49 (86) | 43 (77) | 39 (21·4) |
| Bifurcated | 4 (7) | 12 (21) | 136 (74·7) |
| Tube/other‡ | 4 (7) | 1 (2) | 7 (3·8) |
| Open repair commenced | n = 57 | n = 48 | n = 293 |
| Aortouni‐iliac | 0 (0) | 0 (0) | 1 (0·3) |
| Bifurcated | 7 (12) | 22 (46) | 55 (18·8) |
| Tube | 50 (88) | 26 (54) | 237 (80·9) |
| Length of time in theatre for those who | n = 111 | n = 100 | n = 501 |
| commenced EVAR or open repair (min)* | 177 (150–210) | 180 (135–250) | 180 (147–235) |
| Time of surgery for those who commenced | |||
| EVAR or open repair | |||
| In routine working hours§ | 42 of 116 (36·2) | 28 of 105 (26·7) | 173 of 515 (33·6) |
| Monday–Friday | 96 of 116 (82·8) | 81 of 106 (76·4) | 404 of 515 (78·5) |
| Outcomes | |||
| Discharged alive | 84 of 116 (72·4) | 75 of 104 (72·1) | 384 of 613 (62·6) |
| Death within 30 days | |||
| EVAR | 12 of 57 (21) | 10 of 55 (18) | 112 of 316 (35·4) |
| Open repair | 15 of 59 (25) | 12 of 50 (24) | 111 of 297 (37·4) |
| Missing | 0 | 2 | 0 |
| Death within 90 days | |||
| EVAR | 15 of 57 (26) | 11 of 53 (21) | 120 of 316 (38·0) |
| Open repair | 17 of 59 (29) | 17 of 45 (38) | 118 of 296 (39·9) |
| Missing | 0 | 9 | 1 |
| Place of discharge | n = 84 | n = 65 | n = 384 |
| Another hospital | 2 (2) | 3 (5) | 36 (9·4) |
| Home | 64 (76) | 38 (58) | 330 (85·9) |
| Nursing, residential or sheltered home | 18 (21) | 23 (35) | 8 (2·1) |
| Other | 0 (0) | 1 (2) | 10 (2·6) |
| Length of stay for those discharged | n = 84 | n = 75 | n = 384 |
| alive (days)* | 14 (8–22) | 15 (11– 26) | 11 (7–20) |
Values in parentheses are percentages unless otherwise indicated;
values are median (i.q.r.).
From admission in AJAX and ECAR, and from randomization in IMPROVE.
Includes conversion to open repair and extra‐anatomic bypass.
Monday to Friday between 08.30 and 16.30 hours in the UK; Monday to Friday between 08.00 and 17.00 hours in the Netherlands and France. EVAR, endovascular aneurysm repair.
Figure 1.
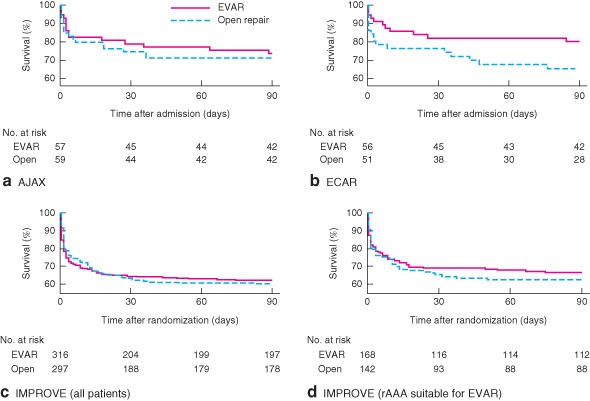
Kaplan–Meier survival plots up to 90 days by trial and randomized group: a AJAX, b ECAR, c IMPROVE and d patients with ruptured abdominal aortic aneurysm (rAAA) suitable for endovascular repair (EVAR) in IMPROVE. a P = 0·866, b P = 0·048, c P = 0·899, d P = 0·890 (log rank test)
At 90 days there was slight evidence of heterogeneity in the odds ratios (I 2=18·9 per cent), with the odds ratio in ECAR being lower than in the other trials, although several patients in this trial had been lost to follow‐up (Fig. 2 a). Despite this, the overall pooled odds ratio for survival at 90 days was not significant (0·85, 95 per cent c.i. 0·64 to 1·13), with a random‐effects estimate of 0·82 (0·56 to 1·18). Adjusted analyses gave similar results and those that restricted the population to ruptured aneurysms suitable for EVAR were more homogeneous (pooled odds ratio 0·74, 0·51 to 1·08) (Fig. 2 b).
Figure 2.
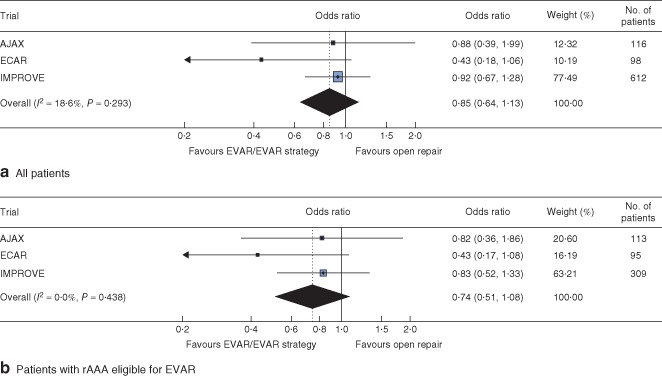
Analysis of 90‐day mortality by randomized group: a all patients and b patients with ruptured abdominal aortic aneurysm (rAAA) eligible for both endovascular aneurysm repair (EVAR) and open repair. Odds ratios are shown with 95 per cent c.i.
Similar results were seen at 30 days, with little evidence of a benefit of an endovascular strategy or EVAR among any of the three trials comparing patients as randomized (Fig. S1a, supporting information), and estimated only in patients with confirmed rupture and aortic anatomy suitable for EVAR (Fig. S1b, supporting information).
There was also little evidence of the treatment effect changing by age or Hardman index at either 30 days (Fig. S2, supporting information) or 90 days (Fig. 3). However, for age, 90‐day results were heterogeneous across the trials (I2 = 74·2 per cent, P = 0·021), with AJAX suggesting a benefit of open repair and ECAR suggesting a benefit of EVAR for older patients. There were no early deaths among women in the ECAR trial. In the pooled analysis, women benefited more from an endovascular strategy than men (ratio of odds ratios 0·49, 95 per cent c.i. 0·24 to 0·99). A similar ratio of odds ratios was estimated at 30 days (0·47, 0·23 to 0·97).
Figure 3.
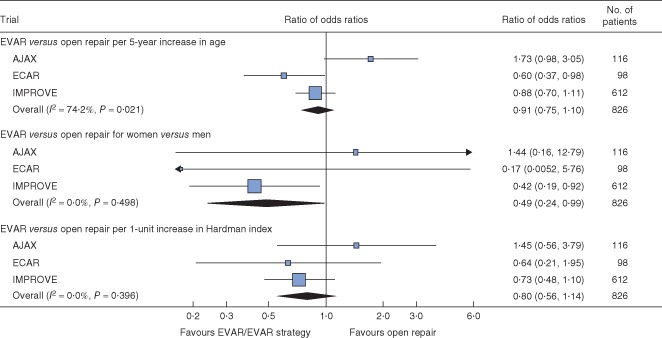
Analysis of 90‐day mortality by randomized group with subgroup analyses for age, sex and Hardman index. Multiple imputation was used for Hardman index. With small numbers in the ECAR trial (2 of 9 deaths within 90 days in women), the trial‐specific subgroup effect for sex was calculated by adding a continuity correction of 0·5 to all cells in the contingency table. Ratios of odds ratios are shown with 95 per cent c.i. EVAR, endovascular aneurysm repair
Although the IMPROVE trial had suggested that the 30‐day mortality for open repair was higher when patients were randomized outside routine working hours, this finding could not be substantiated across the three trials.
Occlusion balloons were not used widely, but were employed more often in ECAR (18 of 107, 16·8 per cent), than in either AJAX (4 of 116, 3·4 per cent) or IMPROVE (32 of 522, 6·1 per cent). The reporting of complications was very different in the three trials; however, abdominal hypoperfusion syndromes (including abdominal compartment syndrome) were reported in all trials (Table 3). Only ECAR routinely reported abdominal compartment syndrome according to established methods17 and searched actively for colonic ischaemia, perhaps explaining the higher rates reported in this trial. Abdominal compartment syndrome was reported more often in the EVAR groups of AJAX and ECAR, but not in the endovascular strategy group of IMPROVE (pooled odds ratio 1·64, 95 per cent c.i. 0·87 to 3·09). However, in all trials there was an indication of a lower incidence of isolated mesenteric and/or colonic ischaemia in the endovascular strategy or EVAR groups; the pooled odds ratio (0·57, 0·32 to 1·01) narrowly failed to achieve statistical significance.
Table 3.
Reported incidence of abdominal compartment syndrome and other mesenteric hypoperfusion syndromes, colonic ischaemia and mesenteric ischaemia16 by randomized group
| Trial | Syndrome | EVAR/endovascular strategy | Open repair |
|---|---|---|---|
| AJAX |
ACS CMI |
5 of 57 (9) 2 of 57 (4) |
2 of 59 (3) 5 of 59 (8) |
| ECAR |
ACS CMI |
8 of 56 (14) 4 of 56 (7) |
1 of 51 (2) 8 of 51 (16) |
| IMPROVE* |
ACS CMI |
14 of 259 (5·4) 14 of 259 (5·4) |
13 of 243 (5·3) 19 of 243 (7·8) |
Values in parentheses are percentages.
The denominator is the number of ruptured aneurysms repaired. EVAR, endovascular aneurysm repair; ACS, abdominal compartment syndrome; CMI, chronic mesenteric ischaemia.
There was wide variation in discharge policy across the three trials, with many patients in ECAR being discharged to other care facilities, whereas more patients in IMPROVE were discharged directly to home. Overall, for patients discharged alive from the vascular surgical centre, the duration of primary hospital stay was shorter for patients in the endovascular strategy or EVAR groups (pooled hazard ratio for discharge 1·24, 95 per cent c.i. 1·04 to 1·47) versus open repair (Fig. 4; Fig. S3, supporting information).
Figure 4.
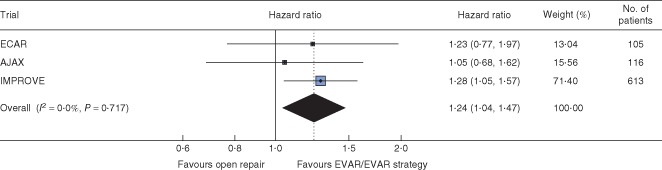
Hazard ratios, with 95 per cent c.i., for time to discharge alive from primary admission hospital by randomized group. EVAR, endovascular aneurysm repair
The morphological parameters for aneurysms in the three trials are shown in Table 1. Patients in IMPROVE had larger aneurysm diameters and a very different distribution of aneurysm neck lengths compared with the other trials (Fig. S4, supporting information). As previously observed for the IMPROVE trial18, aneurysm neck length (Fig. 5), but not AAA diameter, neck diameter or proximal neck angle (Figs. S5–S7, supporting information), appeared to be a predictor of 30‐day mortality, particularly after open repair. For open repair, for each 15‐mm increase in neck length, 30‐day mortality decreased (odds ratio 0·69, 95 per cent c.i. 0·53 to 0·89); with both operations combined the association was slightly weaker (odds ratio 0·80, 0·66 to 0·97).
Figure 5.
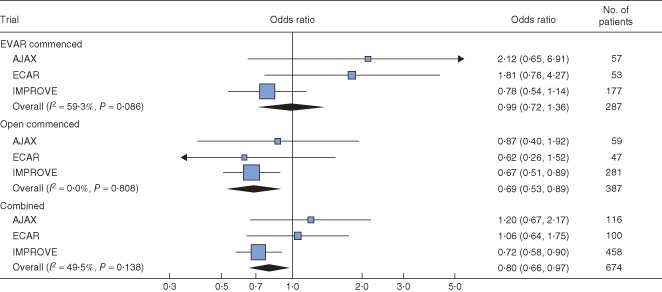
Effect of aneurysm neck length on 30‐day mortality, by treatment commenced. The analysis was restricted to patients with ruptured abdominal aortic aneurysm who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation was used to account for missing data. Odds ratios of 30‐day mortality per 15‐mm increase in aneurysm neck length are shown with 95 per cent c.i. EVAR, endovascular aneurysm repair
Discussion
The best evidence comes from synthesis of the results of randomized trials. Three recent randomized trials of endovascular versus open repair of ruptured AAA showed no significant survival benefit at 90 days for endovascular repair (pooled odds ratio 0·85, 95 per cent c.i. 0·64 to 1·13), but with slight heterogeneity. Perhaps the heterogeneity was expected given that the trials had very different designs, although estimating the extent of between‐study heterogeneity is difficult with only three trials19. The additional flexibility of individual‐patient meta‐analysis also allowed only specific patient subsets to be included in the meta‐analysis. For instance, when the analysis included only those patients in IMPROVE with confirmed rupture eligible for both treatments, as in the AJAX and ECAR trials, at 90 days the trial odds ratios were more homogeneous and the pooled odds ratio was lower, but with a wider confidence interval (0·74, 95 per cent c.i. 0·51 to 1·08) because a smaller number of patients contributed to this analysis. Similarly, at 30 days there was no evidence that EVAR offered a survival advantage in patients with confirmed rupture eligible for both endovascular and open repair.
Another benefit of an individual‐patient meta‐analysis is the ability to identify subgroups of patients who may benefit from a specific treatment. Women appeared to be under‐represented in both the ECAR and AJAX trials, so the estimate of the influence of sex was dominated by the larger IMPROVE trial. Women appear to gain twice as much benefit from an endovascular strategy or EVAR versus open repair than men, although the pooled effect was of borderline significance. The reasons for this potential benefit in women are still uncertain, although morphology may play a role18. This begins to suggest that, although further research is needed, EVAR should be a more common treatment option for women. The weak trend for patients with higher Hardman index (more unfit) to have better 30‐day survival after EVAR was not substantiated across the three trials and there was no clear effect of age.
The three trials were set in different healthcare systems, as clearly shown by the time to discharge and destination after discharge. Patients in France and the Netherlands stayed in hospital longer and were more often discharged to a step‐down care facility than patients in IMPROVE, where more patients were discharged directly to home. Nevertheless the time to discharge was shorter in the endovascular strategy or EVAR groups. In contrast, for each trial, it took longer to get a patient to EVAR than to open repair, the difference being greatest in France. This too is likely to be a reflection of both the stability of the patient, with ECAR having the most stable patients, and the different healthcare organizations. There is weak evidence that delays in time to treatment influence survival adversely20, 21. The longer time taken to start definitive EVAR could have contributed to some of the anticipated benefit of endovascular repair on 30‐day mortality being eroded.
The specificity of CT for the detection of ruptured AAA has been questioned22. In the AJAX trial three patients who underwent open repair were found to have an intact AAA but a different pathology, such as bleeding liver tumour or Crohn's disease. At endovascular repair, the underlying cause of admission might not have been ascertained correctly in a few patients, potentially increasing the mortality of those in the EVAR/endovascular strategy groups.
The largest trial, IMPROVE, also conducted cohort analyses and two of the observations reported were assessed in individual patients from the three trials. The findings from IMPROVE included the observation of higher mortality risk for open repair outside routine working hours23 and the strong influence of aneurysm neck length on mortality after open repair18. Overall there was no evidence for a higher mortality rate among patients treated outside routine working hours. The strong relationship between short aneurysm neck length and high mortality after open repair may help explain why the mortality rate after open repair remains high; around one‐quarter of the patients contributed by the IMPROVE trial had a juxtarenal aneurysm (aneurysm neck length 0–9 mm) and were not eligible for conventional EVAR. Open juxtarenal aneurysm repair requires cross‐clamping of the aorta above the renal arteries, with inevitable compromise of the visceral circulation, especially in shocked patients. In contrast, for longer aneurysm necks (15 mm or more), an infrarenal aortic clamp is likely to be used for open repair, with operative mortality similar to that for EVAR18. In the future, the reporting of results for open repair of ruptured AAA should be in two categories: infrarenal aneurysms and juxtarenal aneurysms.
Some other clinical messages are consistent across all three trials. The reported rate of abdominal compartment syndrome was low, similar to the 8 per cent reported in a recent meta‐analysis24, and only the ECAR trial suggested a higher rate after EVAR. Abdominal compartment syndrome is characterized by intra‐abdominal hypertension. As bladder pressures were measured only in the ECAR trial, underdetection of abdominal compartment syndrome is likely. However, other non‐occlusive mesenteric hypoperfusion syndromes are recognized, including mesenteric and colonic ischaemia, and these tended to be more common after open repair16. The total rate of non‐occlusive hypoperfusion complications reported was much higher than the 8 per cent noted for abdominal compartment syndrome.
This individual‐patient meta‐analysis across three European trials concluded that there is no early survival benefit for an endovascular strategy or EVAR following ruptured AAA, although there is a very weak indication in favour of EVAR at 90 days for patients with ruptured AAA, who are eligible for both treatments. The meta‐analysis continues to suggest that women may have improved early survival with an EVAR/endovascular strategy and that patients in the endovascular strategy or EVAR groups benefit from earlier hospital discharge. These data might be sufficient for patients to prefer EVAR, but longer‐term data from all three trials will be needed to inform clinical decision‐making.
Collaborators
Ruptured Aneurysm Trialists are listed by trial.
AJAX trial investigators: R. Balm, M. J. W. Koelemay, M. M. Idu, C. Kox, D. A. Legemate, L. C. Huisman, M. C. M. Willems, J. A. Reekers, O. M. van Delden, K. P. van Lienden (Academic Medical Centre, Amsterdam, The Netherlands; 39 patients); L. L. Hoornweg, J. J. Reimerink, S. C. van Beek (trial coordinators; Academic Medical Centre, Amsterdam, The Netherlands); A. C. Vahl, V. J. Leijdekkers, J. Bosma, A. D. Montauban van Swijndregt, C. de Vries, V. P. M. van der Hulst, J. Peringa, J. G. A. M. Blomjous, M. J. T. Visser, F. H. W. M. van der Heijden (Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands; 46 patients); W. Wisselink, A. W. J. Hoksbergen, J. D. Blankensteijn, H. M. E. Coveliers, J. H. Nederhoed, F. G. van den Berg, B. B. van der Meijs, M. L. P. van den Oever, R. J. Lely, M. R. Meijerink, I. Westra (VU University Medical Centre, Amsterdam, The Netherlands; 31 patients); A. Voorwinde, J. M. Ultee, R. C. van Nieuwenhuizen (Sint Lucas Andreas Ziekenhuis, Amsterdam, The Netherlands; referring centre); B. J. Dwars, T. O. M. Nagy (Slotervaartziekenhuis, Amsterdam, The Netherlands; referring centre); P. Tolenaar, A. M. Wiersema (Boven IJ Ziekenhuis, Amsterdam, The Netherlands; referring centre); J. A. Lawson, P. J. van Aken, D. A. A. Stigter (Ziekenhuis Amstelland, Amstelveen, The Netherlands; referring centre); T. A. A. van den Broek, G. A. Vos (Waterlandziekenhuis, Purmerend, The Netherlands; referring centre); W. Mulder, R. P. Strating (Zaans Medisch Centrum, Zaandam, The Netherlands; referring centre); D. Nio, G. J. M. Akkersdijk, A. van der Elst (Spaarne Ziekenhuis; Hoofddorp, The Netherlands); P. van Exter (regional ambulance services).
ECAR trial investigators: P. Desgranges, J.‐P. Becquemin, E. Allaire, F. Cochennec, J. Marzelle, N. Louis, J. Schneider, M. Majewski (Centre Hospitalier Universitaire (CHU) Henri Mondor, Créteil, France); Y. Castier, G. Leseche, F. Francis (CHU Bichat, Paris, France); E. Steinmetz, J.‐P. Berne, C. Favier (CHU Dijon, Dijon, France); S. Haulon, M. Koussa, R. Azzaoui, D. Piervito (Centre Hospitalier Régional Universitaire (CHRU) Lille, Lille, France); Y. Alimi, M. Boufi, O. Hartung, P. Cerquetta (Hôpital Nord Marseille, Marseille, France); P. Amabile, P. Piquet, J. Penard, M. Demasi (CHU Marseille, Marseille, France); P. Alric, L. Canaud, J.‐P. Berthet (CHU Montpellier, Montpellier, France); P. Julia, J.‐N. Fabiani, J. M. Alsac (CHU Hôpital Européen Georges‐Pompidou, Paris, France); P. Gouny, A. Badra, J. Braesco (CHU Brest, Brest, France); J.‐P. Favre, J.‐N. Albertini (CHU Saint Etienne, Saint Etienne, France); R. Martinez (CHRU Tours, Tours, France); R. Hassen‐Khodja, M. Batt, E. Jean, M. Sosa, S. Declemy (CHU Nice, Nice, France); L. Destrieux‐Garnier (Centre Hospitalier Régional Annecy, Annecy, France); P. Lermusiaux, P. Feugier (CHU Lyon, Lyon, France).
IMPROVE trial investigators: management committee – J. T. Powell (Chair; Imperial College London, UK); R. Ashleigh (University Hospital of South Manchester, Manchester, UK), M. Gomes (London School of Hygiene and Tropical Medicine, London, UK), R. M. Greenhalgh (Imperial College London, UK), R. Grieve (London School of Hygiene and Tropical Medicine, London, UK), R. Hinchliffe (St George's Hospital, London, UK), M. Sweeting (University of Cambridge, Cambridge, UK), M. M. Thompson (St George's Hospital, London, UK), S. G. Thompson (University of Cambridge, Cambridge, UK), P. Ulug (Imperial College London, UK). N. J. Cheshire (Imperial College Healthcare NHS Trust, London, UK; 20 patients); J. R. Boyle (Addenbrooke's Hospital, Cambridge, UK; 40 patients); F. Serracino‐Inglott, J. V. Smyth (Manchester Royal Infirmary, Manchester, UK; 69 patients); M. M. Thompson, R. J. Hinchliffe (St George's Hospital, London, UK; 75 patients); R. Bell (Guy's and St Thomas' Hospital, London, UK; 81 patients); N. Wilson (Kent and Canterbury Hospital, Canterbury, UK; 23 patients); M. Bown, M. Dennis (Leicester Royal Infirmary, Leicester, UK; 18 patients); M. Davis (Royal Free Hospital, London, UK; 1 patient); R. Ashleigh (University Hospital of South Manchester, Manchester, UK; 21 patients); S. Howell (Leeds General Infirmary, Leeds, UK; 23 patients); M. G. Wyatt (Freeman Hospital, Newcastle upon Tyne, UK; 23 patients); D. Valenti (King's College Hospital, London, UK; 2 patients); P. Bachoo (Aberdeen Royal Infirmary, Aberdeen, UK; 4 patients); P. Walker (James Cook University Hospital, Middlesbrough, UK; 5 patients); S. MacSweeney (Queen's Medical Centre, Nottingham, UK; 34 patients); J. N. Davies (Royal Cornwall Hospital, Truro, UK; 5 patients); D. Rittoo, S. D. Parvin (Royal Bournemouth Hospital, Bournemouth, UK; 22 patients); W. Yusuf (Royal Sussex County Hospital, Brighton, UK; 5 patients); C. Nice (Queen Elizabeth Hospital, Gateshead, UK; 5 patients); I. Chetter (Hull Royal Infirmary, Hull, UK; 32 patients); A. Howard (Colchester General Hospital, Colchester, UK; 24 patients); P. Chong (Frimley Park Hospital, Frimley, UK; 14 patients); R. Bhat (Ninewells Hospital, Dundee, UK; 8 patients); D. McLain (Royal Gwent Hospital, Newport, UK); A. Gordon, I. Lane (University Hospital of Wales, Cardiff, UK; 4 patients); S. Hobbs (New Cross Hospital, Wolverhampton, UK; 3 patients); W. Pillay (Doncaster Royal Infirmary, Doncaster, UK; 8 patients); T. Rowlands, A. El‐Tahir (Royal Derby Hospital, Derby, UK; 13 patients); J. Asquith (University Hospital of North Staffordshire, Stoke‐on‐Trent, UK; 15 patients); S. Cavanagh (York Hospital, York, UK; 3 patients); L. Dubois, T. L. Forbes (London Health Sciences Centre, University of Western Ontario, London, Ontario, Canada; 13 patients).
Supporting information.
Additional supporting information may be found in the online version of this article:
Appendix S1 Multiple imputation models (Word document)
Table S1 Variables considered for multiple imputations and imputation model considered (Word document)
Fig. S1 Thirty‐day mortality by randomized group: a for 834 patients, b restricted to 525 patients with ruptured abdominal aortic aneurysm eligible for both endovascular and open repair (Word document)
Fig. S2 Thirty‐day mortality by randomized group with subgroup analyses for age, sex and Hardman index (Word document)
Fig. S3 Cumulative incidence of time to primary hospital discharge by trial and randomized group (Word document)
Fig. S4 Aortic neck length by trial (Word document)
Fig. S5 Effect of abdominal aortic aneurysm diameter on 30‐day mortality, by treatment commenced (Word document)
Fig. S6 Effect of aortic neck diameter on 30‐day mortality, by treatment commenced (Word document)
Fig. S7 Effect of proximal neck angulation on 30‐day mortality, by treatment commenced (Word document)
Supporting information
Appendix S1 Multiple imputation models
TableS1 Variables considered for multiple imputations and imputation model considered
FigS1 Thirty‐day mortality by randomized group: a for 834 patients, b restricted to 525 patients with ruptured abdominal aortic aneurysm eligible for both endovascular (EVAR) and open aneurysm repair
FigS2 Thirty‐day mortality by randomized group with subgroup analyses for age, sex and Hardman index. Multiple imputation was used for Hardman index. With small numbers in the ECAR trial (0 of 10 deaths within 30 days in women), the trial‐specific subgroup effect for sex was calculated by adding a continuity correction of 0.5 to all cells in the contingency table. EVAR, endovascular aneurysm repair
FigS3 Cumulative incidence of time to primary hospital discharge by trial and randomized group, and restricting IMPROVE data to ruptured abdominal aortic aneurysm (AAA) suitable for endovascular aneurysm repair (EVAR)
FigS4 Aortic neck length by trial, and restricting IMPROVE data to ruptured abdominal aortic aneurysm suitable for endovascular repair (EVAR). All patients with rupture in the common iliac arteries of an aortoiliac aneurysm were excluded
FigS5 Effect of abdominal aortic aneurysm (AAA) diameter on 30‐day mortality, by treatment commenced. The analysis was restricted to patients with ruptured AAA who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation used to account for missing data
FigS6 Effect of aortic neck diameter on 30‐day mortality, by treatment commenced. The analysis was restricted to ruptured patients with ruptured abdominal aortic aneurysm who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation was used to account for missing data
FigS7 Effect of proximal neck angulation on 30‐day mortality, by treatment commenced. The analysis was restricted to ruptured patients with ruptured abdominal aortic aneurysm who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation was used to account for missing data
Acknowledgements
This analysis was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme (project number 07/37/64). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Health Technology Assessment programme, NIHR, National Health Service or the Department of Health. The AJAX trial was supported by Netherlands Heart Foundation project 2002B197. The ECAR trial was supported by the French Ministry of Health. The IMPROVE trial was supported by Health Technology Assessment project 07/37/64 (as above).
Disclosure: The authors declare no conflict of interest.
Summary of main findings presented to the Annual Meeting of the Vascular Society of Great Britain and Ireland, Glasgow, UK, November 2014
References
- 1. Hinchliffe RJ, Bruijstens L, MacSweeney ST, Braithwaite BD. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm – results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg 2006; 32: 506–513. [DOI] [PubMed] [Google Scholar]
- 2. Mastracci TM, Garrido‐Olivares L, Cinà CS, Clase CM. Endovascular repair of ruptured abdominal aortic aneurysms: a systematic review and meta‐analysis. J Vasc Surg 2008; 47: 214–221. [DOI] [PubMed] [Google Scholar]
- 3. Harkin DW, Dillon M, Blair PH, Ellis PK, Kee F. Endovascular ruptured abdominal aortic aneurysm repair (EVRAR): a systematic review. Eur J Vasc Endovasc Surg 2007; 34: 673–681. [DOI] [PubMed] [Google Scholar]
- 4. Reimerink JJ, Hoornweg LL, Vahl AC, Wisselink W, van den Broek TA, Legemate DA et al Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg 2013; 258: 248–256. [DOI] [PubMed] [Google Scholar]
- 5. Desgranges P, Kobeiter H, Katsahian S, Boufi M, Gouny P, Favre J‐P et al ECAR (Endovasculaire ou Chirurgie dans les Anévrysmes aorto‐iliaques Rompus): a French randomized controlled trial of endovascular vs. open surgical repair of ruptured aorto‐iliac aneurysms. Eur J Vasc Endovasc Surg 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. IMPROVE trial investigators , Powell JT, Sweeting MJ, Thompson MM, Ashleigh R, Bell R et al Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014; 348: f7661. [DOI] [PubMed] [Google Scholar]
- 7. Björck M. Surgery for ruptured abdominal aortic aneurysm. BMJ 2014; 348: g95. [DOI] [PubMed] [Google Scholar]
- 8. Hardman DT, Fisher CM, Patel MI, Neale M, Chambers J, Lane R et al Ruptured abdominal aortic aneurysms: who should be offered surgery? J Vasc Surg 1996; 23: 123–129. [DOI] [PubMed] [Google Scholar]
- 9. Acosta S, Ogren M, Bergqvist D, Lindblad B, Dencker M, Zdanowski Z. The Hardman index in patients operated on for ruptured abdominal aortic aneurysm: a systematic review. J Vasc Surg 2006; 44: 949–954. [DOI] [PubMed] [Google Scholar]
- 10. Hoornweg LL, Wisselink W, Vahl A, Balm R; Amsterdam Acute Aneurysm Trial Collaborators . The Amsterdam Acute Aneurysm Trial: suitability and application rate for endovascular repair of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2007; 33: 679–683. [DOI] [PubMed] [Google Scholar]
- 11. Desgranges P, Kobeiter H, Castier Y, Sénéchal M, Majewski M, Krimi A. The Endovasculaire vs Chirurgie dans les Anévrysmes Rompus PROTOCOL trial update. J Vasc Surg 2010; 51: 267–270. [DOI] [PubMed] [Google Scholar]
- 12. Powell JT. Time to IMPROVE the management of ruptured abdominal aortic aneurysm: IMPROVE trialists. Eur J Vasc Endovasc Surg 2009; 38: 237–238. [DOI] [PubMed] [Google Scholar]
- 13. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30: 377–399. [DOI] [PubMed] [Google Scholar]
- 16. Björck M, Wanhainen A. Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg 2010; 23: 54–64. [DOI] [PubMed] [Google Scholar]
- 17. Björck M, Wanhainen A. Management of abdominal compartment syndrome and the open abdomen. Eur J Vasc Endovasc Surg 2014; 47: 279–287. [DOI] [PubMed] [Google Scholar]
- 18. IMPROVE Trial Investigators . The effect of aortic morphology on peri‐operative mortality of ruptured abdominal aortic aneurysm. Eur Heart J 2015; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012; 41: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groves EM, Khoshchehreh M, Le C, Malik S. Effects of weekend admission on the outcomes and management of ruptured aortic aneurysms. J Vasc Surg 2014; 60: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salhab M, Farmer J, Osman I. Impact of delay on survival in patients with ruptured abdominal aortic aneurysm. Vascular 2006; 14: 38–42. [DOI] [PubMed] [Google Scholar]
- 22. Hoornweg LL, Wisselink W, Vahl AC, Reekers JA, van Delden OM, Legemate DA et al Interobserver and intraobserver variability of interpretation of CT‐angiography in patients with a suspected abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 2008; 35: 295–300. [DOI] [PubMed] [Google Scholar]
- 23. IMPROVE trial investigators ; Powell JT, Hinchliffe RJ, Thompson MM, Sweeting MJ, Ashleigh R, Bell R. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 2014; 101: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karkos CD, Menexes GC, Patelis N, Kaliogirou TE, Giagtzidis IT, Harkin DW. A systematic review and meta‐analysis of abdominal compartment syndrome after endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2014; 59: 829–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Multiple imputation models
TableS1 Variables considered for multiple imputations and imputation model considered
FigS1 Thirty‐day mortality by randomized group: a for 834 patients, b restricted to 525 patients with ruptured abdominal aortic aneurysm eligible for both endovascular (EVAR) and open aneurysm repair
FigS2 Thirty‐day mortality by randomized group with subgroup analyses for age, sex and Hardman index. Multiple imputation was used for Hardman index. With small numbers in the ECAR trial (0 of 10 deaths within 30 days in women), the trial‐specific subgroup effect for sex was calculated by adding a continuity correction of 0.5 to all cells in the contingency table. EVAR, endovascular aneurysm repair
FigS3 Cumulative incidence of time to primary hospital discharge by trial and randomized group, and restricting IMPROVE data to ruptured abdominal aortic aneurysm (AAA) suitable for endovascular aneurysm repair (EVAR)
FigS4 Aortic neck length by trial, and restricting IMPROVE data to ruptured abdominal aortic aneurysm suitable for endovascular repair (EVAR). All patients with rupture in the common iliac arteries of an aortoiliac aneurysm were excluded
FigS5 Effect of abdominal aortic aneurysm (AAA) diameter on 30‐day mortality, by treatment commenced. The analysis was restricted to patients with ruptured AAA who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation used to account for missing data
FigS6 Effect of aortic neck diameter on 30‐day mortality, by treatment commenced. The analysis was restricted to ruptured patients with ruptured abdominal aortic aneurysm who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation was used to account for missing data
FigS7 Effect of proximal neck angulation on 30‐day mortality, by treatment commenced. The analysis was restricted to ruptured patients with ruptured abdominal aortic aneurysm who underwent CT, commenced treatment and did not have a common iliac aneurysm. All analyses were adjusted for age, sex, Hardman index, admission mean arterial BP, treatment commenced and randomized group. Multiple imputation was used to account for missing data


