Abstract
Objective
The current review summarizes recent advances in the origin of brown adipocytes in rodents and humans.
Methods
This review describes recent insights into induction of the brown adipocyte phenotype (BAP) in white fat (WAT) revealed by murine studies during the early postnatal period and reversible temperature transitions. The origin of adipocytes and identity of progenitors as indicated by lineage tracing experiments are reviewed.
Results
We describe a genetic model for brown adipocyte development that involves the appearance of brown adipocytes in WAT at 21 days of age and a mechanism of post‐weaning involution relevant for acquisition of the BAP in fully functional WAT in mice. Under normal physiological conditions, the BAP is dormant with the potential to be stimulated by changes in the external environment. Current evidence for the acquisition of brown adipocytes by interconversion of mature adipocytes versus de novo recruitment of progenitors suggests that mechanisms for acquisition of the BAP in WAT in mice are depot‐specific and controlled by allelic variation.
Conclusions
Although the BAP is highly variable among mice, there is no information on genetic variability in the expression of brown adipocytes in humans. Thus, deeper understanding of genetic mechanisms underlying development of functional brown adipocytes is crucial.
Introduction
Obesity develops as a result of chronic energy imbalance, when energy consumption exceeds energy expenditure (EE). Most of the currently available pharmacological treatments of obesity reduce food intake or decrease the efficiency of food absorption in the intestines. Recent identification of metabolically active brown adipose tissue (BAT) in adult humans 1, 2, 3, 4 has markedly increased the interest in the therapeutic potential of BAT and its role in the regulation of energy homeostasis. BAT increases EE in response to sympathetic stimulation in murine models of obesity. Thus, increasing BAT activity in individuals with obesity could be an effective therapeutic alternative for patients who are incapable of physical activity or fail to follow a dietary regimen.
This review summarizes recent advances in brown adipocyte (BA) development and/or activation in rodents and humans. The goal being to identify effective strategies for induction of BAs that can serve as an anti‐obesity therapy based on maximizing the capacity for thermogenesis. Using murine models we outline the critical time for development of brown and white adipose tissue (WAT), including their lineages, molecular signatures, and depot‐specific differences. We describe the progression in the development of functional BAs in WAT from the early postnatal period that first involves a biosynthetic phase from birth until 21 days of age, followed by development of a mechanism of involution in the post‐weaning period that is essential for the dynamic brown adipocyte phenotype (BAP) that waxes and wanes with the requirements for thermogenesis. We propose that the BAP in WAT is dormant and can be stimulated as soon as WAT develops its fully functional structure. Then we discuss current knowledge on the origin of BAs in WAT and acquisition of the BAP in adult WAT, evaluating mechanisms for the interconversion of mature adipocytes versus de novo recruitment of progenitors, including depot and genetic variability. Finally, we review the molecular identity of human BAT and the recruitment and/or activation of BAT in humans.
Structural and Functional Features of WAT Versus BAT
Adipose tissue in mammals is classified into two major types serving opposite functions in energy balance regulation. WAT is responsible for storing excess energy as triacylglycerols, de novo synthesis of triacylglycerols from glucose and mobilization of energy in the form of free fatty acids. As an endocrine organ, WAT contributes to the regulation of energy homeostasis by secretion of molecules active in the control of food intake, insulin sensitivity and inflammatory responses 5. The primary role of BAT is to uncouple oxidative phosphorylation for defense against hypothermia. Non‐shivering thermogenesis is essential for thermoregulation in small animals and neonates, characterized by a large surface‐to‐volume ratio, and the arousal from hibernation in mammals 6. The majority of a cell volume of white adipocytes (WAs) is occupied by a single, large lipid droplet, with a ring of cytoplasm compacted to a thin layer surrounding the lipid particle and a nucleus displaced to the periphery of the cell. In contrast, BAs contain multiple small lipid droplets and mitochondria, dispersed within the cytoplasm, that contain the uncoupling protein 1 (UCP1), which dissipates the electrochemical gradient driving ATP synthesis, increases the activity of the respiratory chain and generates heat.
Development of Classical BAs
Interscapular BAT (iBAT) in rodents develops during late embryogenesis with the first morphological evidence for BAT depot observed by 15‐16 days of gestation and the maximal mitotic activity of BAs reported during 17‐19 days of fetal development 7. Studies in rats revealed a progressive increase in Ucp1 and CoII mRNA levels from 18 days of embryogenesis until after birth 8. Ucp1 expression in iBAT was first detected in 18‐day‐old fetuses of C57Bl/6 (B6) and A/J mice with a subsequent increase immediately before birth and peak values for Ucp1 mRNA and protein levels occurring on postnatal day 1 and 10, respectively 9. Although iBAT Ucp1 mRNA levels remain relatively stable throughout life in mice reared at 23°C 9, its expression increases slightly in response to acute cold stimulation 10, 11. Chronic cold exposure induces proliferation of interscapular BAs 12, while a transfer from cold to 28°C enhances iBAT apoptosis 13, indicating a physiological balance between proliferation, survival and degradation of BAs. Recent studies showed that cold exposure induced Pdfra+ BA progenitors only in the narrow dorsal region of iBAT 14. Interscapular BAs in mice arise within the central dermomyotome from En1+ progenitors on embryonic day 9.5 15 and Pax7+ precursors between 9.5 to 11.5 days of gestation 16.
The expression of Zic1, that differentiates interscapular BAs from BAs in WAT in mice 17, 18, was over 100‐, 10‐, and 6‐fold greater in iBAT than in supraclavicular, periadrenal, or perirenal regions in infants, respectively, indicating that classical BAT is located in the same anatomical region in mice and humans 19. Human iBAT was most active during infancy and early childhood and disappeared progressively with increasing age 20. Additionally, BAs were found in axillary and perirenal fat pads and Ucp1 expression was highest in infants and children between 1‐15 years of age, respectively, and decreased significantly during adult life 21. The main locations for BAT in adult humans were found in the supraclavicular region, around neck, vasculature, epicardium, and solid organs such as kidney, adrenal, pancreas, and liver 22, 23.
Depot‐Specific Differences in White Adipose Tissue Development
The subcutaneous WAT (sWAT) in mice appears first during the last days of gestation while lipids accumulate during the first days of life. WAs from subcutaneous and retroperitoneal WAT (rWAT) contained lipid droplets already on postnatal day 1; however, they were smaller than mature adipocytes in adult mice 24. Lipid‐filled subcutaneous WAs were identified by postnatal day 2, with a unilocular structure detected on postnatal day 5, while WA biomarkers (Pparg, Cebpa, Fabp4) were expressed on the embryonic day 17.5, prior to lipid accumulation 25. There was little fat deposition in inguinal WAT (iWAT) from 2‐day‐old B6 mice, whereas lipid accumulation increased from 5 until 10 days of age 26. Studies using a doxycycline‐inducible system revealed that differentiation of sWAT began between the 14th and 18th day of embryogenesis and the number of subcutaneous WAs remained relatively stable during life while visceral WAs were formed during the first weeks of postnatal development over a relatively long period of time 27. Epididymal WAT (eWAT) contained a small number of fibroblast‐like cells between postnatal day 1 and 3 while lipid accumulation occurred in a 3‐day‐old mouse 28. Lipid droplets in eWAT were evident in 7‐day‐old mice with mature WAs being observed on postnatal day 14 24. Although traces of lipid and biomarker expression of WAs from subcutaneous and visceral fat (vWAT) depots are detected during late gestation, mature adipose tissue appears post‐natally. In contrast, mature WAT in humans appears during fetal development at 14 weeks of gestation and develops progressively until 24 weeks of embryogenesis 29.
Despite differences in the specific time frame for development, visceral, and sWAT are characterized by distinct secretion of adipokines, insulin sensitivity and rates of lipolysis 30. Adipocyte precursors from sWAT and vWAT have different molecular signature, capacity for differentiation and responsiveness to growth factors as well as genetic and environmental stimuli 31. The analysis of inguinal, retroperitoneal, mesenteric, and epididymal fat collected from adult B6 mice revealed differences in total amount of protein, level of proteins associated with ATP synthesis, glycolysis and glyceroneogenesis, as well as average adipocyte size 32. It was shown that iWAT and eWAT have different developmental origins which could underlie differences between these two fat depots 33.
WAT expansion occurs by increased number (hyperplasia) and/or the average volume of adipocytes (hypertrophy). Feeding a high‐fat diet (HFD) to B6 mice rapidly increased the expression of Mest and Sfrp5 34, the biomarkers for WAT expansion in adult mice, in eWAT and iWAT indicating that in response to a positive energy balance mice accumulate fat by hypertrophy rather than hyperplasia. Hypertrophy is the main contributor to the growth of vWAT in response to feeding a HFD while the average number of adipocytes is strain‐specific 35. Both the number and mean size of WAs in rodents reared under standard conditions is established by the end of adolescence 36. Recent studies further indicated that the amount 27 and the average volume 37 of subcutaneous WAs remained relatively constant in adult mice fed a standard diet. Human studies showed that fat mass gain in people with obesity results from hypertrophy, while the mean number of WAs is stable and characteristic for each individual 38. In addition, approximately 10% of adipocytes are renewed each year indicating an active cell turnover. Therefore, cell proliferation in response to a HFD regimen might be associated with WAT remodeling including cell death and renewal necessary to sustain a stable level of adipocytes.
Early Postnatal Development of BAs in WAT
In addition to interscapular BAs, clusters of BAs, called “brown in white” ‐ brite 39 or beige 40, are dispersed within WAT. In mice raised at 23°C BAs appear spontaneously in rWAT during early postnatal development with a peak of expression observed at 21 days of age 9. BAs were transiently induced in WAT from 20‐day‐old B6 and 129S6sv/ev (129) mice reared at 23°C 41. We demonstrated that development of BAs in iWAT and rWAT occurs independently of the ambient temperature, between 10 and 21 days of age, with a greater BAP reported in 21‐day‐old mice raised at 17°C compared to animals reared at 29°C from birth until weaning 11. Neither 17°C nor administration of thyroid hormone stimulated precocious induction of BAs in WAT suggesting that their appearance is determined genetically. Consistent with this genetic model for development, iWAT in 10‐day‐old B6 and AxB8 mice can induce the BAP in response to a β3‐adrenergic receptor agonist treatment indicating that at 10 days of age WAT in mice has the molecular machinery to induce the BAP. Therefore, the low Ucp1 expression observed in 10‐day‐old mice raised at 17°C is due to the absence of input from the sympathetic nervous system in WAT. In contrast, under‐nutrition during the lactation period decreased the BAP in 21‐day‐old mice 42, suggesting that nutritional status during the early postnatal period affects the development of functional WAT and its thermogenic capacity. However, similar to transient effects of ambient temperature, the suppression of the BAP from under‐nutrition between birth and 21 days of age was not retained in adult mice; that is, BAP could be fully induced in adult mice when exposed to 4°C.
Involution of the BAP in WAT
A vital characteristic of BA development is the spontaneous disappearance of the BAP. Involution of the BAP in rWAT occurred at approximately 35 and 56 days of age in B6 and A/J mice, respectively 9. BAs strongly induced in WAT from 21‐day‐old mice reared at 17°C, 23°C and 29°C in rWAT and iWAT, disappeared in 56‐day‐old B6 and AxB8 mice 11, 42. Furthermore, the BAP was reduced in rWAT and iWAT in 30‐day‐old B6 and 129 mice compared with WAT from 20‐day‐old mice 41. This BAP, which includes a differentiated phenotype at weaning followed by spontaneous involution, is determined by a fixed genetic program in mammals subjected to transient modulation in response to nutrition and ambient temperature. We propose that fully‐functional adipocytes able to acquire the BAP in response to sympathetic stimulation develop in a two‐step process, first, a biosynthetic phase involving the appearance of BAs in WAT at weaning, and second, a phase called involution, in which BAs are degraded when an environment with elevated ambient temperature precludes the need for thermogenesis (Figure 1). Acquisition and disappearance of the BAP in WAT re‐occurs in adult mice during cold exposure and re‐adjustment to the warmth. The BAP was strongly induced in iWAT from adult AxB8 mice in response to 10 days at 4°C and decreased substantially within 14 days of re‐acclimation to thermoneutrality with a complete involution of BAs observed after 21 days at 29°C 43. This indicates that under normal physiological conditions the BAP in WAT is dormant and can be activated, e.g., upon cold exposure, when the need for thermogenesis is increased and is ceased upon termination of external cues.
Figure 1.
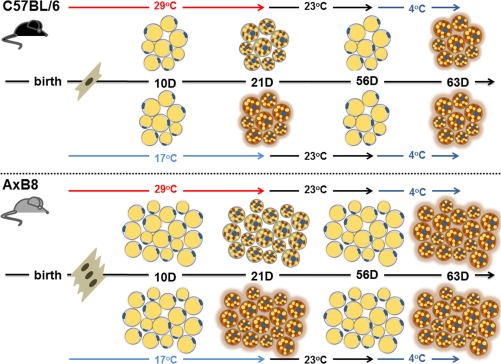
A model for development of functional BAs in WAT. Thermogenic capacity determined by the number of adipocytes capable of activating the BAP in WAT is distinct among different strains of mice. The BAP in WAT is induced at 21 days of age and is transiently greater in mice reared at 17°C. The BAP disappears post‐weaning. Induction of the BAP in adult life is dependent on activation of the sympathetic nervous system, e.g., by cold exposure independently of environmental temperature during the lactation period.
Genetic Variability in Acquisition of the BAP in WAT
The number of BAs in WAT is highly variable within inbred strains of mice and distinct WAT depots 44. The adenylyl cyclase activity in cell membranes collected from eWAT was markedly greater in A/J than B6 mice 45. The expression of BA biomarkers, Ucp1 and Cidea, was markedly higher in sWAT from 129‐S1 and A/J strains compared to B6 mice 46. A/J mice were characterized by significantly greater Ucp1 expression in rWAT and eWAT compared to B6 mice, while strain‐dependent differences in the BAP in iWAT were less definitive 9, 44. After 7 days of cold exposure (4°C) Ucp1 expression in rWAT was more prominent in AxB8 mice, while BAs were equally induced in iWAT from AxB8 and B6 mice 11. The BAP was greater in vWAT and sWATof 129 than in B6 mice; however, these differences were more profound in rWAT than iWAT 41. Similarly, sWAT from 129SVE mice reared under standard conditions was characterized by higher expression of BA biomarkers, e.g., Ucp1, Pgc1a and Cidea, compared to eWAT 40.
Generation and analysis of recombinant inbred strains of mice revealed that induction of the BAP in WAT depends on synergistic interaction among multiple genetic loci and new recombination of alleles present in the parental strains causes transgressive variation and increases Ucp1 expression much beyond the maximal levels reported for A/J and B6 mice 44, 47, 48. Upon cold exposure Ucp1 mRNA levels varied over 70‐fold between AxB8 mice, characterized by the highest number of BAs in WAT, and AxB10 mice, in which Ucp1 expression was even lower than that reported for B6 mice 44. Variation in Ucp1 mRNA levels in rWAT between A/J and B6 mice was determined by a genetic interaction between nine quantitative trait loci (QTLs), mapped to eight different chromosomes, which also regulated the expression of other BA biomarkers, e.g., Pgc1a, Ppara, and Dio2 49. Thus, these three factors, regulated by QTLs described previously, are components of a regulatory system for the induction of BAs by sympathetic signaling. Therefore, as in mice, variation in the amount and activity of human BAT might be determined genetically and points to the need for comprehensive genetic analyses that could facilitate the development of an effective strategy to induce BAs and increase thermogenic capacity.
WA and Classical BA Progenitors in Adult Mice
The origin of BAs and WAs has been extensively studied in adult mice. Genetic lineage‐tracing studies indicated that Myf5+ cells give rise to BAs and myocytes indicating a common origin for iBAT and the skeletal muscle in mice 50. All BAT‐derived adipocytes cultured in vitro originated from progenitors expressing Pax3, an upstream regulator of Myf5 expression during embryonic development of the skeletal muscle 51. The vast majority of adipocyte precursors and all mature interscapular BAs from 6‐week‐old mice expressed both Myf5 and Pax3 52. In addition, stem cells residing in skeletal muscle of adult mice differentiate into BAs in vitro, again suggesting that BAs and myocytes originate from the same precursors 53. Transcriptome and lineage‐tracing analyses in PTENmyf5cKO mice showed that Myf5+ precursors gave rise to a subset of interscapular and retroperitoneal WAs with fewer Myf5 + cells found in inguinal and perigonadal WAT 54. 31%, 11%, and 14% of stromal vascular fraction (SVF) cells from anterior subcutaneous, inguinal, and epididymal fat, respectively, derived from Myf5,Sca1‐expressing progenitors residing in WAT 55. In addition, around 60% of WAs in rWAT arise from the Pax3 + cells, while less than 15% of inguinal WAs originated from the Pax3+ lineage 56 indicating that the expression of Pax3 and Myf5 is not sufficiently specific to discriminate between BAs, myocytes, and WAs.
Experiments tracking VE‐cadherin expression at different developmental stages indicated that BAs and WAs in adult WAT originate from endothelial precursors located in interscapular, subcutaneous, and epididymal fat depots 57. Cells expressing Zfp423 were located in the vicinity of blood vessels and perivascular cells, suggesting that brown and white preadipocytes arise from endothelial lineage in vivo 58. Another lineage‐tracing study showed that retroperitoneal and inguinal WAs originate from a pool of proliferating PDFRβ+ progenitors residing within mural cells of WAT vasculature 59. A close anatomical relationship between clusters of differentiated adipocytes and WAT vasculature was reported already in 1982 28. A well‐organized network of blood vessels in eWAT was developed already on postnatal day 4 and preceded the appearance of lipid‐containing adipocytes, which occurred first on the proximal branches of vasculature in 7‐day‐old mice. Clusters of lipid‐filled adipocytes covered the entire network of blood vessels on postnatal day 11 to 14 while inhibition of the VEGFA signaling pathway delayed the development of eWAT 24. Given a tight anatomical and functional association between growing vascular system and adipose tissue, it might be difficult to accurately distinguish whether endothelial progenitors located within WAT gives rise specifically to blood vessels or might also differentiate into WAs. Recently, genetic tracing studies using Cdh5‐Cre:mT/mG, Tie2‐Cre:mT/mG and Vav1‐Cre:mT/mG mice in which GFP fluorescence is detected only in membranes of cells expressing endothelial or hematopoietic lineage markers, respectively, showed that mature adipocytes under normal conditions were derived from a population of CD24+;PdfRα + adipocyte progenitors residing within WAT, not from endothelial or hematopoietic precursors 60. In addition, an inducible Cre‐LoxP system led to identification of bipotential PDGFRα+ stromal stellate‐like cells able differentiate to BAs or WAs depending on the external signals 61.
Although identification of the origin for BAs and WAs in adult tissues is relevant it seems to have little practical aspect at present. Given that master transcription factors and signaling molecules exert their functions in multiple cell types, e.g., adipose, muscle, vasculature, targeting them to increase cell proliferation in a tissue‐specific manner seems problematic. While designing a strategy for BA induction as an anti‐obesity therapy, one should consider when adipose tissue acquires its functional structure that could be stimulated to make a difference to the overall metabolism. Although iBAT is fully active upon birth, lipid storage and thermogenic capacity of WAT develop post‐natally. Since results obtained from lineage‐tracing studies used to identify BA‐ and/or WA‐specific progenitors in adult tissues are often contradictory, it seems crucial to determine the exact time and molecular mechanisms underlying development of functional WAT. Given variation in the genetic predisposition to diet‐induced obesity in humans this could lead to the development of an effective therapeutic strategy in which thermogenic activity of BAs in WAT would be stimulated as soon as WAT function was established.
Identification of Progenitors Specific for BAs in WAT in Adult Mice
The origin of BAs in adult WAT remains inconclusive. BAs stimulated in WAT cultures in vitro or in eWAT from cold‐exposed adult mice did not express BA biomarkers, e.g., Zic1, miR‐206, and Lhx8; nevertheless they were characterized by a distinct molecular signature including the expression of Hoxc9 and Shox2 17, 39. A global microarray analysis of gene expression in inguinal BA cell cultures identified Fgf21, Car4, and Cited1 genes as specific for BAs in WAT in contrast to Zic1, Lhx8, and Epstl1, characteristic for interscapular BAs 62. Forskolin‐differentiated BAs from sWAT were characterized by a unique molecular signature, e.g., Tbx1, Slc27a1, Cd40 and unlike other SVF cells expressed Cd137 and Tmem26 40. Recent validation of putative biomarkers for BAs demonstrated that among all proposed genes only Zic1 and Hoxc9 expression could accurately distinguish between interscapular BAs, BAs in WAT, and WAs 18.
A population of Sca1+/CD45‐/Mac1‐ precursors in sWAT expressed Ucp1 in response to BMP7 treatment in vitro 46. The expression of interscapular brown (Ucp1, Cidea, Prdm16, Pgc1a) and brown in WAT (Tmem26, Tbx1) adipocyte biomarkers were significantly lower in Myf5+ precursors compared to Myf5‐ cells in sWAT 55, suggesting that BAs in iWAT arise from Myf5‐ cells. The expression of Ucp1, Cidea, Cox7a, Cox8b, and Pgc1a in Pax3‐ cells, which comprised 85% of WAs in iWAT, was 45‐, 30‐, 8‐, and 3‐fold greater compared to Pax3+ cells indicating that BAs in WAT might arise from Pax3‐ lineage 56. In response to 10 days of cold stimulation, a subset of subcutaneous BAs arose from Myh11 + cells leading the authors to suggest a smooth‐muscle like origin 63. Chemical and genetic tracing studies showed that 82% of the BAs in eWAT originated from precursors that expressed Pdfrα, CD34, and Sca1, while only 5.8% of BAs in iWAT originated from de novo proliferation in response to a β3‐adrenergic receptor agonist administration 61. Using a doxycycline‐inducible tagging system it was shown that upon adrenergic stimulation the majority of BAs in sWAT arose from de novo differentiation, while β3‐adrenergic stimulation significantly increased white adipogenesis in eWAT 27. Basal and stimulated Ucp1 expression in eWAT is significantly lower compared to iWAT 44, 61 indicating a greater number of BAs in sWAT. Therefore, proliferation of epididymal WAs in response to sympathetic stimulation might increase the total number of adipocytes and stimulate the overall thermogenic capacity of vWAT.
These observations explain the existence of precursors specific for BAs in WAT that argue against a mechanism for acquisition of the BAP based upon the temperature‐modulated mature WA‐to‐BA interconversion. However, any in vitro experiment does not reflect physiological conditions. Freshly isolated SVF cells from anterior sWAT did not express Cd137 and Tmem26 while their expression was induced during proliferation in vitro 56, suggesting that these cell surface biomarkers do not mark BA progenitors in WAT in vivo. Since gene expression patterns of most of the BA‐specific biomarkers in vitro were not replicated under physiological conditions 18, the origin of BAs in WAT cannot be determined exclusively based on in vitro studies which need a stringent validation in vivo.
WA‐to‐BA Conversion
Transdifferentiation is commonly defined as a conversion of one differentiated cell type to another cell type, usually from the same lineage, without returning to a pluripotent state 64. It may occur as a result of epigenetic changes, somatic mutation, and/or environmental cues that affect the expression of master control genes necessary to maintain a physiological function of mature cells. Transdifferentiation represents one of the mechanisms by which mature cells adapt to changes in the external environment. Therefore, as an adaptation to cold exposure mature WAs might acquire the BAP, but as opposed to known examples of transdifferentiation this adaptive mechanism is reversible.
The evidence that WAT acquires the BAP in response to cold exposure and re‐adapt to the warmth by involution of the BAP was reported over 20 years ago 65, 66. Since then, multiple studies indicated that BAs in WAT appear through a direct WA‐to‐BA conversion accompanied by molecular and morphological changes, from unilocular to multilocular structure, and increased thermogenic activity 67, 68; however, this mechanism seems to be depot‐specific. BAs in iWAT appear as a result of conversion of pre‐existing unilocular adipocytes that acquired the BAP upon a β3‐adrenergic receptor agonist treatment 61 or cold stimulation 14. Similarly, overexpression of Prdm16 activated the BAP in iWAT while there were no morphological and/or molecular changes in eWAT 69. Deletion of the cannabinoid receptor type 1 induced conversion of inguinal WAs into thermogenically active BAs, evident by increased expression of BA biomarkers, mitochondrial biogenesis and oxygen consumption, while Ucp1 and Pgc1a mRNA levels were decreased in eWAT 70. Cold exposure up‐regulated the expression of BA biomarkers in iWAT but not eWAT; however, it was only a transient effect lost after 5 weeks of re‐acclimation at 23°C 10. During adaptation to the warmth BAs converted into WAs, which re‐acquired the BAP upon repeated cold exposure, suggesting that a mechanism of physiological adaptation to variation in ambient temperature in iWAT occurs in both directions. The induction of Ucp1 mRNA in iWAT reaches half of its maximal levels after only 12 h of cold exposure and decreases by 40% after 1 day at 29°C 43. Therefore, it is likely that the BAP, at least in sWAT from adult mice, is acquired during WA‐to‐BA conversion rather than recruitment of BAs from progenitors.
According to current knowledge on the origin of BAs in WAT we cannot determine a definite and uniform mechanism for acquisition of the BAP. In support of morphological and kinetic studies described above, any mechanism for induction of BAs in WAT based on progenitors needs to be consistent with kinetics of induction of Ucp1 43 and Pgc1a 47, for iWAT and rWAT, respectively, that becomes maximally expressed within 12 h after cold stimulus. Two independent studies suggest that BAs in iWAT arise from direct conversion of pre‐existing WAs 10, 61 while one demonstrated cold‐induced de novo proliferation of BAs in sWAT 27. Since the average number of adipocytes is characteristic for a WAT depot and each individual, de novo proliferation of BAs needs to be accompanied by corresponding cell degradation. RFP+ labeling in iWAT was evident even after re‐adaptation to warm environment suggesting the disappearance of the BAP not dead cell removal 10. Upon re‐adaptation to thermoneutrality mitochondria of adipocytes in iWAT undergo dynamic structural changes 43 further indicating a phenotypic BA‐to‐WA switch rather than adipocyte death. Since eWAT consists of bi‐potential progenitor cells able to increase thermogenic or storage capacity in response to distinct external signals 61 the existence of one mechanism for the induction of the BAP does not necessarily exclude the other; however, it seems to be depot‐specific.
The Molecular Signature of Human BAT
Two murine experiments demonstrated that BAs in WAT versus classical BAs were similar in terms of thermogenic potential in response to cold stimulation 43, 71; however, only mitochondria from iBAT retained their normal function in response to changes in ambient temperature and were not degraded at thermoneutrality 43. While, the activity of iBAT is relatively similar among distinct strains of mice the number of BAs and UCP1 content within WAT is a highly variable genetic trait 9, 11. Although, thermogenic activity of human BAT varies enormously with respect to sex, age, adiposity, and within individuals from the same age and ethnic group 1, 3, 72, it is unknown whether this variation is specifically restricted to BAs in WAT or also includes classical BAs.
The molecular identity of human supraclavicular BAs resembled murine BAs in WAT rather than iBAT 40, while human neck BAT was similar to murine iBAT 23 (Table 1). Supraclavicular, retroperitoneal or intra‐abdominal human BAT, expressed biomarkers specific for BAs in WAT, e.g., HOXC9 or CITED1 62. Human brown preadipocytes expressed multiple genes of the smooth muscle lineage 73 similar to BAs in mouse WAT 63. Recent analysis of clonal brown preadipocyte cell lines generated from SVF obtained during biopsies of supraclavicular BAT confirmed molecular resemblance between human BAT and murine BAs in WAT; however, several genes encoding molecules that control thermogenesis, mitochondrial function and fatty acid oxidation were expressed in human BAT biopsies as well as mouse iBAT and BAs in WAT 73. An independent study showed that supraclavicular BAT in humans expressed biomarkers for classical BAs and markers specific for BAs in WAT 74 indicating that human BAT might be composed of a mixed population of BAs. Two types of BAs, those in the interscapular region and those dispersed within unilocular WAs, have been distinguished in neonatal and adult humans 19.
Table 1.
Anatomical and molecular characterization of murine and human classical and BAs dispersed within WAT
| Mice | Humans | ||||
|---|---|---|---|---|---|
| Cellular origin | Anatomical distribution | Molecular biomarkers | Anatomical distribution | Molecular biomarkers | |
| Classical BAs |
En1+, Pax7+
Myf5+, Pax3+ |
Interscapular |
Zic1
18
Zic1, miR‐206, Lhx8 17, 39 Zic1, Lhx8, Epstl1 17, 39, 62 Eva1, Pdk4 40, 62 |
Interscapular Deep neck |
ZIC1
19
ZIC1, LHX8 23 |
| BAs dispersed in WAT | Myf5 ‐, Pdfrα+rWAT: Myf5+, Pax3+ |
Subcutaneous WAT Visceral WAT |
Hoxc9
18
Hoxc9, Shox2 17, 39 Fgf21, Car4, Cited1 62 Cd40, Tbx1, Cd137, Tmem26, Slc27a1 40 |
Supraclavicular Periadrenal |
Supraclavicular: miR‐206, miR‐133b, LHXB8, ZIC1, TBX1, TMEM26 74 MTUS1, KCNK3 73 TMEM26, SHOX2, TBX1 19 Periadrenal: HOXC9, EVA1, ZIC1 19 |
β‐Adrenergic Stimulation of BAT in Humans
Current research utilizing 18fluoro‐2‐deoxyglucose positron emission tomography (18FDG‐PET) scans showed that the amount of BAT is negatively correlated with adiposity in adult humans 1, 2, 3. Short‐term exposure (2 h) to 16‐17°C increased resting metabolic rate in 10/10 lean and 13 out of 14 overweight or individuals with obesity, whose BAT activity was, in general, significantly lower than in healthy subjects 2. Daily exposure to 15.5°C for 2.5 h increased glucose uptake, oxidative metabolism and blood flow in the supraclavicular BAT and enhanced EE in 9 individuals characterized by high basal BAT activity 75. Long‐term exposition to 15‐16°C for 2 h per day significantly increased EE through non‐shivering thermogenesis, glucose uptake and the amount of BAT in 16 out of 17 individuals indicating the recruitment of human BAT upon chronic cold stimulation 76. Exposure to 17°C for 2 h per day for 6 weeks resulted in a two fold increase in BAT activity, stimulation of EE and a significant decrease in fat mass 77 indicating that long‐term mild cold stimulation exerts favorable effects on adiposity in humans.
In contrast to rodent studies, isoprenaline treatment in humans had no effect on BAT activity, despite an increase in EE 78. Administration of ephedrine did not alter BAT activity as measured by PET/CT scans, while it significantly increased heart rate and blood pressure, as opposed to cold exposure which increased BAT thermogenesis, induced EE and oxygen capacity measured by indirect calorimetry, decreased heart rate and had little effect on circulating blood metabolites 79. Administration of mirabegron increased BAT thermogenesis and resting metabolic rate with fewer side‐effects on the cardiovascular system as compared to other sympathomimetic drugs 80. While developing an effective and safe pharmacological approach to stimulate BAT in humans is a time‐consuming process, there is promising evidence that environmental stimuli, e.g., mild cold‐exposure, represent an alternative and feasible way to increase EE in individuals with obesity 76.
Conclusion
Since thermogenic potential of iBAT in mice remains relatively stable throughout life, maintaining iBAT in adult humans might provide a constant level of BAT activity; however, adult human BAT resembles murine BAs in WAT. Therefore, development of a powerful strategy to fight obesity epidemics would be to increase the number and/or thermogenic capacity of BAs in WAT, rather than target the activity of iBAT. The mean number of adipocytes in mice 27 and humans 38 is relatively constant, indicating that the total number of adipocytes is determined during the early postnatal period 11. Strain‐ and depot‐specific differences in the ability to acquire the BAP in WAT in mice and enormous interindividual variation in the amount and activity of BAT in humans indicate that this trait is determined genetically. Therefore, the maximal amount and thermogenic capacity of BAs in humans might be strictly regulated and fixed for a depot and each individual. Thus, it is crucial to determine when BAs in humans acquire their functional structure that could be stimulated to have a significant effect on adiposity.
Funding agencies: This manuscript was financially supported by LPK's grant from the Foundation for Polish Science, Programme WELCOME, no. WELCOME/2010‐4/3 titled Nutrition and ambient temperature during early development can reduce susceptibility to obesity.
Disclosure: The authors declare no conflict of interests.
References
- 1. Saito M, Okamatsu‐Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold‐activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500‐1508. [DOI] [PubMed] [Google Scholar]
- 3. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444‐E452. [DOI] [PubMed] [Google Scholar]
- 5. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013;9:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 1969;49:330–425. [DOI] [PubMed] [Google Scholar]
- 7. Nnodim JO, Lever JD. The pre‐ and postnatal development and ageing of interscapular brown adipose tissue in the rat. Anat Embryol 1985;173:215‐223. [DOI] [PubMed] [Google Scholar]
- 8. Giralt M, Martin I, Iglesias R, Vinas O, Villarroya F, Mampel T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Unaltered iodothyronine 5'‐deiodinase activity is necessary for the response to environmental temperature at birth. Eur J Biochem/FEBS 1990;193:297–302. [DOI] [PubMed] [Google Scholar]
- 9. Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007;48:41–51. [DOI] [PubMed] [Google Scholar]
- 10. Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi‐directional interconversion of brite and white adipocytes. Nat Cell Biol 2013;15:659‐667. [DOI] [PubMed] [Google Scholar]
- 11. Chabowska‐Kita A, Trabczynska A, Korytko A, Kaczmarek MM, Kozak LP. Low ambient temperature during early postnatal development fails to cause a permanent induction of brown adipocytes. FASEB J 2015;29:3238–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bukowiecki L, Collet AJ, Follea N, Guay G, Jahjah L. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol 1982;242:E353‐E359. [DOI] [PubMed] [Google Scholar]
- 13. Lindquist JM, Rehnmark S. Ambient temperature regulation of apoptosis in brown adipose tissue. Erk1/2 promotes norepinephrine‐dependent cell survival. J Biol Chem 1998;273:30147‐30156. [DOI] [PubMed] [Google Scholar]
- 14. Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold‐induced brown adipocytes in adult mice. FASEB J 2014;29:286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atit R, Sgaier SK, Mohamed OA, et al. Beta‐catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006;296:164‐176. [DOI] [PubMed] [Google Scholar]
- 16. Lepper C, Fan CM. Inducible lineage tracing of Pax7‐descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010;48:424‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 2012;302:E19–E31. [DOI] [PubMed] [Google Scholar]
- 18. de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 2015;308:E1085‐E1105. [DOI] [PubMed] [Google Scholar]
- 19. Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013;19:631‐634. [DOI] [PubMed] [Google Scholar]
- 20. Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972;112 (Pt 1):35‐39. [PMC free article] [PubMed] [Google Scholar]
- 21. Lean ME. Brown adipose tissue in humans. Proc Nutr Soc 1989;48:243‐256. [DOI] [PubMed] [Google Scholar]
- 22. Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013;62:1783‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013;19:635‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han J, Lee JE, Jin J, et al. The spatiotemporal development of adipose tissue. Development 2011;138:5027‐5037. [DOI] [PubMed] [Google Scholar]
- 25. Birsoy K, Berry R, Wang T, et al. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development 2011;138:4709‐4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kozak LP, Newman S, Chao PM, Mendoza T, Koza RA. The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure. PLoS One 2010;5:e11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013;19:1338‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cook JR, Kozak LP. Sn‐glycerol‐3‐phosphate dehydrogenase gene expression during mouse adipocyte development in vivo. Dev Biol 1982;92:440‐448. [DOI] [PubMed] [Google Scholar]
- 29. Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev 1984;10:1–11. [DOI] [PubMed] [Google Scholar]
- 30. Wajchenberg BL, Giannella‐Neto D, da Silva ME, Santos RF. Depot‐specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 2002;34:616‐621. [DOI] [PubMed] [Google Scholar]
- 31. Macotela Y, Emanuelli B, Mori MA, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012;61:1691‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sackmann‐Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring) 2012;20:101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014;16:367‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP. Mesoderm‐specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J 2008;22:3925‐3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jo J, Gavrilova O, Pack S, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 2009;5:e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res 1974;15:474‐483. [PubMed] [Google Scholar]
- 37. Hemmeryckx B, Loeckx D, Dresselaers T, Himmelreich U, Hoylaerts MF, Lijnen HR. Age‐associated adaptations in murine adipose tissues. Endocr J 2010;57:925‐930. [DOI] [PubMed] [Google Scholar]
- 38. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783‐787. [DOI] [PubMed] [Google Scholar]
- 39. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator‐activated receptor gamma (PPAR gamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1‐containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153‐7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lasar D, Julius A, Fromme T, Klingenspor M. Browning attenuates murine white adipose tissue expansion during postnatal development. Biochim Biophys Acta 2013;1831:960‐968. [DOI] [PubMed] [Google Scholar]
- 42. Kozak LP, Koza RA, Anunciado‐Koza R, Mendoza T, Newman S. Inherent plasticity of brown adipogenesis in white fat of mice allows for recovery from effects of post‐natal malnutrition. PLoS One 2012;7:e30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gospodarska E, Nowialis P, Kozak LP. Mitochondrial turnover: a phenotype distinguishing brown adipocytes from interscapular brown adipose tissue and white adipose tissue. J Biol Chem 2015;290:8243‐8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998;102:412‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collins S, Daniel KW, Petro AE, Surwit RS. Strain‐specific response to beta 3‐adrenergic receptor agonist treatment of diet‐induced obesity in mice. Endocrinology 1997;138:405‐413. [DOI] [PubMed] [Google Scholar]
- 46. Schulz TJ, Huang TL, Tran TT, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA 2011;108:143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coulter AA, Bearden CM, Liu X, Koza RA, Kozak LP. Dietary fat interacts with QTLs controlling induction of Pgc‐1 alpha and Ucp1 during conversion of white to brown fat. Physiol Genomics 2003;14:139‐147. [DOI] [PubMed] [Google Scholar]
- 48. Koza RA, Hohmann SM, Guerra C, Rossmeisl M, Kozak LP. Synergistic gene interactions control the induction of the mitochondrial uncoupling protein (Ucp1) gene in white fat tissue. J Biol Chem 2000;275:34486‐34492. [DOI] [PubMed] [Google Scholar]
- 49. Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol 2005;25:8311‐8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu W, Liu Y, Lai X, Kuang S. Intramuscular adipose is derived from a non‐Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol 2012;361:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanchez‐Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yin H, Pasut A, Soleimani VD, et al. MicroRNA‐133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 2013;17:210‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanchez‐Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab 2012;16:348‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5‐lineage progenitors in white adipose tissues. J Lipid Res 2013;54:2214‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu W, Shan T, Yang X, et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci 2013;126 (Pt 16):3527‐3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tran KV, Gealekman O, Frontini A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012;15:222‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gupta RK, Mepani RJ, Kleiner S, et al. Zfp423 Expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 2012;15:230‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science 2008;322:583‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013;15:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta 3‐adrenoceptor activation and high‐fat feeding. Cell Metab 2012;15:480‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Long JZ, Svensson KJ, Tsai L, et al. A smooth muscle‐like origin for beige adipocytes. Cell Metab 2014;19:810‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thowfeequ S, Myatt EJ, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Dev Dyn 2007;236:3208‐3217. [DOI] [PubMed] [Google Scholar]
- 65. Loncar D. Convertible adipose tissue in mice. Cell Tissue Res 1991;266:149‐161. [DOI] [PubMed] [Google Scholar]
- 66. Cousin B, Cinti S, Morroni M, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992;103 (Pt 4):931‐942. [DOI] [PubMed] [Google Scholar]
- 67. Barbatelli G, Murano I, Madsen L, et al. The emergence of cold‐induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010;298:E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 68. Himms‐Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL‐316243‐treated rats derive directly from white adipocytes. Am J Physiol‐Cell Ph 2000;279:C670–C681. [DOI] [PubMed] [Google Scholar]
- 69. Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wagner IV, Perwitz N, Drenckhan M, Lehnert H, Klein J. Cannabinoid type 1 receptor mediates depot‐specific effects on differentiation, inflammation and oxidative metabolism in inguinal and epididymal white adipocytes. Nutr Diabetes 2011;1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013;5:1196‐1203. [DOI] [PubMed] [Google Scholar]
- 72. Yoneshiro T, Aita S, Matsushita M, et al. Brown adipose tissue, whole‐body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13‐16. [DOI] [PubMed] [Google Scholar]
- 73. Shinoda K, Luijten IH, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015;21:389‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013;17:798–805. [DOI] [PubMed] [Google Scholar]
- 75. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold‐activated human brown fat. J Nucl Med 2013;54:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395‐3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404−3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vosselman MJ, van der Lans AA, Brans B, et al. Systemic beta‐adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 2012;61:3106‐3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA 2012;109(25):10001‐10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cypess AM, Weiner LS, Roberts‐Toler C, et al. Activation of human brown adipose tissue by a beta3‐adrenergic receptor agonist. Cell Metab 2015;21:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]


