Abstract
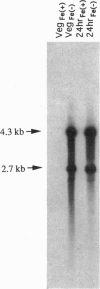
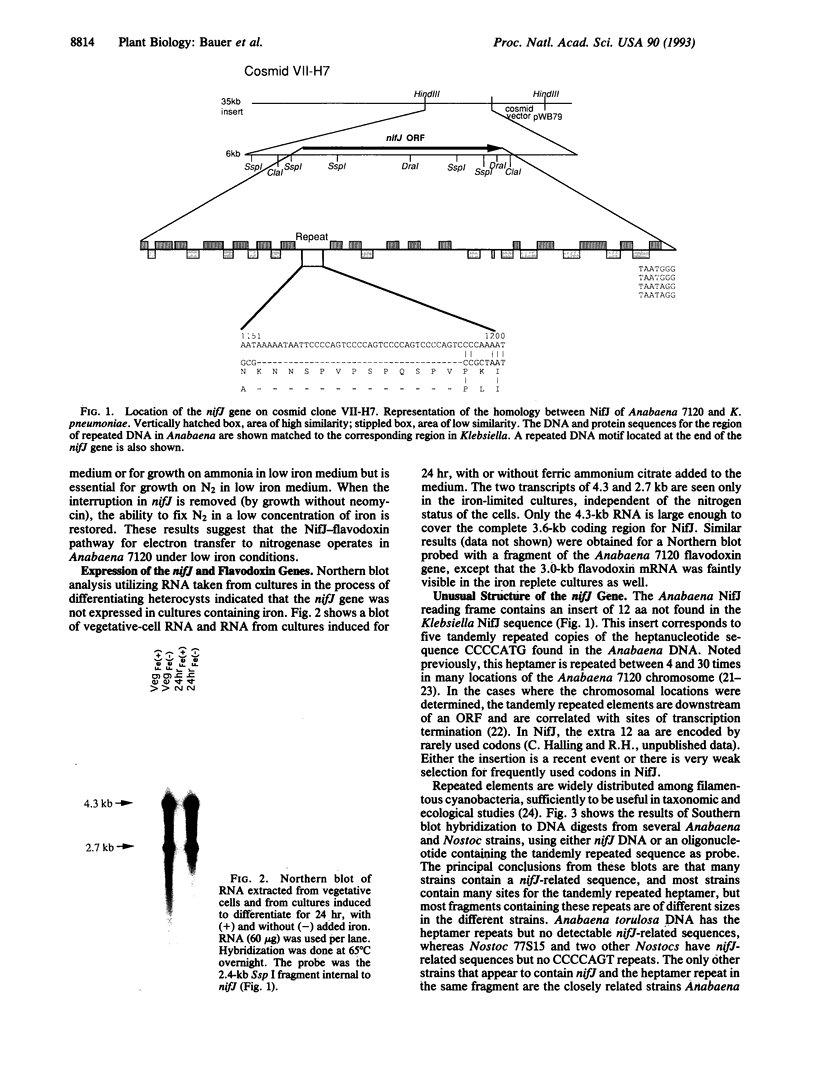
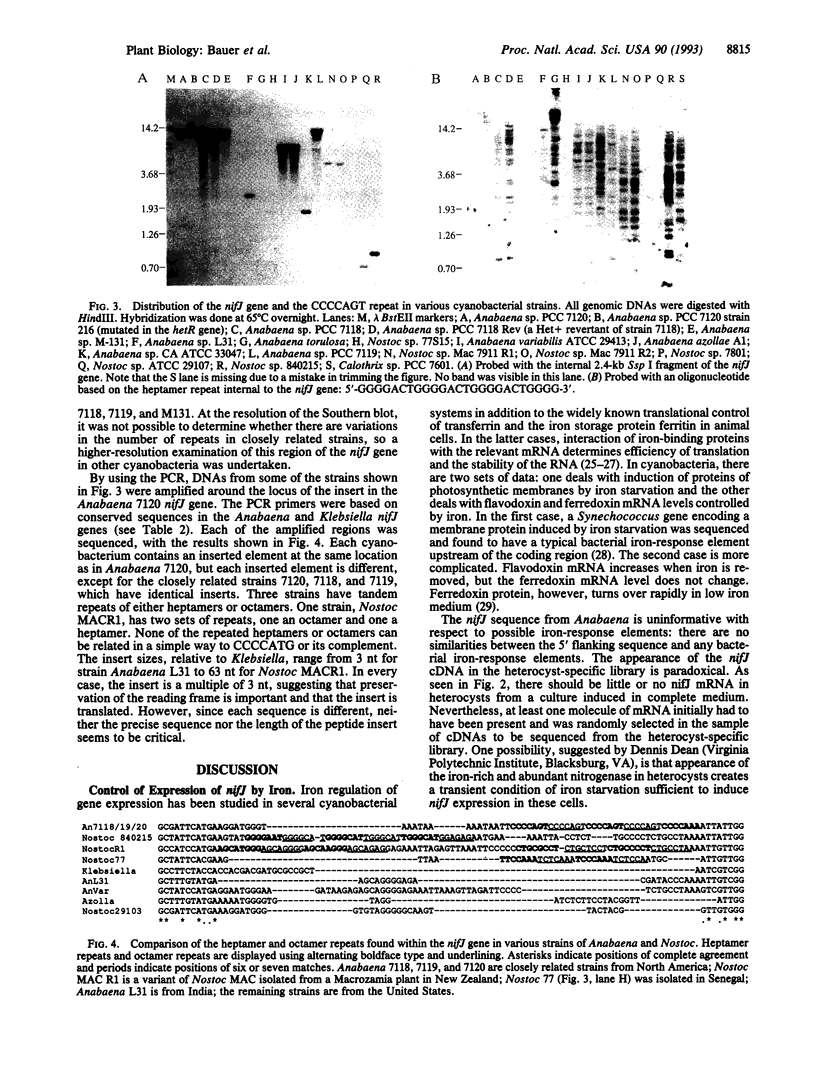
The nifJ gene of Klebsiella pneumoniae encodes an oxidoreductase required for the transfer of electrons from pyruvate to flavodoxin, which reduces nitrogenase. The nifJ gene of Anabaena 7120, isolated from a cosmid bank, was found to contain an open reading frame encoding a 1197-aa protein. The deduced amino acid sequence shows 50% identity to the Klebsiella homolog. The nifJ gene in Anabaena 7120 was inactivated by chromosomal interruption. The resulting mutant was unable to grow on medium depleted of both iron and combined nitrogen but grew normally, fixing nitrogen, when iron was present. NifJ transcripts of 2.7 and 4.3 kb are induced by iron depletion irrespective of nitrogen status. One particular stretch of the Anabaena 7120 nifJ gene encodes 12 aa with no complementary matches in the Klebsiella protein. This insert contains five tandem repeats of the heptamer CCCCAGT. These heptamers, as well as heptamers and octamers of other related sequences, have been located in a number of cyanobacterial genomes but are usually not found within the coding region of a gene. The site of the Anabaena 7120 heptamers in the nifJ genes of other filamentous cyanobacteria contains a surprising diversity of repeated sequences, both octamers and heptamers. The corresponding protein inserts range in length from 1 to 21 aa, relative to Klebsiella NifJ.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovy A., de Vrieze G., Lugones L., van Horssen P., van den Berg C., Borrias M., Weisbeek P. Iron-dependent stability of the ferredoxin I transcripts from the cyanobacterial strains Synechococcus species PCC 7942 and Anabaena species PCC 7937. Mol Microbiol. 1993 Feb;7(3):429–439. doi: 10.1111/j.1365-2958.1993.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Brahamsha B., Haselkorn R. Isolation and characterization of the gene encoding the principal sigma factor of the vegetative cell RNA polymerase from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Apr;173(8):2442–2450. doi: 10.1128/jb.173.8.2442-2450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991 Feb;5(2):321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- Cannon M., Cannon F., Buchanan-Wollaston V., Ally D., Ally A., Beynon J. The nucleotide sequence of the nifJ gene of Klebsiella pneumoniae. Nucleic Acids Res. 1988 Dec 9;16(23):11379–11379. doi: 10.1093/nar/16.23.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. H. The nitrogen fixation genes of Klebsiella pneumoniae: a model system. Microbiol Sci. 1984 May;1(2):29–33. [PubMed] [Google Scholar]
- Golden J. W., Mulligan M. E., Haselkorn R. Different recombination site specificity of two developmentally regulated genome rearrangements. Nature. 1987 Jun 11;327(6122):526–529. doi: 10.1038/327526a0. [DOI] [PubMed] [Google Scholar]
- Hill S., Kavanagh E. P. Roles of nifF and nifJ gene products in electron transport to nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1980 Feb;141(2):470–475. doi: 10.1128/jb.141.2.470-475.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Wolk C. P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990 Jun;172(6):3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. D., Haselkorn R. Isolation, sequence and transcription of the gene encoding the photosystem II chlorophyll-binding protein, CP-47, in the cyanobacterium Anabaena 7120. Plant Mol Biol. 1989 Oct;13(4):441–457. doi: 10.1007/BF00015556. [DOI] [PubMed] [Google Scholar]
- Leonhardt K. G., Straus N. A. Sequence of the flavodoxin gene from Anabaena variabilis 7120. Nucleic Acids Res. 1989 Jun 12;17(11):4384–4384. doi: 10.1093/nar/17.11.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt K., Straus N. A. An iron stress operon involved in photosynthetic electron transport in the marine cyanobacterium Synechococcus sp. PCC 7002. J Gen Microbiol. 1992 Aug;138(Pt 8):1613–1621. doi: 10.1099/00221287-138-8-1613. [DOI] [PubMed] [Google Scholar]
- Mazel D., Houmard J., Castets A. M., Tandeau de Marsac N. Highly repetitive DNA sequences in cyanobacterial genomes. J Bacteriol. 1990 May;172(5):2755–2761. doi: 10.1128/jb.172.5.2755-2761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Haselkorn R. Nitrogen fixation (nif) genes of the cyanobacterium Anabaena species strain PCC 7120. The nifB-fdxN-nifS-nifU operon. J Biol Chem. 1989 Nov 15;264(32):19200–19207. [PubMed] [Google Scholar]
- Munro H. N. Iron regulation of ferritin gene expression. J Cell Biochem. 1990 Oct;44(2):107–115. doi: 10.1002/jcb.240440205. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Malkin R. Iron-sulfur centers and activities of the photosynthetic electron transport chain in iron-deficient cultures of the blue-green alga aphanocapsa. Plant Physiol. 1983 Nov;73(3):724–728. doi: 10.1104/pp.73.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]