Abstract
Objective
To identify factors associated with antiretroviral therapy (ART) attrition among patients initiating therapy in 2005–2011 at two large, public-sector department-level hospitals, and to inform interventions to improve ART retention.
Methods
This retrospective cohort study used data from the iSanté electronic medical record (EMR) system. The study characterized ART attrition levels and explored the patient demographic, clinical, temporal, and service utilization factors associated with ART attrition, using time-to-event analysis methods.
Results
Among the 2 023 patients in the study, ART attrition on average was 17.0 per 100 person-years (95% confidence interval (CI): 15.8–18.3). In adjusted analyses, risk of ART attrition was up to 89% higher for patients living in distant communes compared to patients living in the same commune as the hospital (hazard ratio: 1.89, 95%CI: 1.54–2.33; P < 0.001). Hospital site, earlier year of ART start, spending less time enrolled in HIV care prior to ART initiation, receiving a non-standard ART regimen, lacking counseling prior to ART initiation, and having a higher body mass index were also associated with attrition risk.
Conclusions
The findings suggest quality improvement interventions at the two hospitals, including: enhanced retention support and transportation subsidies for patients accessing care from remote areas; counseling for all patients prior to ART initiation; timely outreach to patients who miss ART pick-ups; “bridging services” for patients transferring care to alternative facilities; routine screening for anticipated interruptions in future ART pick-ups; and medical case review for patients placed on non-standard ART regimens. The findings are also relevant for policymaking on decentralization of ART services in Haiti.
Keywords: HIV, antiretroviral therapy, highly active, lost to follow-up, decentralization, Haiti
Haiti has the most extensive epidemic of human immunodeficiency virus (HIV) in the Caribbean region, with a national adult HIV prevalence rate of 2.2% (1). In the early 2000s, several small-scale programs in Haiti demonstrated success in treating HIV-positive patients with antiretroviral therapy (ART), inspiring rapid scale-up of ART programs within Haiti and globally (2). By the end of 2011, a total of 34 927 Haitians were receiving ART, representing approximately 60% of the adults and children requiring therapy (3). Retention of ART patients is critical to the program’s success at both the individual and population levels.
Since 2004, the Ministry of Public Health and Population (MSPP) in Haiti has maintained an electronic medical record (EMR) system called iSanté that contains longitudinal health records for patients living with HIV (4). A concerning level of ART attrition is evident in iSanté data, with approximately 25% of ART patients recorded as officially discontinued. The earthquake of January 2010, which devastated the already-fragile health services infrastructure in Port-au-Prince and its surroundings, may have exacerbated ART attrition. Prior studies have examined ART attrition in patient cohorts in Port-au-Prince prior to the earthquake (5–7), but their findings do not necessarily reflect ART outcomes in other parts of Haiti or outcomes during the post-earthquake period.
The objectives of this retrospective cohort study were to identify factors associated with ART attrition among patients initiating therapy in 2005–2011 at two large, public-sector department-level hospitals and to inform interventions to improve ART retention at these sites. Note that in Haiti “departments” are the first and largest sub-national administrative level.
MATERIALS AND METHODS
Study setting and patient population
The study cohort included ART patients at Hôpital St. Michel (HSM) in Jacmel (South East Department) and Hôpital St. Antoine (HSA) in Jérémie (Grande-Anse Department), sites where study authors are engaged in on-going work in health systems strengthening. Both sites are the leading secondary hospitals in their respective departments, which are rural and mountainous. Both departments are relatively poor: in the Grande-Anse Department, half of the population is estimated to fall in the lowest quintile of wealth for Haiti, compared to 31% in the South East Department (1). The commune (the third administrative level in Haiti) of Jacmel, where HSM is located, is 444 km2, while the commune of Jérémie, where HSA is located, is 427 km2. The 2010 earthquake was centered < 30 km from HSM; it destroyed several buildings on the hospital campus and killed approximately 300–500 people in the town of Jacmel (8). In contrast, HSA is located > 150 km from the earth-quake epicenter and did not experience direct damage.
The study cohort included patients who were 15 years of age or older at the time of HIV program registration, and who started a multidrug ART regimen. The study excluded several two-drug ART in women, regimens that were presumed to represent time-limited therapies for preventing mother-to-child HIV transmission (PMTCT) under prevailing treatment guidelines.
Data source
All study data came from the consolidated server of the iSanté EMR system. iSanté is one of three EMR in Haiti, and was used by 71 HIV care and treatment sites covering approximately 70% of all ART patients in Haiti in 2011 (9). Data are typically captured on standard paper-based iSanté patient encounter forms, and then retrospectively entered into the system. In addition to capturing data within an EMR, sites are required to keep paper-based registers with summary data on all ART patients. iSanté data extracts were obtained on 1 February 2012. The average lag-time for electronic data entry is < 1 week, meaning that the data extract was an up-to-date representation for the cohort. Data cleaning involved inspection and adjudication of elements that violated field validation rules or plausible data relationships. Elements that could not be adjudicated or that were determined to be errors were coded as missing.
Outcome variable
The outcome variable was time in days from ART start to attrition. ART start date was defined as the first dispense date for the multi-drug ART regimen. A variety of definitions of ART attrition have been used in international research (5, 10–12). The primary definition of attrition was the failure to return for pharmacy pick-up within 30 days of the expected ART refill date (“30+ day pharmacy definition”). In sensitivity analyses, three alternative definitions were considered: (a) no return pharmacy pick-up within 60 days (“60+ day pharmacy definition”); (b) no return for any type of outpatient HIV visit within 90 days (“90+ day encounter definition”), the MSPP standard definition; and (c) no return for any type of outpatient HIV visit within 180 days (“180+ day encounter definition”), a proposed universal definition suggested by empirical findings on loss-to-follow-up (LTFU) among more than 180 000 patients in 19 countries (10).
To calculate ART attrition from pharmacy data, ART refill due dates were defined for every interval between pharmacy visits, based on dispense dates and number of dose-days dispensed. Missing dispense dates (missing in 4.1% of pharmacy records) were imputed using the date of the clinical visit where the prescription was made (concordant in 97.8% of cases where both dates were available). The minimum number of dose-days dispensed for any single medication within a regimen was used to calculate the refill due date for the overall regimen. Missing dose-days dispensed (missing in 3.9% of pharmacy records) were imputed using the patient-specific median dose-days or, if this was unavailable, the median dose-days for the study cohort.
A patient was counted as a case of attrition upon the first incident of failure to return in a timely manner. To partially account for patient transfers within the network of iSanté sites, a matching algorithm was used to identify those who presented for care at another iSanté site before the pharmacy refill or encounter due date. These patients were then counted as transfer cases rather than ART attrition cases, and their observations were censored as of the due date. Transfers of care to facilities outside of the iSanté data system could not be verified.
Haiti lacks a national death registry for ascertainment of mortality, and both hospitals lack resources for tracing patients who failed to return, meaning that official ART disenrollment forms citing a reason for discontinuation were only available for a small fraction. For these reasons, deaths could not be distinguished from cases of LTFU. In sum, the outcome measure represented a composite of several possible reasons for attrition, with accounting for patient transfers to other iSanté network facilities.
Covariates
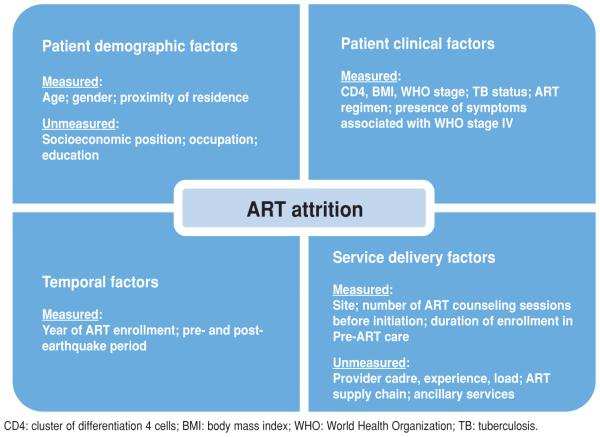
Factors assessed for association with ART attrition were: temporal factors, patient demographic factors, patient clinical factors, and service utilization factors (Figure 1). Cluster of differentiation 4 cells (CD4), body mass index (BMI), disease stage by World Health Organization (WHO) guidelines, tuberculosis (TB) status, and presence of symptoms associated with WHO Stage IV were entered into analytic models as baseline covariates, measured during a baseline period of 180 days prior to ART initiation. TB status was a binary variable indicating a confirmed TB diagnosis, use of TB prophylaxis, or suspicion of TB during the baseline period. ART regimen was the initial regimen prescribed. Proximity of patient residence to the hospital used the commune of residence at initial registration in HIV care, and defined categories of residing in the same, adjacent, or non-adjacent (more distant) commune as the hospital.
FIGURE 1. Conceptual model for HIV antiretroviral therapy (ART) attrition at two hospitals in Haiti, 2005–2011.
Statistical methods
This study used time-to-event analysis methods (13). Observations of patient outcomes were censored for transfer cases or for patients not meeting the attrition definition as of 1 January 2012. Descriptive statistics were used to characterize the sample and identify the number of patients meeting the primary and alternative ART attrition definitions. The Kaplan-Meier method was used to estimate average rates of attrition, attrition levels at specific time points, and attrition levels by patient sub-groups. Unadjusted and adjusted semi-parametric Cox regression models were used to obtain hazard ratio (HR) estimates for observed covariates. The adjusted model included a post-earthquake indicator as a time-varying exposure to allow for different attrition risk before and after the earthquake. To test for a differential earthquake effect by site, an interaction term for hospital site and the post-earthquake period was also included. To avoid assumptions of linearity in relationships, baseline BMI and baseline CD4 were classified as categorical variables with logical or clinically-relevant cut-points (see Table 1 for cut-points).
TABLE 1.
Patient characteristics and rates of antiretroviral therapy (ART) attrition at two hospitals in Haiti, 2005–2011
| Patients |
Attrition a |
Rate per 100 person-years |
95% CI |
|||
|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | Lower Upper | P valueb | |
| Overall | 2 023 | 100 | 694 | 17.0 | (15.8, 18.3) | |
| Hospital sites | ||||||
| Hôpital St. Antoine in Jérémie | 1 052 | 52 | 434 | 21.5 | (19.6, 23.6) | |
| Hôpital St. Michel in Jacmel | 971 | 48 | 260 | 12.6 | (11.1, 14.2) | < 0.001 |
| Gender | ||||||
| Male | 877 | 43 | 295 | 16.2 | (14.5, 18.2) | |
| Female (not pregnant) | 1 043 | 52 | 367 | 17.5 | (15.8, 19.4) | |
| Female (pregnant) | 103 | 5 | 32 | 19.2 | (13.6, 27.1) | 0.83 |
| Age group (years) | ||||||
| < 25 | 182 | 9 | 62 | 17.2 | (13.4, 22.1) | |
| 25–44 | 1 254 | 62 | 428 | 16.6 | (15.1, 18.3) | |
| ≥ 45 | 587 | 29 | 204 | 17.8 | (15.5, 20.4) | 0.87 |
| Residence | ||||||
| Same commune | 1 301 | 64 | 389 | 13.4 | (12.1, 14.8) | |
| Adjacent commune | 419 | 21 | 158 | 20.8 | (17.8, 24.3) | |
| Non-adjacent commune | 291 | 14 | 140 | 34.0 | (28.8, 40.1) | |
| Missing | 12 | 1 | 7 | 60.9 | (29.1, 127.8) | < 0.001 |
| Body mass index | ||||||
| ≤ 18.5 | 529 | 26 | 180 | 16.2 | (14.0, 18.7) | |
| > 18.5 | 1 338 | 66 | 471 | 17.4 | (15.9, 19.1) | |
| Missing | 156 | 8 | 43 | 15.9 | (11.8, 21.4) | 0.63 |
| ART start | ||||||
| 2005–2006 | 343 | 17 | 192 | 18.1 | (15.7, 20.8) | |
| 2007–2008 | 645 | 32 | 261 | 14.6 | (12.9, 16.4) | |
| 2009–2010 | 660 | 33 | 208 | 19.8 | (17.3, 22.7) | |
| 2011 | 375 | 19 | 33 | 18.3 | (13.0, 25.8) | < 0.001 |
| Baseline CD4 | ||||||
| < 100 | 485 | 24 | 164 | 14.8 | (12.7, 17.2) | |
| 100–249 | 743 | 37 | 253 | 15.9 | (14.0, 18.0) | |
| 250+ | 453 | 22 | 129 | 18.4 | (15.5, 21.9) | |
| Missing | 342 | 17 | 148 | 21.8 | (18.6, 25.6) | < 0.001 |
| ART regimenc | ||||||
| AZT-3TC-EFV | 1 122 | 55 | 371 | 16.4 | (14.8, 18.2) | |
| AZT-3TC-NVP | 690 | 34 | 268 | 18.7 | (16.6, 21.1) | |
| d4T-3TC-EFV or TDF-3TC-EFV | 88 | 4 | 18 | 9.8 | (6.2, 15.5) | |
| d4T-3TC-NVP or TDF-3TC-NVP | 89 | 4 | 24 | 13.7 | (9.2, 20.5) | |
| Non-standard | 34 | 2 | 13 | 36.6 | (21.2, 63.0) | 0.03 |
| World Health Organization clinical stage | ||||||
| Stage I or II | 1 187 | 59 | 387 | 16.4 | (14.8, 18.1) | |
| Stage III or IV | 776 | 38 | 280 | 17.4 | (15.4, 19.5) | |
| Missing | 60 | 3 | 27 | 25.7 | (17.6, 37.4) | 0.05 |
| Tuberculosis status | ||||||
| No | 1 581 | 78 | 530 | 17.7 | (16.3, 19.3) | |
| Yes | 442 | 22 | 164 | 15.0 | (12.8, 17.4) | 0.96 |
| Stage 4 symptom | ||||||
| No | 1 728 | 85 | 630 | 17.1 | (15.8, 18.5) | |
| Yes | 295 | 15 | 64 | 15.9 | (12.5, 20.3) | 0.01 |
| Pre-ART duration (months) | ||||||
| < 1 | 677 | 33 | 255 | 20.8 | (18.4, 23.6) | |
| 1–6 | 689 | 34 | 239 | 15.9 | (14.0, 18.0) | |
| > 6 | 657 | 32 | 200 | 14.7 | (12.8, 16.9) | < 0.01 |
| Counseling sessions | ||||||
| 0 | 1 332 | 66 | 481 | 16.9 | (15.4, 18.4) | |
| 1 | 539 | 27 | 166 | 17.5 | (15.0, 20.4) | |
| ≥ 2 | 152 | 8 | 47 | 16.6 | (12.5, 22.1) | 0.35 |
ART attrition is defined as being 30 or more days late for an ART pharmacy re-fill (“30+ day pharmacy definition”).
P value based upon log-rank test of equality of survivor functions.
ART regimen: AZT = zidovudine; 3TC = lamivudine; EFV = efavirenz; NVP = nevirapine; d4T = stavudine; TDF = tenofovir.
To handle missing data on the covariates proximity of residence (< 1% missing), WHO Stage (3.0 % missing), BMI (7.7% missing), and CD4 (17.0% missing), multiple imputation using chained equations were employed. This method has been shown to minimize bias in risk estimates relative to other possible methods for handling missing covariate data (14–16). The imputation model included all model variables; five imputed datasets were created (17). Categorical variable constructs were tested with joint testing of coefficients. To assess the sensitivity of the study findings to this strategy for handling missing data, the analyses compared models using complete case analysis, missing indicator dummy variables for covariates with missing data, and manual imputation using extreme values, for each of the attrition definitions. All analyses were performed using Stata®/MP12 (StataCorp LP, College Station, Texas, United States).
Ethical review
The study protocol was reviewed and approved by the University of Washington (Seattle, Washington, United States), the Haiti National Bioethics Committee (Port-au-Prince, Haiti), and the United States Centers for Disease Control and Prevention (Atlanta, Georgia, United States). The study used only de-identified patient data.
RESULTS
The study cohort included 2 023 patients who had received a multidrug ART regimen at the two hospital sites by the end of 2011. The cohort excluded 113 pediatric patients, 15 patients of unknown age or year of birth, and 1 patient of unknown gender. Characteristics of the patient population are shown in Table 1. Overall, 694 patients (34.3%) met the 30+ day pharmacy attrition definition; 331 (16.4%) met the 60+ day pharmacy attrition definition; 966 (47.8%) met the 90+ day encounter definition; and 321 (15.9%) met the 180+ day encounter definition. Only a small number of patients who stopped receiving care at the two sites made timely transfers to an alternative facility (1 timely transfer under the 30+ day pharmacy definition, and 4 timely transfers under the alternative definitions).
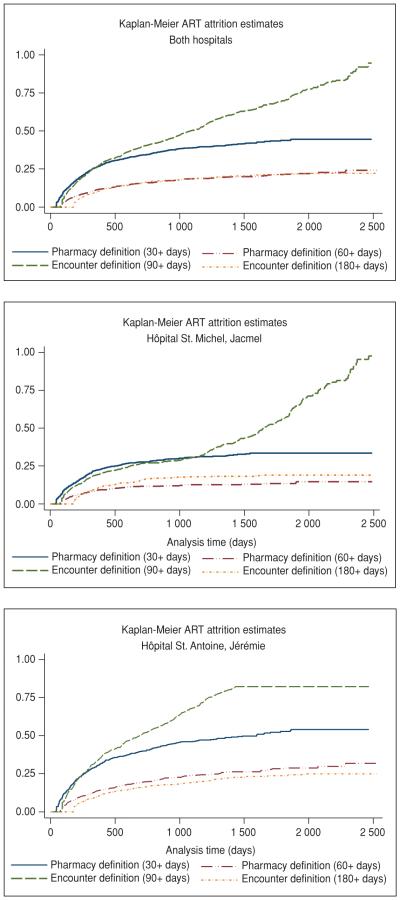
Under all definitions, attrition rose steeply during the first 12 months after ART initiation, with 26.6% of patients abandoning ART under the 30+ day pharmacy definition (95% confidence interval [95%CI]: 24.6%–28.7% (Figure 2). While attrition under the 30+ day pharmacy definition tapered considerably after 12 months, it increased more steadily over time under the 90+ day encounter definition (Figure 2). Attrition under the 60+ day pharmacy definition was about half of that under the 30+ day pharmacy definition at each time point. This indicates that a sizable proportion of attrition reflected temporary interruptions in care. Attrition under the 60+ day pharmacy definition and the 180+ encounter definition was similar. ART attrition was notably higher at HSA in Jérémie than at HSM in Jacmel, for every definition and for every time point (Figure 2).
FIGURE 2. Antiretroviral therapy (ART) attrition estimates at two hospitals in Haiti, 2005–2011.
Rates of attrition per 100 person-years by patient subgroups are shown in Table 1, based on the 30+ day pharmacy definition. In unadjusted analyses, factors associated with attrition were: hospital site at HSA (P < 0.001); location of residence (P < 0.001); ART start year (P < 0.001); baseline CD4 (P < 0.001); ART regimen (P = 0.03); not having a WHO Stage IV symptom reported (P = 0.01); and pre-ART duration (P < 0.01). There were no meaningful differences in rates of attrition by gender, age, BMI, WHO Stage, TB status, and number of counseling sessions prior to ART initiation, in unadjusted analyses.
In the adjusted analysis, there was no evidence that the relative risk of attrition between the two hospitals changed following the 2010 earthquake (P = 0.66 for interaction term). The results of the main-effects adjusted Cox model are presented in Table 2. After adjustment for all covariates, the following were strongly associated with ART attrition: proximity of residence, hospital site, and year of ART start (representing a cohort effect by calendar year). Adjusted ART attrition risk was 69% higher for patients residing in an adjacent commune (Hazard ratio [HR]: 1.69, 95%CI: 1.39–2.06; P < 0.001) and 89% higher for patients residing in a non-adjacent commune (HR: 1.89, 95%CI: 1.54–2.33; P < 0.001), compared to patients residing in the same commune as the hospital. Adjusted ART attrition risk was meaningfully lower at HSM in Jacmel versus at HSA in Jérémie, both before and after the earthquake (HR: 0.58, 95%CI: 0.49–0.70; P < 0.001). The adjusted model showed a risk of attrition that decreased successively with calendar time of ART start (P < 0.001), but there was no evidence of a specific temporal effect related to the earth-quake (HR: 0.94 for post-earthquake compared to pre-earthquake, 95%CI: 0.73–1.22; P = 0.66). Having shorter duration of enrollment in HIV care prior to ART initiation (P < 0.01), having a non-standard ART regimen (P < 0.05), having no counseling sessions prior to ART initiation (P < 0.10), and having a BMI > 18.5 (P < 0.10) were also associated with higher attrition risk in the adjusted analysis.
TABLE 2.
Adjusted hazard ratios for antiretroviral therapy (ART) attrition at two hospitals in Haiti, 2005–2011a
| Risk factor | Hazard ratio |
95% confidence interval |
P value |
|---|---|---|---|
| Sites | |||
| Hôpital St. Michel in Jacmel vs. Hôpital St. Antoine in Jérémie |
0.58 | (0.49, 0.70) | < 0.001 |
| Post- vs. pre-earthquake | 0.94 | (0.73, 1.22) | 0.66 |
| Gender (male = reference) | |||
| Female (not pregnant) | 0.99 | (0.81, 1.21) | 0.92 |
| Female (pregnant) | 1.08 | (0.72, 1.62) | 0.71 |
| Age (10 years or more) | 1.05 | (0.97, 1.13) | 0.20 |
| Proximity (same commune = reference)b | |||
| Adjacent commune | 1.69 | (1.39, 2.06) | < 0.001 |
| Non-adjacent commune | 1.89 | (1.54, 2.33) | < 0.001 |
| Body mass index (< 18.5 = reference) | |||
| >18.5 | 1.19 | (0.99, 1.42) | 0.06 |
| Year of ART start (2005–2006 = reference)b | |||
| 2007–2008 | 0.66 | (0.54, 0.81) | < 0.001 |
| 2009–2010 | 0.60 | (0.44, 0.81) | 0.001 |
| 2011 | 0.37 | (0.23, 0.61) | < 0.001 |
| Baseline CD4 (< 100=reference) | |||
| 100–249 | 1.06 | (0.86, 1.30) | 0.58 |
| ≥ 250 | 1.15 | (0.86, 1.54) | 0.33 |
| ART regimenc (AZT-3TC-EFV=reference)d | |||
| AZT-3TC-NVP | 1.14 | (0.92, 1.42) | 0.23 |
| d4T or TDF + 3TC-EFV | 0.67 | (0.42, 1.08) | 0.10 |
| d4T or TDF + 3TC-NVP | 0.86 | (0.55, 1.34) | 0.51 |
| non-standard regimens | 2.04 | (1.16, 3.59) | 0.01 |
| World Health Organization clinical stage (Stage I or II = reference) |
|||
| Stage III or IV | 1.16 | (0.99, 1.37) | 0.07 |
| Tuberculosis status (No suspicion, prophylaxis or diagnosis = reference) |
|||
| Yes | 0.91 | (0.74, 1.10) | 0.32 |
| Any Stage IV symptom (No = reference) | |||
| Yes | 0.84 | (0.64, 1.11) | 0.22 |
| Pre-ART duration (30 day increase) | 0.99 | (0.98, 1.00) | 0.01 |
| Counseling sessions prior to ART start (None = reference)e | |||
| 1 | 0.84 | (0.70, 1.01) | 0.07 |
| ≥ 2 | 0.74 | (0.54, 1.01) | 0.06 |
Main effects model using 30+ day pharmacy definition for ART attrition.
P value using joint testing of coefficients: P ≤ 0.001.
ART regimen: AZT = zidovudine; 3TC = lamivudine; EFV = efavirenz; NVP = nevirapine; d4T = stavudine; TDF = tenofovir.
P value using joint testing of coefficients: P ≤ 0.05.
P value using joint testing of coefficients: P ≤ 0.10.
The findings of elevated attrition risk by distance of patient residence and by hospital site were robust across sensitivity analyses using alternative attrition definitions and alternative strategies for handling missing data (Table 3). The findings on attrition risk by calendar period, ART regimen, and number of counseling sessions prior to ART initiation were more sensitive to the attrition definition and to the strategy for handling missing data. Calendar period was significantly associated with attrition risk in all models except the model using the 60+ day pharmacy definition; however, ART regimen and number of counseling sessions were not significantly associated factors in the sensitivity analyses.
TABLE 3.
Sensitivity analyses for alternative definitions of antiretroviral therapy (ART) attrition: Adjusted hazard ratios (HR), Haiti, 2005–2011a
| 30+ day pharmacy |
60+ day pharmacy |
90-day visit |
180-day visit |
|||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | HR | P value | HR | P value | HR | P value | HR | P value |
| Site | ||||||||
| Hôpital St. Michel in Jacmel vs. Hôpital St. Antoine in Jérémie |
0.58 | < 0.001 | 0.53 | < 0.001 | 0.46 | < 0.001 | 0.95 | 0.69 |
| Post vs. pre-earthquake | 0.94 | 0.66 | 0.77 | 0.14 | 0.38 | < 0.001 | 0.51 | < 0.01 |
| Gender (male=reference) | ||||||||
| Female (not pregnant) | 0.99 | 0.92 | 1.07 | 0.67 | 1.08 | 0.40 | 1.02 | 0.90 |
| Female (pregnant) | 1.08 | 0.71 | 1.20 | 0.54 | 1.16 | 0.42 | 1.44 | 0.22 |
| Age (10 years or more) | 1.05 | 0.20 | 0.98 | 0.75 | 0.92 | 0.02 | 0.84 | < 0.01 |
| Proximity (same commune=reference) | ||||||||
| Adjacent commune | 1.69 | < 0.001 | 1.44 | 0.02 | 1.03 | 0.77 | 1.20 | 0.25 |
| Non-adjacent commune | 1.89 | < 0.001 | 2.37 | < 0.001 | 1.65 | < 0.001 | 2.58 | < 0.001 |
| Body mass index (< 18.5=reference) | ||||||||
| >18.5 | 1.19 | 0.06 | 1.18 | 0.21 | 1.18 | 0.04 | 1.40 | 0.02 |
| Year of ART start (2005-2006=reference) | ||||||||
| 2007–2008 | 0.66 | < 0.001 | 0.91 | 0.56 | 2.02 | < 0.001 | 1.14 | 0.36 |
| 2009–2010 | 0.60 | < 0.01 | 1.09 | 0.71 | 1.67 | < 0.001 | 0.81 | 0.41 |
| 2011 | 0.37 | < 0.001 | 0.54 | 0.13 | 2.20 | < 0.01 | 0.88 | 0.77 |
| Baseline CD4 (< 100=reference) | ||||||||
| 100–249 | 1.06 | 0.58 | 1.01 | 0.97 | 1.06 | 0.54 | 0.75 | 0.05 |
| ≥ 250 | 1.15 | 0.33 | 0.98 | 0.92 | 1.04 | 0.74 | 0.78 | 0.18 |
| ART regimenb (AZT-3TC-EFV=reference) | ||||||||
| AZT-3TC-NVP | 1.14 | 0.23 | 0.94 | 0.70 | 0.87 | 0.16 | 0.79 | 0.15 |
| d4T or TDF + 3TC-EFV | 0.67 | 0.10 | 0.47 | 0.07 | 1.01 | 0.97 | 1.08 | 0.81 |
| d4T or TDF + 3TC-NVP | 0.86 | 0.51 | 0.78 | 0.45 | 0.73 | 0.09 | 0.66 | 0.21 |
| non-standard regimens | 2.04 | 0.01 | 1.04 | 0.94 | 0.54 | 0.07 | 1.17 | 0.73 |
| World Health Organization clinical stage (Stage I or II = reference) |
||||||||
| Stage III or IV | 1.16 | 0.07 | 1.19 | 0.15 | 1.11 | 0.13 | 1.09 | 0.51 |
| Tuberculosis status (No suspicion, prophylaxis or diagnosis = reference) |
||||||||
| Yes | 0.91 | 0.32 | 0.95 | 0.75 | 0.94 | 0.48 | 1.03 | 0.81 |
| Any Stage IV symptom (No=reference) | ||||||||
| Yes | 0.84 | 0.22 | 0.77 | 0.21 | 0.94 | 0.64 | 1.16 | 0.47 |
| Pre-ART duration (30 day increase) | 0.99 | 0.01 | 0.99 | 0.07 | 0.99 | < 0.01 | 0.98 | 0.01 |
| Counseling sessions prior to ART start (None = reference) | ||||||||
| 1 | 0.84 | 0.07 | 0.92 | 0.52 | 1.11 | 0.22 | 1.05 | 0.73 |
| ≥ 2 | 0.74 | 0.06 | 0.83 | 0.42 | 0.99 | 0.93 | 0.92 | 0.75 |
Main effects model using alternative definitions for ART attrition; items in bold represent statistically significant hazard ratios.
ART regimen: AZT = zidovudine; 3TC =l amivudine; EFV = efavirenz; NVP = nevirapine; d4T = stavudine; TDF = tenofovir.
DISCUSSION
This study describes ART attrition before and after the 2010 earthquake in two large public-sector hospitals in rural departments of Haiti. The detailed, routine pharmacy data in iSanté enables measurement of attrition via the 30+ day pharmacy definition, an outcome measure that reflects continuity of actual ART use, and that is sensitive to any interruptions of clinical significance. Our exploratory findings point to several quality improvement interventions with potential to improve ART program outcomes at both hospitals.
That ART attrition risk was sharply higher at HSA in Jérémie compared to HSM in Jacmel, a finding that is consistent with other studies from Haiti showing the heterogeneity of ART attrition in different facilities and geographic areas (5, 9, 18). For example, unpublished studies by the MSPP found that in 2010, ART attrition after 12 months ranged from 13%–48% among facilities with at least 50 ART patients (9); and ranged from 6%–51% by department among ART enrollees in 2007, 2010, and 2011 (18). The relatively high rate of ART attrition observed at HSA may reflect the relative disadvantage of the GrandAnse Department (1).
The finding of elevated risk of attrition for patients residing at a greater distance from the hospital was noteworthy. Since the start of the national ART program, Haiti has steadily decentralized ART to bring services closer to the people in need, growing the number of ART sites from two in 2003 to more than 63 in 2011 (3, 9). However, as late as 2011, 36% of patients newly enrolling in ART at HSA and HSM still resided outside the commune of the hospital. This finding is consistent with evidence from other low-resource settings where patient travel distance and transportation barriers play a role in ART attrition (19). Studies in Malawi, South Africa, and Mozambique have all demonstrated more favorable ART retention in decentralized primary care centers compared with larger centralized hospitals (12, 20, 21). We can presume that travel distance plays a strong role in explaining these findings.
In Haiti, as in other countries, the question of the right level of ART decentralization is complex. There is tension between the goal of increasing patient access to ART via decentralization, and the goal of maintaining quality of care via concentration of resources in larger, regionalized care centers. Within the global health services literature, there is a compelling body of evidence indicating that greater patient volume is associated with improved health outcomes in HIV care (22–24), as well as other areas such as surgery (25), cardiac care (26), and neonatal care (27). Patients, who travel long distances for ART services, whether by necessity or preference, need supportive services to counteract elevated risk of attrition, particularly considering the limited resources available for continued ART decentralization in Haiti.
The pattern of decreased attrition risk with maturation of the national ART program is consistent with evidence from other low-resource countries (5, 11, 12, 28, 29), likely reflecting greater provider experience and access to medical technologies over time. ART attrition did not increase following Haiti’s devastating earthquake of 2010 at either site, which reflects tremendous public health efforts to rapidly re-establish ART services following the earthquake (30).
Our finding that adjusted attrition risk was 26% lower for patients receiving two or more counseling sessions before ART initiation compared to those who did not receive counseling differ from that of a Ugandan study reporting no benefit from counseling sessions (31). In the Haitian context, counseling sessions may effectively identify and mitigate some barriers to care.
Quality improvement interventions
Together, our findings suggest several interventions to improve retention of patients on ART. First, targeting patients who access care from remote areas (non-adjacent communes) with enhanced retention support by community health workers may be fruitful. Mobilizing greater material resources for transportation subsidies may also be important. Offering counseling for all patients prior to ART initiation could help ensure that patients’ access barriers are more universally and systematically identified. Scheduling outreach to patients who are late for ART refills based on automated alerts arising from ART dispensing data, without waiting until 90 days elapse between visits, could also mitigate attrition. Providing “bridging services” whereby community health workers accompany transferring patients to the new facility in a timely manner could also help ensure that ART use remains uninterrupted during the transfer process. Routine screening at each ART pick-up for anticipated inability to return in a timely manner for the next refill, possibly due to upcoming travel, would allow providers to adjust the duration of ART dispenses accordingly. Finally, conducting medical case review for patients placed on non-standard ART regimens could help improve ART outcomes for these patients. Of course, the associations observed in this study cannot be interpreted as causal, due to the possibility that unmeasured patient characteristics drive the observed relationships. Still, the suggested quality improvement interventions are worthy of further testing at the two hospitals.
Study limitations
Data quality within routine health information systems in low-resource settings, such as iSanté, is typically considered to be weak (32). However, a data validation exercise comparing iSanté electronic data with data from paper charts and ART registers within a randomly selected sample of 200 ART patients from the two hospitals, found a high concordance (≥ 90%) of both outcome and covariate data between sources for patient demographic data, weight, CD4, and ART medication prescriptions (33). Further, our estimates of attrition closely matched estimates independently obtained by the MSPP from paper-based ART registers at each of the two sites, for the cohort of patients enrolled on ART in 2010 (9). In sum, the consistency of both outcomes and covariate data between iSanté and other data sources underscores that the iSanté data were fit for use for this study.
The lack of cause-specific information about attrition represents a second limitation. Our estimates of absolute attrition levels may be upwardly biased due to our inability to fully account for transfers of care to sites outside of the iSanté network. However, given that iSanté sites account for more than half of patients enrolled in the national ART program, the number of uncounted transfer cases in our cohort was likely small. This means that neither the absolute attrition estimates nor the hazard ratio estimates are likely to be strongly affected by missing information about patient transfers.
The absence of socioeconomic data on income, occupation, and education is an important limitation of the iSanté data system, as is its lack of data on other influential patient and system-level factors, namely, mental health status, provider experience level, availability and use of ancillary services such as nutrition support, and history of ART stock-outs (Figure 1). Still, iSanté represents a remarkably rich data source for operational research and monitoring of quality improvement interventions.
Conclusions
ART attrition differed notably between the two hospitals and was strongly associated with distance of patient residence from the facility. The latter finding has important policy implications with respect to decentralization of services and argues for minimizing transportation-related access barriers. As Haiti expands its criteria for ART eligibility, challenges of maximizing retention will intensify. Quality improvement interventions that target retention support to patients residing in remote areas, and that promptly provide outreach to patients who miss ART refills, merit testing at the two facilities, as well as at other similar facilities in Haiti. This study demonstrates the potential of the iSanté system to not only inform the design of interventions for improved ART retention, but also to monitor and evaluate their success over time.
Acknowledgements
The authors would like to thank Newton Jeudy (Medical Director, Hôpital St. Michel de Jacmel, Ministry of Public Health and Population), Jean Marie Duvilaire (Medical Director, Hôpital St. Antoine de Jérémie, Ministry of Public Health and Population), Jean Ronald Cadet (National AIDS Control Program, Ministry of Public Health and Population), and Garilus France (Planning and Evaluation Unit) from the Ministry of Public Health and Population for their support with the conceptualization of the study. The authors also thank Jean Gabriel Balan (I-TECH Haiti), Marcia Weaver (University of Washington), and Barbara Marston (United States Centers for Disease Control and Prevention) for thoughtful comments on the presentation of study results. The authors acknowledge the contributions of Bill Lober (I-TECH Director of Informatics) for design of the iSanté data system, and Steven Wagner (I-TECH Informatics Team) for generating the iSanté data extracts. The study would not have been possible without the dedicated efforts of the disease-reporting officers and health care workers at Hôpital St. Michel and Hôpital St. Antoine who entered patient data into iSanté over many years.
This research was supported by the United States President’s Emergency Plan for AIDS Relief (Washington, DC, United States) through the Health Resources and Services Administration (Grant no. U91HA0680), and the U.S. Centers for Disease Control and Prevention (Grant no. 5U2GGH000549-03) to the International Training and Education Center for Health (I-TECH) at the University of Washington (Seattle, Washington, United States). The research was also supported by the Center for AIDS Research (CFAR) at the University of Washington, which is in turn supported by the United States National Institutes of Health (Bethesda, Maryland, United States) (Grant no. P30AI027757). The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their supporting agencies.
Footnotes
Conflict of Interest. None.
REFERENCES
- 1.Cayemittes M, Busangu MF, Bizimana JdD, Barrère B, Sévère B, Cayemittes V, et al. Intitut Haitien d’Enfance; Port-au-Prince: Apr, 2013. Enquête mortalité, morbidité et mtilisation des services, Haiti 2012. [Google Scholar]
- 2.Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H. Antiretroviral therapy in resource-poor settings: decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43(suppl. 1):S123–S6. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- 3.Ministère de la Santé Publique et de la Population, Programme National de Lutte contre le SIDA . Déclaration d’Engagement sur le VIH/SIDA: rapport de situation national. MSPP/PNLS; Port-au-Prince: 2012. [Google Scholar]
- 4.Matheson AI, Baseman JG, Wagner SH, O’Malley GE, Puttkammer NH, Emmanuel E, et al. Implementation and expansion of an electronic medical record for HIV care and treatment in Haiti: an assessment of system use and the impact of large-scale disruptions. Int J Med Inform. 2012;81(4):244–56. doi: 10.1016/j.ijmedinf.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Koenig SP, Rodriguez LA, Bartholomew C, Edwards A, Carmichael TE, Barrow G, et al. Long-term antiretroviral treatment outcomes in seven countries in the Caribbean. J Acquir Immune Defic Syndr. 2012;59(4):e60–71. doi: 10.1097/QAI.0b013e318245d3c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353(22):2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 7.Leger P, Charles M, Severe P, Riviere C, Pape JW, Fitzgerald DW. 5-Year survival of patients with AIDS receiving antiretroviral therapy in Haiti. N Engl J Med. 2009;361(8):828–9. doi: 10.1056/NEJMc0809485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. In Haiti, the Jacmel cathedral clock stopped at 5:37 pm. 20 January 2010. Available from: www.mysinchew.com/node/34251. Accessed on 17 November 2014.
- 9.Ministère de la Santé Publique et de la Population, Programme National de Lutte contre le SIDA Rétention à 12 mois des nouveaux patients sous traitement antirétroviral en 2010 en Haïti. Bulletin de Surveillance Epidemiologique VIH/SIDA. 2013;3:5–17. [Google Scholar]
- 10.Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8(10):e1001111. doi: 10.1371/journal.pmed.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15:1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosmer D, Lemeshow S, May S. Applied Survival Analysis. 2nd Wiley; New York: 2007. [Google Scholar]
- 14.Groenwold RHH, White IR, Donders ART, Carpenter JR, Altman DG, Moons KGM. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–9. doi: 10.1503/cmaj.110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7(2):147–77. [PubMed] [Google Scholar]
- 16.Knol MJ, Janssen KJM, Donders ART, Egberts ACG, Heerdink ER, Grobbee DE, et al. Unpredictable bias when using the missing indicator method or complete case analysis for missing confounder values: an empirical example. J Clin Epidemiol. 2010;63(7):728–36. doi: 10.1016/j.jclinepi.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Buuren SV, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Ministère de la Santé Publique et de la Population/Programme National de Lutte contre le SIDA . MSPP/PNLS; Port-au-Prince: 2014. Rapport de l’enquete sur retention sous traitement antiretroviral et determination des indicateurs d’alerte precoce. [Google Scholar]
- 19.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in South-western Uganda: a qualitative study. AIDS Behav. 2009;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massaquoi M, Zachariah R, Manzi M, Pasulani O, Misindi D, Mwagomba B, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Lambdin BH, Micek MA, Koepsell TD, Hughes JP, Sherr K, Pfeiffer J, et al. Patient volume, human resource levels, and attrition from HIV treatment programs in Central Mozambique. J Acquir Immune Defic Syndr. 2011;57:e33–e9. doi: 10.1097/QAI.0b013e3182167e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handford CD, Rackal JM, Tynan A-M, Rzeznikiewiz D, Glazier RH. The association of hospital, clinic and provider volume with HIV/AIDS care and mortality: systematic review and meta-analysis. AIDS Care. 2012;24(3):267–82. doi: 10.1080/09540121.2011.608419. [DOI] [PubMed] [Google Scholar]
- 23.Kitahata MM, Van Rompaey SE, Dillingham PW, Koepsell TD, Deyo RA, Dodge W, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med. 2003;18:95–103. doi: 10.1046/j.1525-1497.2003.11049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitahata MM, Van Rompaey SE, Shields AW. Physician experience in the care of HIV-infected persons is associated with earlier adoption of new antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;24:106–14. doi: 10.1097/00126334-200006010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 26.Grumbach K, Anderson GM, Luft HS, Roos LL, Brook R. Regionalization of cardiac surgery in the United States and Canada: geographic access, choice, and outcomes. JAMA. 1995;274:1282–8. [PubMed] [Google Scholar]
- 27.Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276:1054–9. [PubMed] [Google Scholar]
- 28.Auld AF, Mbofana F, Shiraishi RW, Sanchez M, Alfredo C, Nelson LJ, et al. Four-year treatment outcomes of adult patients enrolled in Mozambique’s rapidly expanding antiretroviral therapy program. Plos One. 2011;6(4):e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabapathy K, Ford N, Chan KN, Kyaw MK, Elema R, Smithuis F, et al. Treatment outcomes from the largest antiretroviral treatment program in Myanmar (Burma): a cohort analysis of retention after scale-up. J Acquir Immune Defic Syndr s. 2012;60(2):e53–e62. doi: 10.1097/QAI.0b013e31824d5689. [DOI] [PubMed] [Google Scholar]
- 30.Puttkammer NH, Zeliadt SB, Balan JG, Baseman JG, Destiné R, Domerçant JW, et al. Before and after the earthquake: a case study of attrition from the HIV antiretroviral therapy program in Haiti. Glob Health Action. 2014;7(0) doi: 10.3402/gha.v7.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siedner MJ, Lankowski A, Haberer JE, Kembabazi A, Emenyonu N, Tsai AC, et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. Plos One. 2012;7(6):e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AbouZahr C, Adjei S, Kanchanachitra C. From data to policy: good practices and cautionary tales. Lancet. 2007;369(9566):1039–46. doi: 10.1016/S0140-6736(07)60463-2. [DOI] [PubMed] [Google Scholar]
- 33.Puttkammer N, Raphael A, Zamor G. International Training and Education Center for Health (I-TECH); Seattle, WA: 2013. Report: iSanté data validation exercise. [Google Scholar]