Abstract
Intracellular nucleic acid delivery has the potential to treat many genetically-based diseases, however, gene delivery safety and efficacy remains a challenging obstacle. One promising approach is the use of polymers to form polymeric nanoparticles with nucleic acids that have led to exciting advances in non-viral gene delivery. Understanding the successes and failures of gene delivery polymers and structures is key to engineering optimal polymers for gene delivery in the future. This article discusses the polymer structural features that enable effective intracellular delivery of DNA and RNA, including protection of nucleic acid cargo, cellular uptake, endosomal escape, vector unpacking, and delivery to the intracellular site of activity. The chemical properties that aid in each step of intracellular nucleic acid delivery are described and specific structures of note are highlighted. Understanding the chemical design parameters of polymeric nucleic acid delivery nanoparticles is important to achieving the goal of safe and effective non-viral genetic nanomedicine.
Graphical abstract
Introduction
Because aberrations in the genetic code are the root cause of many inheritable diseases (i.e., cystic fibrosis, hematological disorders, severe combined immunodeficiency disorder) [1] and acquired diseases such as cancer, gene therapy, if fully realized, could provide treatments, and potentially even cures [2]. Although gene therapy is promising, realizing the potential of gene therapy has proven to be a tremendous challenge. Despite more than 2,000 clinical trials worldwide [3], to date only two gene therapies have reached approval by regulatory bodies; namely the Chinese Food and Drug Administration (2003; adenovirus vector delivering the P53 gene for a head and neck squamous cell carcinoma application) and the European Medicines Agency (2012; associated-adenovirus vector delivering the lipoprotein lipase gene for a lipoprotein lipase deficiency application). There have not been any U.S. FDA approvals, underscoring the need for safer and more effective gene delivery vectors.
Because viral vectors have evolved to be highly efficient, many of the past and ongoing clinical trials have focused on viral methods. However, viral methods are known to be associated with insertional mutagenesis, can cause deleterious side effects, and are immunogenic which raise safety concerns. There are a host of attractive non-viral alternatives which enable transient non-integrating gene transfer and mitigate safety concerns. Non-viral methods do not have cargo size restrictions that viruses do, enabling larger genes to be delivered and combinations of genes. Non-viral biomaterials are also able to be manufactured on a larger scale more easily than viruses and are able to be more readily chemically modified for enhanced function as well.
Although non-viral vectors are typically considered safer than viral vectors, further optimization of non-viral vectors is necessary for clinical translation. As biomaterial and nanoparticle structure-function relationships are more thoroughly characterized, rational design principles can be implemented to engineer superior polymeric vectors, aiding the clinical translation of gene therapies.
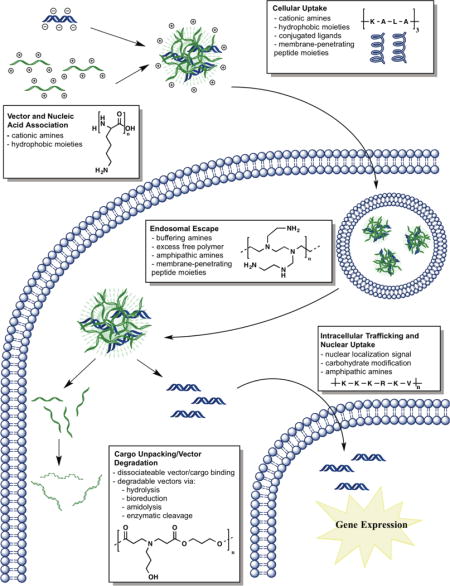
This review article discusses how structural elements of natural (i.e., peptides such as poly(L-lysine) (PLL) and carbohydrates, such as chitosan) and synthetic (i.e., poly(β-amino ester)s (PBAE), poly(ethyleneimine) (PEI), polyurethane, disulfide-containing poly(amido amine)s, alkyl-amine containing polymers, imine-containing polyamines, and polyorthoesters) polymers are used to aid the transport of nucleic acid cargo (i.e., short hairpin RNA (shRNA), plasmid DNA (pDNA), short interfering RNA (siRNA), micro RNA (miRNA), and messenger RNA (mRNA) [4]) to overcome intracellular barriers (Table 1). These barriers include: vector and nucleic acid cargo association for protection of nucleic acid from degradation; cellular uptake; endosomal escape to the cytoplasm; nucleic acid unpacking from the vector carrier; vector degradation to minimize toxicity; and lastly, nuclear uptake in the case of DNA (Figure 1) [4].
Table 1.
Select polymeric structural features to overcome intracellular gene delivery barriers.
| Gene Delivery Barrier | Polymer Structural Features |
|---|---|
| Vector and Nucleic Acid Cargo Association | Electrostatic Binding via Cationic Amine Charges (1°>2°>3°) |
| Cellular Uptake | Upregulated Ligand-Conjugation (i.e., Folic Acid) Site-specific Triggered Release to Target Cell Type (i.e., MMP) Cationic Carrier and Anionic Membrane Affiliation Membrane Adsorption via Lipophilicity/Hydrophobicity (i.e., Akyl Addition) Membrane Penetration via Cell Penetrating Peptides (i.e., KALA) |
| Endosomal Escape | Amine Buffering Moieties Leading to Endosomolysis (3°>2°>1°) Buffering via Excess Free Polymer Disruption via Amphipathic Amines or Penetratable Entities (i.e., CPP) |
| Cargo Unpacking/Vector Degradation | Modifying Binding Strength (i.e., Molecular Weight, Amine Density) Polymer Degradation (i.e., Amidolysis, Hydrolysis, Reduction (siRNA), MMP-cleavable) |
| Intracellular Trafficking and Nuclear Uptake | Optimal Polymer Degradation Kinetics to Minimize Nucleic Acid Time in Free State (Nucleic Acid in Free State is More Prone to Nuclease Degradation) Nuclear Localization Signal Conjugation (i.e., PKKKRKV) Incorporating a DNA-Targeted Sequence into the Cargo Carbohydrate Shuttling through Nuclear Membrane Nuclear Pore Permeabilization via Amphipathic Properties (Emulating Importin Activity) |
Figure 1.
Intracellular nucleic acid delivery steps and polymer design strategies. These steps include: 1) Vector and nucleic acid association; 2) Cellular uptake via a variety of mechanisms (i.e., clathrin-mediated, caveolae, and macropinocytosis) [5]; 3) Endosomal escape to circumvent the lysosomal degradation pathway; 4) Nucleic acid cargo unpacking, in some cases due to degradation of the nucleic acid vector, minimizing toxicity; 5) Intracellular trafficking and nuclear import in the case of DNA.
Vector and nucleic acid cargo association
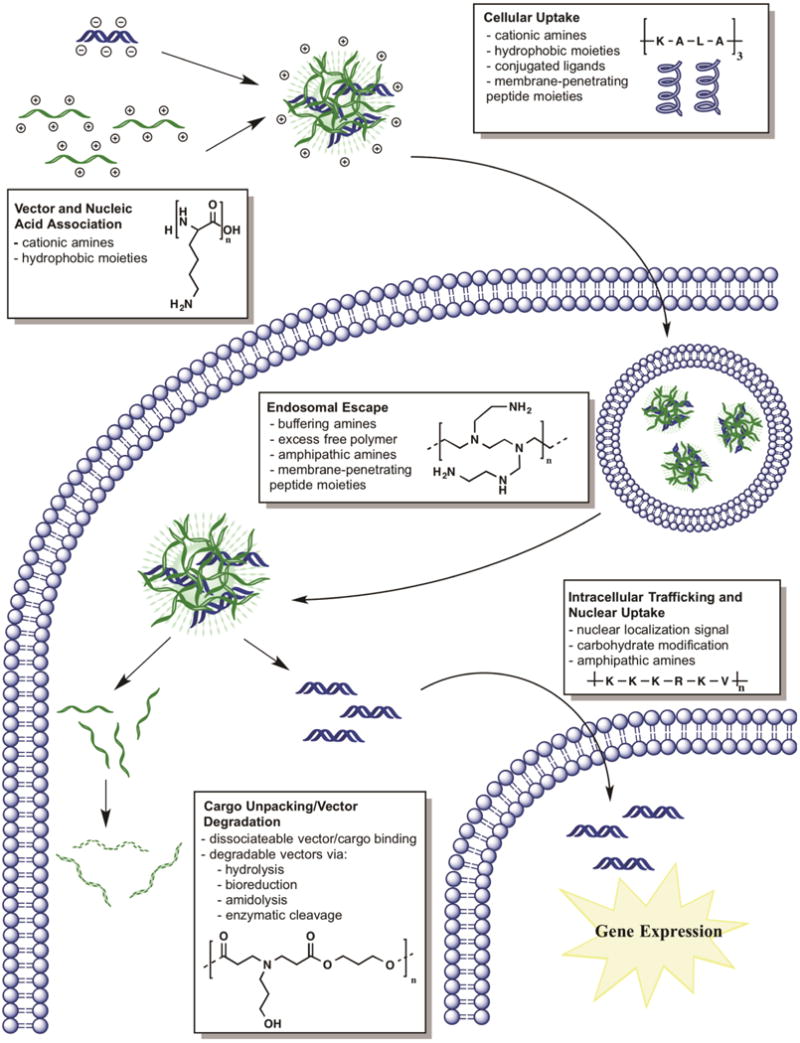
Nucleic acid molecules are difficult to deliver intracellularly due in large part to the ease in which enzymes can degrade them and the difficulty of transporting large negatively charged biomacromolecules across cellular membranes. Therefore, the first step required from a polymer to be used as a non-viral polymeric gene delivery vector is nucleic acid binding, complexation, and/or encapsulation. Typically anionic nucleic acids are ionically complexed to cationic polymer vectors through electrostatic interactions via amine functional groups capable of forming nanoparticles (also referred to as polyplexes) on the order of 25–300 nm [6, 7]. Cationic amine-containing vectors have included natural polymers of amino acids [8] and sugars such as histone proteins [9], peptides [10], and chitosan [1], as well as synthetic polymers such as linear and branched poly(ethyleneimine) (PEI) [11], dendrimers [12], poly(β-amino ester)s (PBAE) [13], peptide nucleic acid (PNA) [14], and many others. Structures of commonly used polymers that form nanoparticles with nucleic acids for intracellular delivery are shown in Figure 2 [1, 13, 15–17]. A number of the effective polymers (i.e., PBAEs, epoxide-containing block copolymers) for gene delivery have been discovered via combinatorial high throughput methods [18–20].
Figure 2.
Chemical structures of select polymers employed for gene delivery [1, 13, 15–17, 21].
Due to charge neutralization by cationic materials, large anionic biomacromolecules such as plasmid DNA can be condensed into a small sized particles. This is critical to protect nucleic acids from nuclease degradation [22] extracellularly and intracellularly. The half-life of uncomplexed nucleic acid extracellularly is on the order of 10 minutes [23] and intracellularly on the order of 1 hour [24]. Therefore, protection of nucleic acid within polymeric nanoparticles is important for successful nucleic acid delivery. It has been shown for example that a polymer complexed single-stranded nucleic acid’s half-life can be improved 3- to 6-fold during a 5 hour incubation in serum and more than 90% of polymer complexed pDNA remained full length in 1.5 and 3 hour incubations in serum and culture medium (10% serum) [25].
In addition, polymer/DNA polyplex complexation is critical to enable nucleic acids to be effectively internalized through a cell’s anionic phospholipid bilayer. Typically zeta potentials of polymer/DNA polyplexes are neutral to positively charged, which allows for better interaction of the polyplex with the anionic cell membrane. Binding of a polymer to a nucleic acid is multivalent due to the many positive charges of amines on a polymer (primarily the primary and secondary amines) associating with the many negatively charged phosphate groups on a nucleic acid. Several groups have quantified these polymer/DNA binding interactions for various cationic polymers and peptides [10, 11, 13, 26]. A generalized finding observed between different polymer structures and molecular weights in these studies is that binding affinity between cationic polymers and DNA is often biphasic, with binding affinity that is too low or too high contributing to poorer transfection efficacy than intermediate binding affinities. For example, with PBAEs it was found that polymer/DNA binding affinity increased with increasing PBAE molecular weight, with decreasing numbers of carbon atoms in the backbone or sidechain of the constitutive monomers, and with the incorporation of amine-containing polymer terminal groups [13]. The transfection levels were biphasic with binding affinity in two evaluated human cell lines, with optimal transfection activity with this class of polymers occurring with binding constants per amine in the range of 1–6×104 M−1. The importance of complexation has also been demonstrated through microfluidics assisted confinement experiments where polyplexes with 40–50% smaller diameters are formulated compared to bulk mixing approaches [27]. These more compact polyplexes were found to transfect 6–31% more cells and have 1.9–6.8-fold higher total exogenous gene expression [27].
Natural cationic peptides such as PLL were among of the first materials used as polymeric transfection agents due to their availability and their ability to form nanoparticles with DNA. PLL’s strong positive charge allowed it to bind to and charge-invert DNA [28–30]. Because histones naturally complex DNA through charged residues, histone proteins, namely H2A, have been used to complex DNA [9]. This approach has shown many fold higher transfection compared to other non-viral methods such as PLL, poly(L-arginine), and liposomes (phosphatidylcholine/serine, 7:3 molar ratio) [9]. The type of histone protein is critical as in this study there was little transfection observed using H1, H2B, H3, and H4. Synthetic polymers useful for binding DNA took their bio-inspired cues from biological DNA binding proteins that have high cationic charge. PEI, for example, has an extremely high cationic density as, throughout its structure, for every two carbon atoms there is an amine group.
Nucleic acid binding can be particularly challenging in the case of shorter therapeutic nucleic acids such as siRNA and miRNA compared to plasmid DNA. These nucleic acids are important for engineered gene regulation as both siRNA and miRNA can induce sequence-specific gene knockdown [31]. However, both RNAs are ~21–25 bp in length, approximately 200 times shorter than the length of plasmids typically used for gene delivery. Double stranded RNA is also stiffer, as a macromolecule, than double stranded DNA [32, 33]. Shorter nucleic acid length reduces the multivalency in the charge interaction between anionic nucleic acids and cationic polymers, and stiffness can prevent RNA from conforming into shapes favorable for polymer binding. To increase polymer/RNA binding and subsequent intracellular delivery, researchers have engineered siRNAs to be longer by using covalent linkages [34], sticky RNA overhangs [35], or have formed much larger structures such as siRNA microsponges [36]. Strategies that focus on polymer engineering have included chemically modifying polymers to be more cationic or hydrophobic. For example, poly(amido amine)s (PAAs) have been modified using ethylene diamine or triethylenetetraamine to increase polymer positive charge and increase siRNA binding [37, 38]. For PBAEs, increasing polymer hydrophobicity [39, 40] or molecular weight [40] led to successful siRNA binding and delivery. Additionally, increasing the PBAE to siRNA weight ratio (wt/wt) of polyplexes to 100:1 or greater has led to successful siRNA binding and intracellular delivery, while PBAE:DNA wt/wt is typically in a lower range from 25–75 wt/wt [41].
Chemically controlling siRNA binding for optimal delivery has also been employed by modifying poly(acrylic acid) polymers with amine-containing molecules. By functionalizing acrylic acid size chains with hydrophobic amine-containing agmatine (Agm) or hydrophilic amine-containing monosaccharide D-galactosamine (Gal), Pelet et al [42]. were able to fine tune siRNA binding to enable enhanced delivery. Polymers containing higher Agm content bound siRNA tightly and effectively knocked down protein expression, but were increasingly toxic as Agm content increased, likely due to the hydrophobicity of Agm [42].
Siegwart et al., via high throughput modular robotic synthesis, investigated 1,536 epoxide-functionalized, amine-containing block polymeric nanoparticle formulations which form hairy core-shells (Figure 3) [20]. The authors designed these materials to have a cationic core for siRNA entrapment and also found that optimal cross-linkers contained tertiary dimethylamine or piperazine, capable of aiding the proton sponge effect through endosomal buffering. Utilizing these materials, the authors were able to deliver both siRNA and pDNA in vitro as well as to murine hepatocytes in vivo [20].
Figure 3.
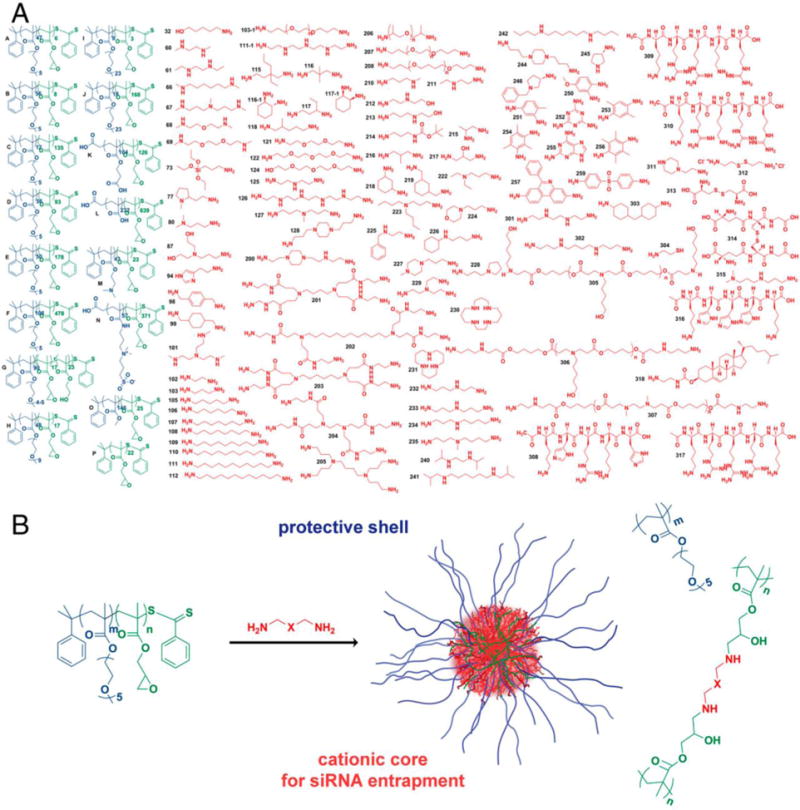
Library used for the formation of hairy core-shell nanoparticles. A) Epoxide-containing block copolymers and the amine-containing molecules used; B) The reaction proceeds via the ring-opening reaction with the amines which form β-hydroxyl groups. Reproduced with permission from Siegwart, et al. PNAS 108(32):12996–3001 [20]. Copyright (2011) National Academy of Sciences, USA.
A small compact nanoparticle size is also important for systemic biodistribution of nanoparticles administered in vivo, particularly as cancer therapeutics. Tumor neovasculature is heterogeneous and more permissive to the transport of nanostructures from the blood than healthy vasculature [43]. Thus, polymers with suitable binding affinity have the potential to bind nucleic acid to form a small and stable nanoparticle that is above the glomerular filtration cutoff (> 10 nm) and below the cutoff for permeability in leaky tumor vasculature (< 200 nm preferably) [44, 45] to pass into the extravascular space. Once into the tumor space, the nanoparticles are more likely to be retained there due to compromised lymphatic drainage (enhanced permeability and retention effect) [43]. For a cancer therapeutic, this is an important nanomedicine feature.
A common structural feature among gene delivery polymers to satisfy the design requirements of complexing nucleic acid is cationic amines. Although primary amines have stronger binding affinity with nucleic acids than secondary or tertiary amines, the presence of primary amines is not a strict requirement for successful condensation and transfection. Generally, the overall charge of polyplex nanoparticles post-nucleic acid complexation is cationic, aiding the interaction with the anionic phospholipid bilayer membrane.
Cellular Uptake
Cellular uptake of polymeric nanoparticles is often through adsorptive endocytosis [46]. Analysis of the cell membrane’s interaction with amphiphilic materials has demonstrated that adsorption of hydrophobic moieties can cause disruption or deformation of the outer layer of the cell membrane [47], and that this disruption can induce endosomal uptake (Figure 4A) [48]. Therefore, promoting cellular uptake is often accomplished using polymers designed to increase the interaction between nanoparticles and the cell membrane.
Figure 4.
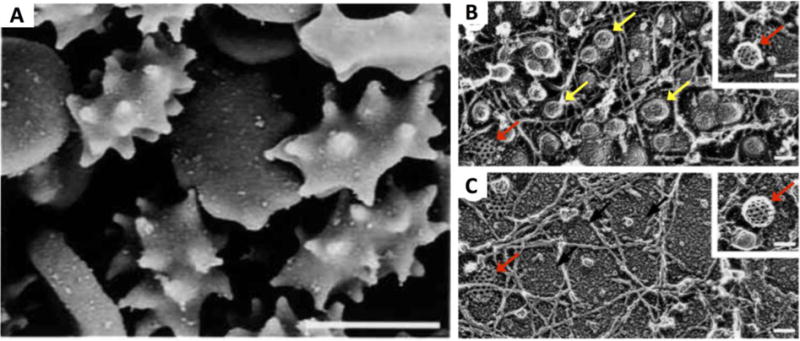
(A) Amphiphilic PEG-cholesterol induces erythrocyte membrane stress and causes protrusions along the cell membrane. (B) Increasing concentrations of amphiphilic polymer changes the primary mode of endosomal uptake in A431 human squamous carcinoma cells from combined caveolae-mediated and clathrin-mediated endocytosis to (C) primarily clathrin-mediated endocytosis. This can be observed by the decreased prevalence of smooth membrane protrusions indicative of caveolae (yellow arrows) in (B) versus (C), whereas the polyhedral structure typical of clathrin membrane protrusions (red arrows) can be seen in both images. Polymer/nucleic acid nanoparticles can likewise be engineered to preferentially enter the cell via one or both of these pathways. Scale bars: panel A: 5μm; all others: 100 nm. Reproduced with permission from [48]. Copyright John Wiley and Sons 2001.
Cationic materials are employed to electrostatically interact with the negative charge of the phospholipid bilayer membrane [49]. In order to accomplish this, it is necessary to cause the nucleic acid to charge-invert through the use of adequate cationic polymer. As previously mentioned, polymers like PLL can bind to anionic DNA to form a cationic polyplex that can then associate with the anionic cell surface [28–30]. It should be noted, however, that very strong positive charges could lead to cytotoxicity, likely due to electrostatic interactions that can deform the cell membrane to an unstable state [50]. It is therefore important to balance cationic polymer structure to optimize uptake without inducing cytotoxicity.
Hydrophobic polymers can also induce cell membrane adsorption, and have been shown to enhance gene delivery [51]. Moreover, cationic polymers that are more hydrophobic require less polymer mass to achieve charge-inversion of DNA, thus enabling uptake [30]. Functionalizing conventional polymers with hydrophobic moieties has promoted enhanced cell uptake. For example, PEI and PLL modified with palmitic acid promoted increased binding to the cell surface, and binding increased as the degree of substitution increased [52]. PAAs modified with alkyl chains have shown increased uptake as the number or length of alkyl modifications increased [12]. Tzeng et al. screened a library of PBAEs for siRNA delivery and found that only the more hydrophobic polymers tested were capable of any measureable gene knockdown [40].
Li, et al. determined which cationic lipid-like structures or lipidoids amongst 200 were able to efficiently deliver pDNA and siRNA (comparable or superior to Lipofectamine 2000 in HEK293T cells; approximately 2% of library) using a high throughput screening technique which involved a single-step alkylation of amines. They found they were able to enhance transfection by combining single-chain with double-chain lipidoids [53].
For optimization of siRNA delivery, bioreducible PBAE monomers have been copolymerized with hydrophobic monomers at varying ratios. Gene knockdown using these polymers demonstrated a biphasic response in which moderate hydrophobicity optimized gene delivery but increasing hydrophobicity led to cytotoxicity [39]. This demonstrates that polymer hydrophobicity, as with positive charge, must be balanced for optimal gene delivery without cytotoxicity.
A principal component analysis of PBAEs elucidated the degree that 24 physicochemical properties drove transfection, cellular uptake, and viability in human glioblastoma cells [54]; Hydrophobicity, as measured by the LogP partition coefficient and by the number of carbons in a polymer’s repeat unit, were both key drivers of cellular uptake and transfection [54]. While hydrophobicity drove principal component 1, molecular weight was found to drive principal component 2, with increased molecular weight being the most critical for successful transfection of the most hydrophilic polymers. Broadly among polymeric gene delivery nanoparticles, a common structural feature varied to optimize cellular uptake is the length of alkyl chains in the backbones or sidechains (i.e., PBAE, epoxide-containing block co-polymers, alkyl-amine-containing polymers, and poly(amido amine)s).
Cell-penetrating peptides (CPPs), a component of certain gene delivery polymers, often employ electrostatic or hydrophobic interactions to interact with the cell membrane. Amphipathic polypeptide GALA, consisting of 4 Glu-Ala-Leu-Ala repeats, is designed to interact with cell membrane lipid bilayers [55]. Peptide KALA, containing 3 Lys-Ala-Leu-Ala repeats, interacts similarly with the cell membrane but is cationic at slightly acidic to neutral pH, enabling nucleic acid binding [56]. Trans-activating transcriptional activator (TAT) peptide derived from HIV also penetrates lipid bilayers and can be functionalized onto polymeric nanoparticles to promote uptake [57, 58]. Manickam et al. designed multimeric TAT peptide linked with disulfide bonds as a gene delivery vehicle designed to enhance uptake [59]. Arginine-rich CPPs have also been employed for DNA delivery via modification of poly(methacrylate) polyplexes [60].
Small changes to polymer structure can dramatically change the efficacy and mechanism of cellular uptake. PBAE polymers end-capped with either amine-containing or acrylate-containing small molecules of similar size, DNA binding strength, and buffering capacity were used to form nanoparticles that were measured to have similar sizes and zeta potentials. Although every measured physical property showed the nanoparticles to be similar, uptake and transfection were negligible in acrylate-terminated polymers while amine-terminated polymers enabled successful uptake and transfection [61].
Small molecule changes to polymer structure can also direct uptake to specific endocytotic pathways (Figure 4B and 4C) [48]. Cellular uptake pathways relevant to polymeric gene delivery include macropinocytosis, clathrin-mediated endocytosis, and caveolae-mediated endocytosis. Non-specific, actin-driven uptake via macropinocytosis envaginates the cell membrane, engulfing the surrounding extracellular fluid [62]. Macropinocytosis has been implicated in nanoparticle uptake but often results in reduced transfection due to endosomal recycling [63]. Clathrin-mediated endocytosis, in which ~100–150 nm pits form via clathrin macromolecular organization, can be targeted by modification of nanoparticles with MC1SP-peptide or transferrin [64]. Unmodified liposomes and lipid-based materials have demonstrated clathrin-mediated endocytosis as their major mechanism of uptake [65]. Caveolae-mediated endocytosis involves ~50–100 nm flask-shaped pits which form within the cell membrane. These pits can be targeted via conjugation of folic acid to nanoparticles [66]. Caveolae-mediated endocytosis has been shown to be the major uptake pathway for several polymeric gene delivery nanoparticles [5, 67, 68]. Caveolae-mediated endocytosis has also been demonstrated to be the major uptake pathway of PBAE nanoparticles to human breast cancer cells, but the pathway which most determined transfection efficacy varied with polymer structure and molecular weight. Overall in PBAE-based studies, clathrin-mediated endocytosis enabled the most efficient transfection, while certain particles uptaken via caveolae encountered inefficient intracellular delivery that generally did not enable transfection [5].
It is important to note that although cellular uptake is a necessary step in successful transfection, uptake does not always lead to transfection. In work by Guerrero-Cazares et al., DNA delivery via PBAE nanoparticles was significantly more effective in primary human brain cancer cells versus human neural progenitor cells, while cell uptake was similar in vitro. Delivery of the same particles to brain cancer cells in vivo demonstrated selective transfection of cancer versus healthy tissue, even though nanoparticle uptake was distributed amongst both tissues [69]. However, in other cases, cell uptake is the key step in cell-type specificity. Modifying amine-containing molecules with glucopyranose block copolymers enabled Wu et al. to selectively target human hepatocellular carcinoma over human cervix adenocarcinoma, while removing the sugar modifications had the opposite targeting effect [70].
To increase the cellular uptake of polymeric nanoparticles in target cells, a ligand may also be conjugated to the nanoparticles that targets an overexpressed receptor of interest. For example, Zhang, et al, and Ogris, et al. have conjugated various ligands to their polymeric gene delivery nanoparticles such as folic acid [71] and epidermal growth factor [72]. In addition, polymeric nanoparticle affinity for a particular receptor may also trigger other biological responses. For example, a viologen-based dendrimer has been known to have affinity for CXC receptor 4, functioning as an antagonist of cancer cell metastasis [73]. Understanding the molecular pathways involved in cellular uptake and intracellular nanoparticle trafficking is an important consideration for future polymer structure design and may enable polymeric nanoparticles to target specific routes of uptake in target cells for enhanced transfection in specific cell types.
Endosomal escape
Following cellular uptake via adsorptive endocytosis, polymeric nanoparticles must escape the endosomal compartment in order for nucleic acids to reach their subcellular targets in the cytosol or nucleus. For amine-containing or titratable polymers, a mechanism that has been well reported in the literature [74, 75], but also still actively debated [76], is known as the “proton sponge” hypothesis. In this mechanism, protons pumped into the endosome do not decrease the endosomal pH as they normally would as this potential pH is buffered by reversibly-protonable polymeric moieties such as tertiary amines. A buildup of positive charge causes an influx of chloride counter-ions within the endosomes. The resulting hypertonic environment causes osmosis and an influx of water triggers endosome lysis and the nanoparticles are released into the cytosol. This process also protects the nucleic acids from damage due to the acidification of the endosome and leads to higher binding affinity between the polymer and nucleic acid as the polymer becomes further protonated. The proton-sponge effect was introduced to explain the gene delivery efficacy of PEI, which contains a high proportion of buffering tertiary and secondary amines, versus PLL which contains relatively more non-buffering primary amines [74]. Although the proton-sponge mechanism is only a theory and has been challenged [76], it remains the most widely accepted explanation for the endosomal escape of polymeric materials [75, 77]. While PLL has generally been shown incapable of endosomal release on its own [78, 79], it can be modified to allow buffering through covalent addition of chemical moieties with titratable amines, such as histidine and arginine, or via coencapsulation of amphipathic amines such as chloroquine [80, 81]. Many PAAs contain chemical groups that are able to be protonated at pH 5.1–7.4 which makes them capable of proton buffering and endosomal lysis [82]. PBAEs also contain titratable tertiary and secondary amines. Although the buffering capacities of common PBAEs are not as high as PEI on a per-mass basis, (1.4–4.6 mmol of H+/g vs. 6.2 mmol of H+/g, respectively) the PBAEs are less cytotoxic and can be used at higher wt/wt ratio to enable nanoparticle concentrations with approximately 5–fold higher total buffering capacity than PEI [61].
Lachelt, et al. were able to fine-tune proton sponge activity using a library of oligo(ethanamino)amides, containing oligoethanamino acids and histidines. This system has the ability to be protonated and has the pH profile need to rupture endosomes [83] which is critical for gene transfer. Furthermore, modification of poly(glycoamidoamine)s with moieties with higher buffering capacities has shown an increase in transfection based on the proton sponge theory [84].
It has been shown that without free polymer in solution, there is relatively very little transfection [85], that supplementation with free polymer restores gene transfection, and also that the physical states of polyplexes are not affected by free polymer [86–88]. Because polyplexes have an overall positive charge they can interact with the negatively charged cell membranes [89]. The excess polymer has been shown to facilitate uptake, decrease inhibitory polyplex interaction with glycosaminoglycans, and to play a role in the buffering of the endosome for the proton sponge effect [89]. Researchers have also shown that cellular uptake of polyplexes may not be dependent on the presence of free polymer and that free polymer may aid mostly with downstream delivery steps. It was shown in CT 26 cells that transfection was highest when free PEI was added 4 hours post plasmid incubation. As a result, the authors concluded that free polymer likely played a larger role in endosomal release or another mechanism downstream from cellular uptake. Figure 5 shows polyplexes (red Cy3-DNA in Figures 5A and 5D) and free linear PEI (green Cy5 in Figures 5B and 5E) in live cells 8 hours post transfection. Figure 5C and 5F show combined fluorescence channels with overlaid differential interference contrast (DIC). The yellow color indicates co-localization of free linear PEI and Cy3-conjugated plasmid. Figures 5A, 5B, and 5C are cells that were transfected simultaneously with free linear PEI whereas Figures 5D, 5E, and 5F are cells that were transfected and had the free linear PEI added 4 hours after transfection [86]. Because there was no difference in the intracellular distribution of Cy3-DNA (Figures 5C and 5F), it was demonstrated that the majority of free Cy5 PEI was colocalized with polyplexes 8 hours after transfection whether or not the free Cy5 PEI was added simultaneously or 4 hours after transfection, which was after when the polyplexes were completely internalized. This suggests that the free polymer did not play a role initially during the cellular uptake of the polyplexes.
Figure 5.
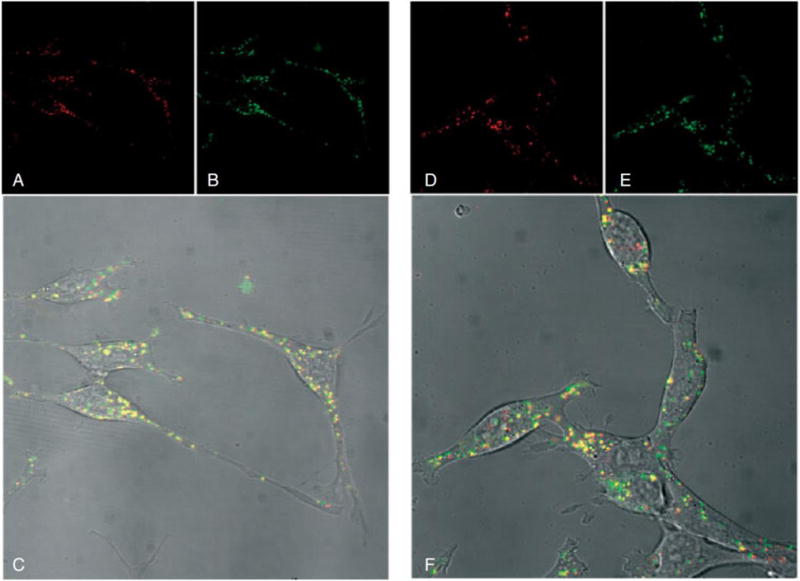
(A and D) CT 26 cells transfected with Cy3 (red) -labeled plasmid and (B and E) uncomplexed Cy5 (green) –labeled linear PEI, which was added either simultaneously (A, B, and C) or 4 hours post-transfection (D, E, and F). Similar intracellular delivery (yellow colocalization) is observed when PEI is added either (C) simultaneously with DNA or (F) 4 hrs later. Reproduced with permission from [86]. Copyright John Wiley and Sons 2004.
Perhaps the most common structural feature of gene delivery polymers to enable endosomal escape is amine groups capable of buffering in physiologically relevant pH (4.5–7.4). Yet, endosomal escape strategies also extend beyond buffering-induced endosomal lysis, often using materials engineered to directly interact with and disrupt the endosomal membrane. For example, anionic polymers have been employed to induce membrane disruption at moderately acidic endosomal pH [90]. CPPs designed to interact with and disrupt the cell membrane can also be engineered to disrupt the endosome. GALA and KALA peptides have been added to nanoparticles to induce endosomal escape [29, 91, 92]. KALA peptide is particularly good at disrupting late endosomes as it is alpha-helical at lower pH (4.5), and has been shown to induce 100% endosomal leakage over the pH range 4.5–8 [56]. The same arginine-rich CPPs that can enhance cell uptake have also been employed to facilitate endosomal escape [60]. In sum, there are multiple polymer strategies to protect nucleic acids from degradation and facilitate their release to the cytoplasm.
Cargo unpacking/vector degradation to minimize toxicity
Nucleic acid unpackaging from the nanoparticle is an imperative step in successful delivery. Research with PAAs and PEI-based polymers has demonstrated that DNA must be unbound from its delivery material in order for transfection to take place [93, 94]. Similarly, siRNA functionalized to gold nanoparticles has been shown to be unable to induce RNA interference (RNAi) when a siRNA release mechanism is not in place [95], but similar nanoparticles that enable release also enable RNAi [96]. As stated earlier, nucleic acid binding must be balanced to promote strong nanoparticle complexation while eventually allowing for cargo release, as transfection efficacy has been shown to have a biphasic relationship with nucleic acid binding affinity [13]. This can be achieved by balancing cationic character with hydrophobicity. Forrest et al. demonstrated that acetylation of primary amines in PEI could increase transfection as much as 21-fold over branched PEI when 43% of primary amines were acetylated [97]. This was later directly correlated with decreased polymer-DNA binding strength [98]. The most common mechanism of nucleic acid release is thermodynamic unbinding of the polymer and nucleic acid. The most common approach of increasing the rate of nucleic acid release is via polymer degradation, which enables high affinity nucleic acid binding to occur during polyplex formation and then this binding can be weakened over time or in a specific targeted intracellular environment. Polymer degradation has the added benefit of reducing cytotoxicity, as the molecular weight of polycations has been positively correlated with cytotoxicity [99]. Polymer molecular weight of conventional DNA delivery polymers such as linear PEI-PEG block copolymers has been shown to correlate positively with cytotoxicity. While increasing PEG chain length can reduce some of this toxicity, a PEG content higher than 50% was shown to also reduce transfection efficacy [100].
Hydrolytic polymer degradation via the addition of ester groups into a polymer’s structure can impart biodegradability to conventional polymers, which are normally non-degradable. Poly[alpha-(4-aminobutyl)-L-glycolic acid] (PAGA) is an analog of PLL with amide (peptide) bonds replaced with esters. DNA delivery using PAGA has shown enhanced transfection over PLL with reduced cytotoxicity [101]. Short, linear 800 kDa PEI linked with ester-containing diacrylate monomers has been shown to enable the same nanoparticle size, charge, DNA binding strength, and polymer MW as commercially-available 25 kDa branched PEI, but also enables hydrolytic degradation, and consequently,16-fold higher transfection and less toxicity [102].
Ester-containing monomers can be used to engineer polymers that are biodegradable without the need of subsequent polymer modification. Solid poly(lactic-co-glycolic acid) PLGA nanoparticles, as opposed to cationic polyplexes, can encapsulate DNA and siRNA via a double emulsion method or by grafting polyamines onto the PLGA backbone. PLGA degrades hydrolytically and its cargo release rate can be easily tuned by adjusting polymer properties such as monomer ratios and chain length [103–105]. PBAEs contain hydrolytically degradable esters whose half-lives are on the order of hours at pH 7.4 at 37°C, but this rate slows at moderately acidic pH and can protect the cargo in endosomes [40].
Hydrolytic biodegradation is also possible using urethanes, imines, and orthoesters. Yang et al. demonstrated DNA delivery using amine-containing polyurethanes [17]. For acid-labile hydrolysis, such as in the acidic tumor microenvironment, imine bonds have been employed to link short PEI chains [106]. Polyorthoesters can also be used for acid-labile hydrolysis as they degrade at pH 5 but not 7 [16, 107].
Disulfide linkages can enable environmentally-triggered release in the cytosol, which is approximately 1000-fold more reducing than the extracellular space [109]. This is particularly beneficial for delivery of mRNA, miRNA, and siRNA, whose site of action is in the cytoplasm [31]. It should be noted that disulfide bioreduction of DNA-carrying nanoparticles has in some cases decreased the expression of exogenously delivered DNA, which is likely due to reducing the amount of intact DNA that ultimately reaches the nucleus [41]. As with esters, addition of disulfides to conventionally nondegradable polymers can enhance nucleic acid delivery while reducing cytotoxicity. Disulfide-linked linear chains of PEI has shown comparable siRNA delivery efficacy versus branched PEI while significantly reducing cytotoxicity [110]. PLL modified with cysteines or with thiol-modified lysines allow for oxidation-induced crosslinking of nanoparticles and enable delivery of DNA or siRNA [111–113]. A comparison was made between linear, dendritic and hyperbranched (randomly branched; one-step preparation on a large scale) PLL structures [114]. The authors of the study found that transcription and translation were proportional to molecular weight, and that at similar molecular weights, the hyperbranched PLL analogs were superior. Disulfide-containing PAAs synthesized from diacrylamide monomers with disulfide linkers have been extensively studied for both DNA and siRNA delivery (Figure 6) [108, 115] and show improved intracellular nucleic acid release leading in many cases to enhanced efficacy. As discussed previously, PAA binding of siRNA can be too weak to form stable nanoparticles, but this can be overcome by modifying the polymers to be more cationic [37, 38]. KALA peptides covalently linked to cysteines have been used to form fusogenic nanoparticles capable of binding siRNA, using peptide conformation to induce cellular uptake and endosomal escape, and then releasing siRNA upon entry to the cytosol [116]. PBAEs containing disulfide bonds in the polymer endcaps [40, 117] or along the polymer backbone [39, 118] have enabled successful siRNA delivery to mesenchymal stem cells and brain cancer cells. Bioreducible PBAEs have demonstrated complete siRNA release within minutes of exposure to cytosolic redox conditions, which is likely what enables them to be used at higher (>100) wt/wts without significant cytotoxicity.
Figure 6.
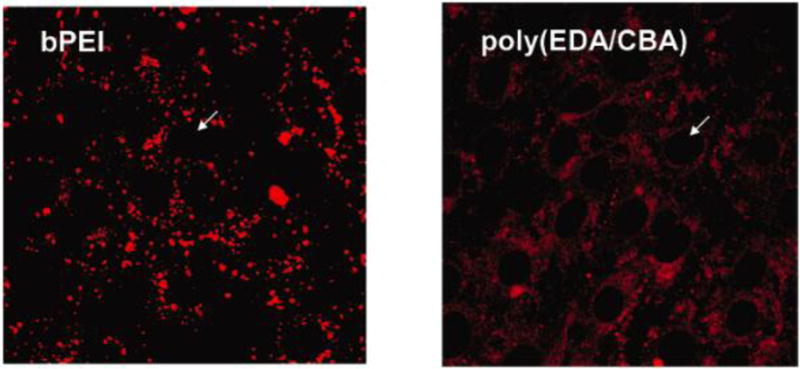
Confocal microscope images of NIH 3T3 bovine aortic endothelial cells 6 h following transfection using either branched PEI (bPEI) or a disulfide-containing poly(amido amine) (poly(EDA/CBA)). Fluorophore-labeled DNA is dispersed throughout the cell when poly(EDA/CBA) was used as the transfection agent, but not bPEI, suggesting that polymer bioreduction enabled improved DNA release. Reprinted (adapted) with permission from [108]. Copyright 2006 American Chemical Society.
Other modes of degradation can enable more specific spatial control of nucleic acid release. Kim et al. used MMP-cleavable peptide linkages to enable tumor-targeted DNA release [119]. External triggers can also be used for spatial control with specific polymer structures. For example, light-responsive polymers enable user-controlled spatial triggering of nucleic acid release [120]. As shown in Table 1, the most common polymeric gene delivery mechanisms utilized for unpacking nucleic acid cargo and mitigating toxicity from the polymer are amidolysis (amide bonds), hydrolysis (ester bonds), and reduction (disulfide bonds).
Intracellular trafficking and nuclear uptake
While the intracellular target for nucleic acids such as mRNA, siRNA, and miRNA is in the cytoplasm, the intracellular target for DNA is the nucleus. For successful gene expression, exogenous DNA must be translocated to the nucleus for transcription and translation to proceed within a relatively quick time frame as the half-life of DNA plasmids in different cells types has been shown to be on the order of 1 hour [121]. The cytoplasm is viscous and molecularly congested at approximately 100 mg/mL of protein, which greatly inhibits transport via diffusion, making the process of transport to the nucleus highly inefficient [122–124]. It has been shown that plasmids >2 kilobases in length were >100-fold slower in the cytoplasm than in water and were essentially immobile in cytoplasm for the study duration [122].
There is typically higher transfection in dividing cells as the nuclear membrane breaks down and plasmids in the cytoplasm can be stochastically encapsulated by the reformation of the nuclear envelope post-mitosis [124, 125]. To further increase nuclear uptake, nuclear localization signals (NLS) have been utilizes. NLSs are structural elements that can be associated with the DNA cargo either directly through conjugation or indirectly, such as through the binding of transcription factors. For example, plasmids can contain nucleotide sequences such as a DNA-targeted sequence (DTS) of a 77 base pair structural element from the simian virus (SV40): 5′-AACCAGCTGT GGAATGTGTG TCAGTTAGGG TGTGGAAAGT CCCCAGGCTC CCCAGCAGGC AGAAGTATGC AAAGCAT-3′ [126]. The DTS is a binding site for an endogenous transcription factor, which contains an embedded NLS. After DNA is released from a polyplex, the transcription factor can bind it. Following a morphological change that can reveal a hidden NLS, the NLS peptide handle can shuttle the DNA cargo into the nucleus via karyopherins. In one approach to incorporate an NLS into a polyplex system, FMOC is used to combine PNA to an SV40 NLS using a hydrophilic linker (PKKKRKV-linker-GCGCTCGGCCCTTCC; linker Fmoc-NC6O3H11-OH) [14]. The PNA conjugated to the NLS can be hybridized to a plasmid and then further ionically complexed with PEI to form polyplexes. The authors of this work were able to achieve 8-fold greater transfection with the NLS containing polyplexes than without the NLS [14].
Research has also revealed how polymer complexation affects pDNA import into nuclei and how polymer structures including linear PEI (JetPEI™) and two other carbohydrate-based poly(glycoamidoamine)s (one containing meso-galactarate (G4) and the other L-tartarate (T4)) affect nuclear import [127]. The authors found JetPEI and G4 exhibited higher transfection and were also able to permeabilize the nuclear envelop more efficiently than T4 [127]. They found that the carbohydrate moieties in the absence of plasmid were able to cross the nuclear membrane as well, suggesting key polymeric structures for rational design [127].
Other bioinspired methods for improved nuclear import have also been utilized. For example, researchers have used trans-cyclohexane-1,2-diol, which is an amphipathic alcohol, to emulate a hypothesized activity of importins at dissolving a hydrophobic phase within nuclear pores to cause the nuclear pores to become more permeable [128]. Using this approach, the authors showed high molecular weight dextrans and pDNA passed through the nuclear envelope without toxicity and enhanced transfection efficacy [128]. Thus, either the DNA plasmid or the polymer can incorporate structural elements to improve nuclear uptake.
Conclusions/Future Perspectives
Gene delivery represents a promising strategy for the treatment of many inheritable and acquired diseases, as it can enable the delivery of therapeutic genes or the knockdown of harmful or detrimental genes. Understanding the chemical properties and function of gene delivery polymers, which facilitate intracellular delivery of DNA and RNA, enables the design of safer and more effective gene therapies. Nucleic acid binding, cellular uptake, endosomal escape, cargo release, intracellular trafficking and nuclear uptake are all necessary steps to transfection. Therefore, engineering gene delivery polymers requires consideration of each obstacle simultaneously.
It should be noted that design of gene delivery vectors discussed herein focused on the optimization of intracellular delivery and trafficking of nucleic acids. Effective gene therapy nanoparticles will also need to address tissue-scale and systemic concerns. These include but are not limited to nanoparticle clearance by the mononuclear phagocytic system, colloidal stability in physiological salt, nanoparticle aggregation in blood serum, and controlled tissue targeting [129–131]. Nonetheless, effective gene delivery strategies must be optimized to carry nucleic acids and direct them to their site of action within the cell. Understanding the polymer chemistry that enables succesful delivery of nucleic acids is a cruicial step in the engineering of optimal gene therapy nanomedicines.
It is interesting to note structural similarities between polymers often used for gene delivery. Chitosan, the epoxide-containing block co-polymers, histone proteins, PNAs, linear and branched poly(amido amine)s, poly(L-lysine), end-modified PBAEs, linear and branched PEI, and poly(L-arginine) all have primary and secondary amines. Most of these structures also contain tertiary amines, with certain naturally derived materials such as chitosan, poly-L-lysine, and poly(L-arginine) being exceptions and in many cases, less effective for gene delivery as a result. While endosomal buffering following the proton sponge hypothesis remains a commonly used polymer design principle for gene delivery, other methods of endosomal disruption, such as incorporation of cell-penetrating peptides, can also be effective. Most gene delivery polymers are chosen from naturally degradable materials (i.e. enzymatically degradable peptides) or are designed to contain linkages that are degradable under physiological conditions (i.e. hydrolytic degradation of esters or reduction of disulfides). One exception to this rule is PEI, which was one of the first off-the-shelf commercial polymers shown effective for polymeric gene delivery even though it also exhibited dose dependent cytotoxicity. Since then, PEI analogs that contain hydrolytic or reducible linkages have shown improved efficacy and reduced cytotoxicity, demonstrating an approach to engineer synthetic gene delivery polymers for improved performance and safety. In some cases, polymeric gene delivery nanoparticle degradability can be engineered to be triggered by a particular microenvironment, such as through the use of matrix metalloproteinase-cleavable polymers which enzymatically degrade in pathologic tissues.
One of the advantages of polymeric gene delivery vectors is that polymers are amenable to high throughput synthesis strategies where a library of polymeric structures can be analyzed in parallel for gene delivery efficacy. Many of the chemically versatile polymer synthesis platforms, such as PBAEs and epoxide containing-block co-polymers, are able to vary hydrophobicity easily by variation in the number of carbons of constituent monomer units and able to vary molecular weight by simple variation to reaction conditions. These library approaches have allowed investigators to probe parameters linked to successful transfection. For the future development of next-generation polymeric gene delivery nanoparticles, it is useful for investigators to know quantitatively how differential changes to polymer structure tune nanoparticle properties and biological efficacy. This will enable rational design of polymers with specific attributes including hydrophobicity, molecular weight, amine content, and degradation kinetics. Critically, research suggests that these attributes may not be universal, but instead significantly dependent on the cargo type (ie DNA vs. siRNA) and the cell type of interest for each application.
A trend in the chemical properties discussed herein is the need for moderation and balance when designing polymers for gene delivery. Increasing polymer positive charge enhances nucleic acid binding while electrostatic interactions that are too strong will prevent cargo release and transfection. Hydrophobic polymers will bind nucleic acids and promote uptake, but hydrophobic interactions with the cell membrane that are excessively destabilizing can lead to toxicity. Biodegradable materials can improve nucleic acid release and reduce cytotoxicity, but may also reduce particle stability, which is particularly important for therapeutic applications. Several biodegradable cationic polymers have been identified as leading candidates due to their delivery efficacy, biodegradability, and safety. Although there are currently no U.S. FDA-approved polymeric nanoparticle systems for DNA or siRNA delivery, there are many ongoing clinical trials [132–135]. Through greater understanding of the underlying mechanisms to control and optimize polymer structure for gene delivery, enhanced therapeutic polymers for intracellular delivery of nucleic acids can be created.
Acknowledgments
The authors would like to acknowledge the NIH (1R01EB016721) for support, the NSF for a graduate research fellowship to CB (DGE-0707427), and the JHU Institute for Nanobiotechnology and the NIH for fellowship support to KK (R25CA153952).
Abbreviations
- CPP
cell-penetrating peptides
- DTS
DNA-targeted sequence
- FDA
Food and Drug Administration
- miRNA
micro RNA
- mRNA
messenger RNA
- NLS
nuclear localization signal
- PAA
poly(amido amine)
- PBAE
poly(β-amino ester)
- pDNA
plasmid DNA
- PEI
polyethyleneimine
- PLGA
poly(lactic-co-glycolic acid)
- PLL
poly-L-lysine
- PNA
peptide nucleic acid
- shRNA
short hairpin RNA
- siRNA
short interfering RNA
- TAT
trans-activating transcriptional activator
- wt/wt
weight/weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quade-Lyssy P, Milanov P, Abriss D, Ungerer C, Konigs C, Seifried E, Schuttrumpf J. Oral gene therapy for hemophilia B using chitosan-formulated FIX mutants. Journal of Thrombosis and Haemostasis. 2014;12:932–942. doi: 10.1111/jth.12572. [DOI] [PubMed] [Google Scholar]
- 2.Bishop CJ, Kim J, Green JJ. Biomolecule Delivery to Engineer the Cellular Microenvironment for Regenerative Medicine. Annals of Biomedical Engineering. 2014;42:1557–1572. doi: 10.1007/s10439-013-0932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestling M, Fenselau C. PVDF: An interface for gel electrophoresis and matrix-assisted laser desorption/ionization mass spectrometry. Biochem Soc Transact. 1994;22:547–551. doi: 10.1042/bst0220547. [DOI] [PubMed] [Google Scholar]
- 4.Sunshine JC, Bishop CJ, Green JJ. Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery. Ther Deliv. 2011;2:493–521. doi: 10.4155/tde.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Sunshine JC, Green JJ. Differential Polymer Structure Tunes Mechanism of Cellular Uptake and Transfection Routes of Poly(beta-amino ester) Polyplexes in Human Breast Cancer Cells. Bioconjugate Chem. 2014;25:43–51. doi: 10.1021/bc4002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge XM, Feng J, Chen S, Zhang C, Ouyang YM, Liu ZG, Yuan WE. Biscarbamate cross-linked low molecular weight Polyethylenimine polycation as an efficient intra-cellular delivery cargo for cancer therapy. Journal of Nanobiotechnology. 2014;12 doi: 10.1186/1477-3155-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P, Phillips A, Amini A, Fabrycki J, Bartholomew RM, Brostoff SW, Carlo DJ. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochimica Et Biophysica Acta-Gene Structure and Expression. 1999;1444:171–190. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 8.Walsh M, Tangney M, O’Neill MJ, Larkin JO, Soden DM, McKenna SL, Darcy R, O’Sullivan GC, O’Driscoll CM. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: Implications for cancer gene therapy. Mol Pharm. 2006;3:644–653. doi: 10.1021/mp0600034. [DOI] [PubMed] [Google Scholar]
- 9.Balicki D, Beutler E. Histone H2A significantly enhances in vitro DNA transfection. Molecular Medicine. 1997;3:782–787. [PMC free article] [PubMed] [Google Scholar]
- 10.Plank C, Tang MX, Wolfe AR, Szoka FC. Branched cationic peptides for gene delivery: Role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther. 1999;10:319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 11.Ketola TM, Hanzlikova M, Leppanen L, Ravina M, Bishop CJ, Green JJ, Urtti A, Lemmetyinen H, Yliperttula M, Vuorimaa-Laukkanen E. Independent versus Cooperative Binding in Polyethylenimine-DNA and Poly(L-lysine)-DNA Polyplexes. Journal of Physical Chemistry B. 2013;117:10405–10413. doi: 10.1021/jp404812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos JL, Oliveira H, Pandita D, Rodrigues J, Pego AP, Granja PL, Tomas H. Functionalization of poly(amidoamine) dendrimers with hydrophobic chains for improved gene delivery in mesenchymal stem cells. J Control Release. 2010;144:55–64. doi: 10.1016/j.jconrel.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Bishop CJ, Ketola TM, Tzeng SY, Sunshine JC, Urtti A, Lemmetyinen H, Vuorimaa-Laukkanen E, Yliperttula M, Green JJ. The Effect and Role of Carbon Atoms in Poly(beta-amino ester)s for DNA Binding and Gene Delivery. J Am Chem Soc. 2013;135:6951–6957. doi: 10.1021/ja4002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branden LJ, Mohamed AJ, Smith CIE. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat Biotechnol. 1999;17:784–787. doi: 10.1038/11726. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Ge Q, Ting D, Nguyen D, Shen HR, Chen JZ, Eisen HN, Heller J, Langer R, Putnam D. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat Mater. 2004;3:190–196. doi: 10.1038/nmat1075. [DOI] [PubMed] [Google Scholar]
- 17.Yang TF, Chin W, Cherng J, Shau M. Synthesis of novel biodegradable cationic polymer: N,N-diethylethylenediamine polyurethane as a gene carrier. Biomacromolecules. 2004;5:1926–1932. doi: 10.1021/bm049763v. [DOI] [PubMed] [Google Scholar]
- 18.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barua S, Ramos J, Potta T, Taylor D, Huang HC, Montanez G, Rege K. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb Chem High Throughput Screen. 14:908–924. doi: 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, Ma M, Lytton-Jean A, Vegas A, Fenton P, Levins CG, Love KT, Lee H, Cortez C, Collins SP, Li YF, Jang J, Querbes W, Zurenko C, Novobrantseva T, Langer R, Anderson DG. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci U S A. 108:12996–13001. doi: 10.1073/pnas.1106379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SY, Sood N, Putnam D. Combinatorial evaluation of cations, pH-sensitive and hydrophobic moieties for polymeric vector design. Mol Ther. 2009;17:480–490. doi: 10.1038/mt.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen PM, Lollo CP, Phan QC, Amini A, Banaszczyk MG, Fabrycki JM, Wu DP, Carlo AT, Pezzoli P, Coffin CC, Carlo DJ. Strength of conjugate binding to plasmid DNA affects degradation rate and expression level in vivo. BBA-Gen Subjects. 2000;1523:103–110. doi: 10.1016/s0304-4165(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 23.Tran TNH, Huy NT, Murao LA, Nguyen TPL, Thuy TT, Tuan HM, Cao TPN, Tuong VV, Dat TV, Kikuchi M, Yasunami M, Morita K, Vu TQH, Hirayama K. Elevated Levels of Cell-Free Circulating DNA in Patients with Acute Dengue Virus Infection. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughan EE, DeGiulio JV, Dean DA. Intracellular trafficking of plasmids for gene therapy: Mechanisms of cytoplasmic movement and nuclear import. Current Gene Therapy. 2006;6:671–681. doi: 10.2174/156652306779010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiou HC, Tangco MV, Levine SM, Robertson D, Kormis K, Wu CH, Wu GY. Enhanced Resistance to Nuclease Degradation of Nucleic-Acids Complexed to Asialoglycoprotein-Polylysine Carriers. Nucleic Acids Res. 1994;22:5439–5446. doi: 10.1093/nar/22.24.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Grigsby CL, Ho YP, Lin C, Engbersen JF, Leong KW. Microfluidic preparation of polymer-nucleic acid nanocomplexes improves nonviral gene transfer. Sci Rep. 3:3155. doi: 10.1038/srep03155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- 29.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza-Virus Hemagglutinin-Ha-2 N-Terminal Fusogenic Peptides Augment Gene-Transfer by Transferrin Polylysine DNA Complexes — toward a Synthetic Virus-Like Gene-Transfer Vehicle. Proc Natl Acad Sci. 1992;89:7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn PS, Levin Y, Barbosa MC. Charge inversion in DNA–amphiphile complexes: possible application to gene therapy. Physica A: Statistical Mechanics and its Applications. 1999;274:8–18. [Google Scholar]
- 31.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 32.Hagerman PJ. Flexibility of RNA. Annu Rev Biophys Biomol Struct. 1997;26:139–156. doi: 10.1146/annurev.biophys.26.1.139. [DOI] [PubMed] [Google Scholar]
- 33.Kebbekus P, Draper DE, Hagerman P. Persistence length of RNA. Biochemistry. 1995;34:4354–4357. doi: 10.1021/bi00013a026. [DOI] [PubMed] [Google Scholar]
- 34.Mok H, Lee SH, Park JW, Park TG. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nature materials. 2010;9:272–278. doi: 10.1038/nmat2626. [DOI] [PubMed] [Google Scholar]
- 35.Bolcato-Bellemin AL, Bonnet ME, Creusat G, Erbacher P, Behr JP. Sticky overhangs enhance siRNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2007;104:16050–16055. doi: 10.1073/pnas.0707831104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JB, Hong J, Bonner DK, Poon Z, Hammond PT. Self-assembled RNA interference microsponges for efficient siRNA delivery. Nat Mater. 2012;11:316–322. doi: 10.1038/nmat3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vader P, van der Aa LJ, Engbersen JFJ, Storm G, Schiffelers RM. Disulfide-Based Poly(amido amine)s for siRNA Delivery: Effects of Structure on siRNA Complexation, Cellular Uptake, Gene Silencing and Toxicity. Pharmaceut Res. 2011;28:1013–1022. doi: 10.1007/s11095-010-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Aa LJ, Vader P, Storm G, Schiffelers RM, Engbersen JFJ. Optimization of poly(amido amine)s as vectors for siRNA delivery. J Control Release. 2011;150:177–186. doi: 10.1016/j.jconrel.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Kozielski KL, Tzeng SY, Mendoza BAHd, Green JJ. Bioreducible Cationic Polymer-Based Nanoparticles For Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells. ACS Nano. 2014;8:3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Adv Healthcare Mater. 2013;2:468–480. doi: 10.1002/adhm.201200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzeng SY, Yang PH, Grayson WL, Green JJ. Synthetic poly(ester amine) and poly(amido amine) nanoparticles for efficient DNA and siRNA delivery to human endothelial cells. Int J Nanomedicine. 2012;6:3309–3322. doi: 10.2147/IJN.S27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelet JM, Putnam D. A combinatorial library of bi-functional polymeric vectors for siRNA delivery in vitro. Pharmaceut Res. 2013;30:362–376. doi: 10.1007/s11095-012-0876-4. [DOI] [PubMed] [Google Scholar]
- 43.Greish K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. Cancer Nanotechnology: Methods and Protocols. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 44.Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chawla JS, Amiji MM. Cellular uptake and concentrations of tamoxifen upon administration in poly(epsilon-caprolactone) nanoparticles. AAPS PharmSci. 2003;5 doi: 10.1208/ps050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Human gene therapy. 2001;12:861–870. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 47.Baba T, Terada N, Fujii Y, Ohno N, Ohno S, Sato SB. Ultrastructural study of echinocytes induced by poly (ethylene glycol)-cholesterol. Histochemistry and cell biology. 2004;122:587–592. doi: 10.1007/s00418-004-0723-8. [DOI] [PubMed] [Google Scholar]
- 48.Baba T, Rauch C, Xue M, Terada N, Fujii Y, Ueda H, Takayama I, Ohno S, Farge E, Sato SB. Clathrin-Dependent and Clathrin-Independent Endocytosis are Differentially Sensitive to Insertion of Poly (Ethylene Glycol)-Derivatized Cholesterol in the Plasma Membrane. Traffic. 2001;2:501–512. doi: 10.1034/j.1600-0854.2001.20707.x. [DOI] [PubMed] [Google Scholar]
- 49.Verma A, Stellacci F. Effect of Surface Properties on Nanoparticle-Cell Interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 50.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 51.Kurisawa M, Yokoyama M, Okano T. Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. J Control Release. 2000;68:1–8. doi: 10.1016/s0168-3659(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 52.Incani V, Tunis E, Clements BA, Olson C, Kucharski C, Lavasanifar A, Uludag H. Palmitic acid substitution on cationic polymers for effective delivery of plasmid DNA to bone marrow stromal cells. Journal of Biomedical Materials Research Part A. 2007;81:493–504. doi: 10.1002/jbm.a.31249. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Wang F, Wu Y, Davidson G, Levkin PA. Combinatorial synthesis and high-throughput screening of alkyl amines for nonviral gene delivery. Bioconjug Chem. 24:1543–1551. doi: 10.1021/bc400158w. [DOI] [PubMed] [Google Scholar]
- 54.Bishop CJ, Abubaker-Sharif B, Guiriba T, Tzeng SY, Green JJ. Gene delivery polymer structure-function relationships elucidated via principal component analysis. Chem Commun. 51:12134–12137. doi: 10.1039/c5cc04417k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subbarao NK, Parente RA, Szoka FC, Jr, Nadasdi L, Pongracz K. The pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- 56.Wyman TB, Nicol F, Zelphati O, Scaria P, Plank C, Szoka FC., Jr Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 57.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 58.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 59.Manickam DS, Bisht HS, Wan L, Mao GZ, Oupicky D. Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. J Control Release. 2005;102:293–306. doi: 10.1016/j.jconrel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Favretto M, Krieg A, Schubert S, Schubert U, Brock R. Multifunctional poly (methacrylate) polyplex libraries: A platform for gene delivery inspired by nature. J Control Release. 2015;209:1–11. doi: 10.1016/j.jconrel.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Sunshine JC, Peng DY, Green JJ. Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol Pharm. 2012;9:3375–3383. doi: 10.1021/mp3004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–494. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 63.Goncalves C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol Ther. 2004;10:373–385. doi: 10.1016/j.ymthe.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Durymanov MO, Beletkaia EA, Ulasov AV, Khramtsov YV, Trusov GA, Rodichenko NS, Slastnikova TA, Vinogradova TV, Uspenskaya NY, Kopantsev EP, Rosenkranz AA, Sverdlov ED, Sobolev AS. Subcellular trafficking and transfection efficacy of polyethylenimine-polyethylene glycol polyplex nanoparticles with a ligand to melanocortin receptor-1. J Control Release. 2012;163:211–219. doi: 10.1016/j.jconrel.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 66.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 67.Gabrielson NP, Pack DW. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136:54–61. doi: 10.1016/j.jconrel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 68.van der Aa MA, Huth US, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, Hennink WE, Peschka-Suss R, Koning GA, Crommelin DJ. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm Res. 2007;24:1590–1598. doi: 10.1007/s11095-007-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerrero-Cázares H, Tzeng SY, Young NP, Abutaleb AO, Quiñones-Hinojosa A, Green JJ. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano. 2014;8:5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y, Wang M, Sprouse D, Smith AE, Reineke TM. Glucose-Containing Diblock Polycations Exhibit Molecular Weight, Charge, and Cell-Type Dependence for pDNA Delivery. Biomacromolecules. 2014;15:1716–1726. doi: 10.1021/bm5001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang CY, Kos P, Muller K, Schrimpf W, Troiber C, Lachelt U, Scholz C, Lamb DC, Wagner E. Native chemical ligation for conversion of sequence-defined oligomers into targeted pDNA and siRNA carriers. J Control Release. 180:42–50. doi: 10.1016/j.jconrel.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Lepadatu AM, Zhu Y, Ciobanu M, Wang Y, Asaftei SC, Oupicky D. Examination of structure-activity relationship of viologen-based dendrimers as CXCR4 antagonists and gene carriers. Bioconjug Chem. 25:907–917. doi: 10.1021/bc500191q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo — Polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 76.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 78.Curiel DT, Agarwal S, Wagner E, Cotten M. Adenovirus Enhancement of Transferrin Polylysine-Mediated Gene Delivery. Proc Natl Acad Sci. 1991;88:8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine pDNA complexes. Bioconjugate Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 80.Okuda T, Sugiyama A, Niidome T, Aoyagi H. Characters of dendritic poly(L-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials. 2004;25:537–544. doi: 10.1016/s0142-9612(03)00542-8. [DOI] [PubMed] [Google Scholar]
- 81.Benns JM, Choi JS, Mahato RI, Park JS, Kim SW. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(L-histidine)-graft-poly(L-lysine) comb shaped polymer. Bioconjugate Chem. 2000;11:637–645. doi: 10.1021/bc0000177. [DOI] [PubMed] [Google Scholar]
- 82.Haensler J, Szoka FC., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 83.Lachelt U, Kos P, Mickler FM, Herrmann A, Salcher EE, Rodl W, Badgujar N, Brauchle C, Wagner E. Fine-tuning of proton sponges by precise diaminoethanes and histidines in pDNA polyplexes. Nanomed. 10:35–44. doi: 10.1016/j.nano.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Reineke TM. Poly (glycoamidoamine) s for gene delivery. Structural effects on cellular internalization, buffering capacity, and gene expression. Bioconjugate Chem. 2007;18:19–30. doi: 10.1021/bc060029d. [DOI] [PubMed] [Google Scholar]
- 85.Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomaterialia. 2014 doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6:1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 87.Ketola TM, Hanzlikova M, Urtti A, Lemmetyinen H, Yliperttula M, Vuorimaa E. Role of Polyp lex Intermediate Species on Gene Transfer Efficiency: Polyethylenimine-DNA Complexes and Time-Resolved Fluorescence Spectroscopy. Journal of Physical Chemistry B. 2011;115:1895–1902. doi: 10.1021/jp109984c. [DOI] [PubMed] [Google Scholar]
- 88.Yue YA, Jin F, Deng R, Cai JG, Chen YC, Lin MCM, Kung HF, Wu C. Revisit complexation between DNA and polyethylenimine — Effect of uncomplexed chains free in the solution mixture on gene transfection. J Control Release. 2011;155:67–76. doi: 10.1016/j.jconrel.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 89.Hanzlikova M, Ruponen M, Galli E, Raasmaja A, Aseyev V, Tenhu H, Urtti A, Yliperttula M. Mechanisms of polyethylenimine-mediated DNA delivery: free carrier helps to overcome the barrier of cell-surface glycosaminoglycans. J Gene Med. 2011;13:402–409. doi: 10.1002/jgm.1587. [DOI] [PubMed] [Google Scholar]
- 90.Drummond DC, Zignani M, Leroux J-C. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–460. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 91.Niidome T, Ohmori N, Ichinose A, Wada A, Mihara H, Hirayama T, Aoyagi H. Binding of Cationic Alpha-Helical Peptides to Plasmid DNA and Their Gene Transfer Abilities into Cells. J Biol Chem. 1997;272:15307–15312. doi: 10.1074/jbc.272.24.15307. [DOI] [PubMed] [Google Scholar]
- 92.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The Influence of Endosome-Disruptive Peptides on Gene-Transfer Using Synthetic Virus-Like Gene-Transfer Systems. J Biol Chem. 1994;269:12918–12924. [PubMed] [Google Scholar]
- 93.Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release. 2007;121:64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 94.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 95.Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, Breunig M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9:2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 96.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly (beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forrest ML, Meister GE, Koerber JT, Pack DW. Partial acetylation of polyethylenimine enhances in vitro gene delivery. Pharmaceut Res. 2004;21:365–371. doi: 10.1023/b:pham.0000016251.42392.1e. [DOI] [PubMed] [Google Scholar]
- 98.Gabrielson NP, Pack DW. Acetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactions. Biomacromolecules. 2006;7:2427–2435. doi: 10.1021/bm060300u. [DOI] [PubMed] [Google Scholar]
- 99.Hill IR, Garnett MC, Bignotti F, Davis SS. In vitro cytotoxicity of poly(amidoamine)s: relevance to DNA delivery. Biochim Biophys Acta. 1999;1427:161–174. doi: 10.1016/s0304-4165(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 100.Bauhuber S, Liebl R, Tomasetti L, Rachel R, Goepferich A, Breunig M. A library of strictly linear poly (ethylene glycol)–poly (ethylene imine) diblock copolymers to perform structure–function relationship of non-viral gene carriers. J Control Release. 2012;162:446–455. doi: 10.1016/j.jconrel.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 101.Lim YB, Han SO, Kong HU, Lee Y, Park JS, Jeong B, Kim SW. Biodegradable polyester, poly[alpha-(4 aminobutyl)-L-glycolic acid], as a non-toxic gene carrier. Pharmaceut Res. 2000;17:811–816. doi: 10.1023/a:1007552007765. [DOI] [PubMed] [Google Scholar]
- 102.Forrest ML, Koerber JT, Pack DW. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjugate Chem. 2003;14:934–940. doi: 10.1021/bc034014g. [DOI] [PubMed] [Google Scholar]
- 103.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Mark Saltzman W. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen J, Steele TWJ, Merkel O, Reul R, Kissel T. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J Control Release. 2008;132:243–251. doi: 10.1016/j.jconrel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oster C, Wittmar M, Unger F, Barbu-Tudoran L, Schaper A, Kissel T. Design of Amine-Modified Graft Polyesters for Effective Gene Delivery Using DNA-Loaded Nanoparticles. Pharmaceut Res. 2004;21:927–931. doi: 10.1023/b:pham.0000029279.50733.55. [DOI] [PubMed] [Google Scholar]
- 106.Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Advanced Drug Delivery Reviews. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 108.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong ZY, Lin C, Engbersen JFJ, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chem. 2006;17:1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 109.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Bio Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 110.Breunig M, Hozsa C, Lungwitz U, Watanabe K, Umeda I, Kato H, Goepferich A. Mechanistic investigation of poly (ethylene imine)-based siRNA delivery: disulfide bonds boost intracellular release of the cargo. J Control Release. 2008;130:57–63. doi: 10.1016/j.jconrel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 111.McKenzie DL, Smiley E, Kwok KY, Rice KG. Low Molecular Weight Disulfide Cross-Linking Peptides as Nonviral Gene Delivery Carriers. Bioconjugate Chem. 2000;11:901–909. doi: 10.1021/bc000056i. [DOI] [PubMed] [Google Scholar]
- 112.Matsumoto S, Christie RJ, Nishiyama N, Miyata K, Ishii A, Oba M, Koyama H, Yamasaki Y, Kataoka K. Environment-Responsive Block Copolymer Micelles with a Disulfide Cross-Linked Core for Enhanced siRNA Delivery. Biomacromolecules. 2009;10:119–127. doi: 10.1021/bm800985e. [DOI] [PubMed] [Google Scholar]
- 113.Miyata K, Kakizawa Y, Nishiyama N, Harada A, Yamasaki Y, Koyama H, Kataoka K. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J Am Chem Soc. 2004;126:2355–2361. doi: 10.1021/ja0379666. [DOI] [PubMed] [Google Scholar]
- 114.Kadlecova Z, Rajendra Y, Matasci M, Baldi L, Hacker DL, Wurm FM, Klok HA. DNA delivery with hyperbranched polylysine: a comparative study with linear and dendritic polylysine. J Control Release. 169:276–288. doi: 10.1016/j.jconrel.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 115.Jeong JH, Christensen LV, Yockman JW, Zhong ZY, Engbersen JFJ, Kim WJ, Feijen J, Kim SW. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28:1912–1917. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 116.Mok H, Park TG. Self-crosslinked and reducible fusogenic peptides for intracellular delivery of siRNA. Biopolymers. 2008;89:881–888. doi: 10.1002/bip.21032. [DOI] [PubMed] [Google Scholar]
- 117.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33:8142–8151. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kozielski KL, Tzeng SY, Green JJ. A bioreducible linear poly(beta-amino ester) for siRNA delivery. Chem Commun. 2013;49:5319–5321. doi: 10.1039/c3cc40718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim HS, Yoo HS. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J Control Release. 2010;145:264–271. doi: 10.1016/j.jconrel.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 120.Li H-J, Wang H-X, Sun C-Y, Du J-Z, Wang J. Shell-detachable nanoparticles based on a light-responsive amphiphile for enhanced siRNA delivery. Royal Society of Chemistry Advances. 2014;4:1961–1964. [Google Scholar]
- 121.Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, Beatty B, Squire J, O’Brodovich H, Lukacs GL. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 122.Lechardeur D, Verkman AS, Lukacs GL. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv Drug Deliv Rev. 2005;57:755–767. doi: 10.1016/j.addr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 123.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: Volume, viscosity. diffusion, intracellular surface area. International Review of Cytology — a Survey of Cell Biology. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. 192. [DOI] [PubMed] [Google Scholar]
- 124.Ludtke JJ, Sebestyen MG, Wolff JA. The effect of cell division on the cellular dynamics of microinjected DNA and dextran. Mol Ther. 2002;5:579–588. doi: 10.1006/mthe.2002.0581. [DOI] [PubMed] [Google Scholar]
- 125.Mortimer I, Tam P, MacLachlan I, Graham RW, Saravolac EG, Joshi PB. Cationic lipid-mediated transfection of cells in culture requires mitotic activity. Gene Ther. 1999;6:403–411. doi: 10.1038/sj.gt.3300837. [DOI] [PubMed] [Google Scholar]
- 126.Sacramento CB, Moraes JZ, Denapolis PMA, Han SW. Gene expression promoted by the SV40 DNA targeting sequence and the hypoxia-responsive element under normoxia and hypoxia. Brazilian Journal of Medical and Biological Research. 2010;43:722–727. doi: 10.1590/s0100-879x2010007500064. [DOI] [PubMed] [Google Scholar]
- 127.Grandinetti G, Reineke TM. Exploring the Mechanism of Plasmid DNA Nuclear Internalization with Polymer-Based Vehicles. Mol Pharm. 2012;9:2256–2267. doi: 10.1021/mp300142d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vandenbroucke RE, Lucas B, Demeester J, De Smedt SC, Sanders NN. Nuclear accumulation of plasmid DNA can be enhanced by non-selective gating of the nuclear pore. Nucleic Acids Res. 2007;35 doi: 10.1093/nar/gkm440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 130.Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- 131.Ward CM, Read ML, Seymour LW. Systemic circulation of poly(L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: differential circulation in mice and rats and the implications for human gene therapy. Blood. 2001;97:2221–2229. doi: 10.1182/blood.v97.8.2221. [DOI] [PubMed] [Google Scholar]
- 132.Anwer K, Barnes MN, Fewell J, Lewis DH, Alvarez RD. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010;17:360–369. doi: 10.1038/gt.2009.159. [DOI] [PubMed] [Google Scholar]
- 133.Davis ME. The First Targeted Delivery of siRNA in Humans via a Nanoparticle: From Concept to Clinic. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 134.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi Ca, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci. 2014;111 doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]


