Summary
RNA interference (RNAi) is a powerful tool to target and knockdown gene expression in a sequence-specific manner. RNAi can be achieved by the intracellular introduction of siRNA, however, intracellular siRNA delivery remains a challenging obstacle. Herein we describe the use of bioreducible nanoparticles formed using poly(beta-amino ester)s (PBAEs) for safe and efficient siRNA delivery. Methods for polymer synthesis, nanoparticle formation, and siRNA delivery using these particles are described. A template protocol for nanoparticle screening is presented and can be used to quickly optimize siRNA delivery for novel applications.
Keywords: siRNA, gene delivery, nanoparticle, bioreducible, poly(beta-amino ester), Polymer, RNA interference, Transfection
Introduction
Sequence-specific gene knockdown via RNA interference (RNAi) has the potential to treat or cure many diseases caused by overexpression or dysregulation of a given gene.1 RNAi can be induced by intracellular delivery of short interfering RNA (siRNA) complementary to the targeted gene’s mRNA. However, siRNA delivery remains a challenge.2,3 Viral delivery methods, although effective, raise several safety concerns due to their inherent immunogenicity and tumorigenicity.4 Nonviral methods such as lipid-based, inorganic, and polymeric nanoparticles typically can avoid such issues but are often less effective at siRNA delivery.5
Poly(beta-amino ester)s (PBAEs), a class of cationic polymer, are well-established as safe an effective DNA delivery vectors.6–9 PBAEs are promising potential candidates for siRNA delivery, as they can bind negatively charged nucleic acids into nanoparticles, promote cellular uptake, and achieve endosomal escape due to their positive charge and high buffering capacities. However, at 21–23 bp siRNA is much shorter than the average plasmid used for DNA delivery (typically 4–10 kpb), and therefore has much less multivalency to electrostatically bind to cationic polymers. This is one reason why cationic polymers may be unable to stably form nanoparticles with siRNA.10,11 Additionally, siRNA must be released at its site of action in the cytoplasm in order for effective RNAi to take place.12
Bioreducible PBAEs have previously been found to overcome these challenges and to successfully deliver siRNA in human glioblastoma cells.13,14 Methods to deliver siRNA to new cell types are fundamental to the development of safer and more efficient siRNA delivery vehicles. Investigating the siRNA delivery capabilities of these polymers in untested cell types is important to discovering their potential for the treatment of various diseases. This chapter discusses a method to synthesize bioreducible PBAEs and assess their ability to safely and effectively deliver siRNA to a new cell type.
2. Materials
2.1 Bioreducible Monomer Synthesis
Bis(2-hydroxyethyl) disulfide; Sigma-Aldrich
Triethylamine (TEA); Sigma-Aldrich
Acryloyl chloride; Sigma-Aldrich
Tetrahydrofuran (THF), anhydrous; Sigma-Aldrich
Dichloromethane (DCM); Sigma-Aldrich
Sodium carbonate (Na2CO3); Alfa Aesar
Sodium sulfate (Na2SO4); Sigma-Aldrich
Coarse porosity filter paper; Fischer
Round bottom flask, 500 mL; Fischer
Round bottom flask, three-neck, 1000 mL; Fischer
Separatory funnel, 1000 and 500 mL; Fischer
Graduated cylinder, glass, 100 mL
Buchner funnel; Fischer
Glass funell; Fischer
Vaccum flask; Fisher
50 mL Glass syringe with stainless steel needle, 22 gauge; Fischer
Rubber septa
Ring stand
Clamps
Support ring
Stir bar
Magnetic stir plate
Nitrogen gas
Chemical hood
Rotary evaporator apparatus
2.2 Polymer Synthesis
4-amino-1-butanol (S4); TCI (Portland, OR)
2-(3-(aminopropyl)amino)methanol (E6); Fluka Chemicals
1-(3-aminopropyl)-4-methylpiperazine (E7); Alfa Aesar
Teflon-lined screw cap glass vials, 5 mL; VWR
Teflon-coated magnetic micro stir bars; VWR
Magnetic stir plate
Incubator/over capable of reaching 95°C
2.3 Cell Culture
Cell line positive for green fluorescent protein (GFP) or other fluorescent protein (See notes). Example cells in experiments described below will be human glioblastoma cells isolated from intraoperative samples,15 and transfected to constitutively express GFP.9
Cell culture medium: DMEM-F12 containing 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic; Invitrogen
Trypsin; Invitrogen
Phosphate buffered saline (PBS); Invitrogen
Hemocytometer; VWR
Incubator (37°C, 5% CO2); Thermo Scientific
2.4 siRNA Delivery Experiment
siRNA delivery polymers from synthesis in Subheading 2.2.
Dimethyl sulfoxide (DMSO), >99.7%, anhydrous; Sigma-Aldrich
Sodium acetate (NaAc) solution, 3 M, pH 5.2, sterile; Sigma-Aldrich
AllStars Hs Cell Death siRNA; Qiagen
Silencer® eGFP siRNA; Life Technologies
Silencer® Negative Control No. 1 siRNA; Life Technologies
Eppendorf tubes, 0.5 and 1.5 mL, sterile; VWR
Pipette tips, autoclaved
Twelve-channel pipettes (5 – 50 µL and 50 – 300 µL); Fischer
Pipetting reservoirs, sterile; Corning
Clear 96-well plate with lid, flat bottom, tissue culture treated, sterile; CytoOne
Clear 96-well plate with lid, round bottom, sterile; Sarstedt
Fluorescence plate reader
Fluorescence microscope
Flow cytometer, preferably with a HyperCyt® Autosampler
3. Methods
3.1 Bioreducible Monomer Synthesis
Researchers should be familiar with basic organic chemistry laboratory techniques such as separation of organic and aqueous phases, drying organic solutions, and completing a reaction in anhydrous conditions. All work should be conducted in a chemical fume hood, and appropriate safety precautions should be followed for each chemical used.
Fill 1000 mL three-neck round bottom flask with 450 mL THF, and fill a 100 mL graduated cylinder with 50 mL THF. Clamp round bottom flask to ring stand.
Dissolve 12.2 mL hydroxyethyl disulfide and 37.5 mL TEA in THF in round bottom flask. Add a stir bar and begin to magnetically stir.
Cover all outlets of round bottom flask with rubber septa and flush with nitrogen for 5 min by attaching the nitrogen tank to the left-most outlet with a needle and inserting an outlet needle to the right-most outlet. Leave nitrogen flowing and cover round bottom flask with aluminum foil.
Dissolve 24.4 mL acryloyl chloride in 50 mL THF in graduated cylinder.
Clamp glass syringe (without plunger) with needle onto the ring stand and lower so that needle goes through the top rubber septum on the round bottom flask.
Fill syringe with acryloyl chloride/THF solution to allow solution to add to round bottom flask dropwise. Continue adding until all acryloyl chloride is added. A pale yellow precipitate will form during the reaction; this is TEA HCl.
Remove glass syringe from top septum and allow nitrogen to flow for an additional 10 min.
Turn nitrogen off and remove needle. Leave an outlet needle in one septum attached to a balloon. This will keep air from entering the reaction but will allow for any slight pressure changes during the reaction.
Allow reaction to continue while stirring overnight (at least 12 h).
Use a glass funnel and filter paper to remove the TEA HCl, and wash the precipitate with an additional 100 mL THF.
Move solution to a 1000 mL round bottom flask. Remove THF via rotary evaporation. You will be left with a yellow, viscous liquid.
Dissolve liquid in 200 mL DCM and move to separatory funnel.
Wash solution with 200 mL of 0.2 M Na2CO3, mix in separtory funnel, move organic phase (the lower layer) to a round bottom flask. The aqueous phase (upper layer) maybe discarded.
Repeat Na2CO3 washes four additional times, followed by three washes in deionized water.
Weigh a clean, dry 500 mL round bottom flask and record its weight.
Following the final separation, use Na2SO4 to dry the contents of the organic phase. Use a glass funnel and filter paper to remove the Na2SO4..
Move the organic phase into the clean round bottom flask, and remove the DCM using rotary evaporation. Weigh the flask to determine the product yield.
The final product, disulfanediylbis(ethane-2,1-diyl) (BR6), will be a yellow, viscous fluid and should yield approximately 10 g. The authors recommend using NMR to confirm the purity of BR6, in particular checking for any remaining solvent and acrylic acid.
3.2 Polymer Synthesis
Weigh 800 mg of monomer DMSO (or other diacrylate monomer) synthesized in Subheading 3.1, into a 5 mL screw cap glass vial.
Add 1059 mg DMSO to BR6 and vortex to dissolve.
Add amine monomer to acrylate monomer at an acrylate/amine stoichiometric ratio of 1.05:1; this will yield acrylate-terminated base polymers. To make polymers BR6-S4-E6 (R646) or BR6-S4-E7 (R647), add 259 mg of monomer S4 to 800 mg BR6.
Add a teflon-coated magnetic stir bar to the vial.
Polymerize while magnetically stirring in an oven at 90°C for 24 h.
To end-cap the polymers, dilute base polymers to 166.7 mg/mL in DMSO, dilute end-caps to 0.5 M, and combine 480 µL polymer with 320 µL end-cap. This will yield a final polymer concentration of 100 mg/mL. To make polymer R647, for example, dilute base polymer BR6-S4 to 166.7 mg/mL with 480 µL total volume, and dilute 25.2 µL E7 in 320 µL DMSO, then combine.
Shake at room temperature for 1 h.
Store polymers at −20°C in the presence of a desiccant until ready to use.
3.3 siRNA Delivery Experiment
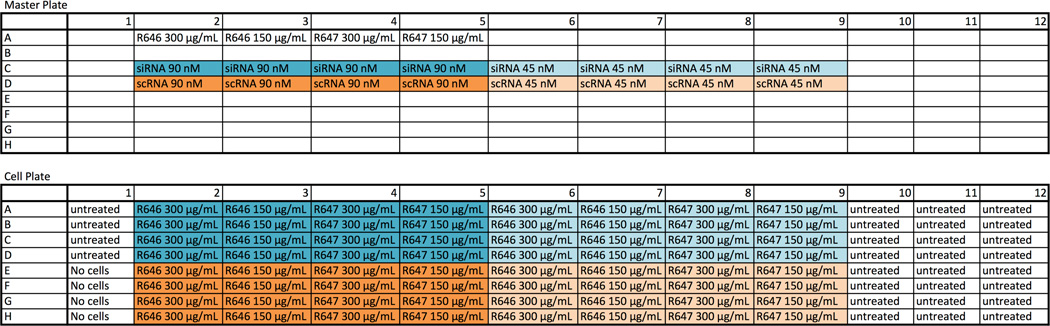
Researchers should be familiar with basic sterile cell culture techniques such as growing, passing, and plating cells. All work should be completed in a laminar flow biosafety cabinet using sterilized reagents and equipment. The following protocol is for testing siRNA formulations in a 96-well plate. Each nanoparticle formulation is tested using both the targeting siRNA and a scrambled control RNA (scRNA), each in quadruplicate. The following example experiment will test two polymers, R646 and R647, each at two different concentrations (300 and 150 µg/mL), and each with an siRNA dose of 90 and 45 nM.
Cell plating. Twenty-four hours prior to transfection, seed cells into a clear tissue culture 96-well plate at 15,000 cells/well in a volume of 100 µL per well. Leave Rows E – H, column 1 free of cells and filled only with 100 µL of media.
Preparation of sodium acetate buffer. Dilute NaAc buffer to 25 mM by diluting 4.2 mL of sterile 3 M NaAc into 495.8 mL sterile deionized water.
siRNA preparation. Thaw siRNA and scRNA stock solutions (each stored at 50 µM). Dilute each using 7.1 µL stock RNA in 322.9 µL NaAc to make a 1080 nM RNA dilution. (This will give you a final concentration of 90 nM once the RNA is diluted by half during nanoparticle formation, and then by six once the particles are added to cells.) To make the 540 nM dilution, dilute 110 µL of 1080 nM RNA in 110 µL NaAc. Aliquot 50 µL of each RNA dilution into a round-bottom 96-well Master Plate following Table 1.
Polymer dilution. Thaw polymers R646 and R647 made in Subheading 3.2 (each stored at 100 mg/mL). Dilute each using 11.9 µL stock polymer in 318.1 µL NaAc to make a 3600 µg/mL dilution (which will yield a final polymer concentration of 300 µg/mL). Dilute 110 µL of the 3600 µg/mL dilution in 110 µL NaAc to make a 1800 µg/mL dilution. Aliquot 105 µL of each polymer to the Master Plate following Table 1.
Nanoparticle formation. Using the multichannel pipette, move 50 µL of polymer from Row A of the Master Plate to the RNA in Row C; pipette vigorously several times. Repeat with RNA in Row D. Allow nanoparticles to form for 10 min.
Adding nanoparticles to cells. Prior to adding nanoparticles, use a fluorescence plate reader to measure the pre-transfection fluorescence of the cells in each well. Remove the media and add 100 µL of serum-free media. Following Table 1 and using a multichannel pipette, add 20 µL of nanoparticles in Row C of the Master Plate to Rows A – D of the Cell Plate. Add 20 µL of nanoparticles in Row D of the Master Plate to Rows E – H of the Cell Plate. Gently shake the Cell Plate on a flat surface to promote mixing. Place cells in a cell culture incubator for the duration of the incubation.
Removing nanoparticles from cells. Following a 1 – 4 h incubation, remove the nanoparticle-containing media using a multichannel pipette. The incubation time should be optimized for any new cell type used. Replenish cells with 100 µL of fresh, previously warmed media, and replace cells in the incubator.
Table 1.
High throughput siRNA delivery experiment plate layout
3.4 Measurement of Gene Knockdown
The following protocol requires cells that are constitutively GFP positive and a flow cytometer equipped with a Hypercyt® Autosampler or other autosampler. If one is not available, the researcher may follow the protocol as written, but will be required to prepare the samples in the traditional manner for flow cytometry, in which individual samples are moved into tubes. The researcher should be familiar with flow cytometry and analysis of flow cytometric data.
Determining the time of peak knockdown. Using the fluorescence plate reader, the GFP knockdown should be tracked each day following transfection until the peak day can be determined. Percent knockdown (%KD) can be determined by first averaging and then subtracting the GFP fluorescence readings of wells containing media only from all samples. Using these new values, the average of siRNA-treated and scRNA-treated wells should be calculated, and %KD can be found by subtracting the siRNA-treated values from scRNA-treated values, normalizing to scRNA-treated values, then multiplying by 100.
Visualization of GFP expression. Repeat the siRNA knockdown experiment, and on the previously-determined peak day of knockdown, use a fluorescence microscope to visualize GFP expression in each group.
Preparing cells for flow cytometry. Prepare the cells for flow cytometry by removing the media from the wells in a 96-well plate, adding 100 µL PBS per well, removing the PBS, and adding 30 µL trypsin. Wait until the cells have released from the plate, add 170 µL of Flow Buffer (2% FBS in PBS), and move all 200 µL in each well to the corresponding wells of a round-bottom 96-well plate. Centrifuge the round-bottom plate to spin cells down, then remove 170 µL of Flow Buffer from each well, and resuspend cells by pipetting to increase the cell concentration. Use the flow cytometer and autosampler to measure the FL1 GFP fluorescence levels of the cells in each sample.
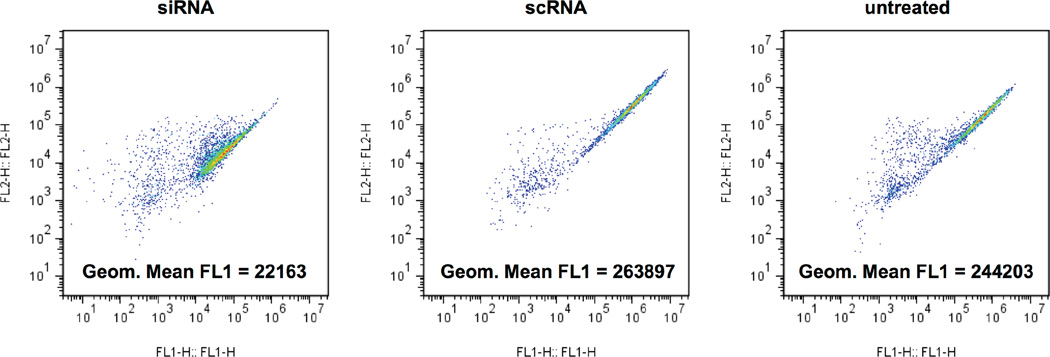
GFP expression calculation. Cells should be gated for as during normal flow cytometric analysis. FL1 geometric mean fluorescence should be calculated for each well (Figure 1). Using these values, calculate %KD as with the data acquired from the plate reader, by normalizing siRNA-treated values to scRNA-treated values.
Figure 1.
Example flow cytometry results of GFP+ glioblastoma cells treated with polymer R647 at 180 µg/mL with 20 nM of either GFP siRNA or scRNA.
4. Notes
The above protocol assumes that the cells used are or can be made to express GFP or another fluorescent protein. If this is difficult or impossible, several other options can be utilized. One option is to instead use a death positive control siRNA such as AllStars Death Control siRNA available from Qiagen. If this siRNA is used, siRNA delivery efficacy can be determined by comparing the toxicity of siRNA-treated cells to scRNA-treated cells, either by cell counting or a proliferation assay.
If siRNA delivery efficacy is lower than desired, increasing polymer or siRNA concentration or increasing incubation time may increase efficacy.
If toxicity is higher than desired, lowering siRNA or polymer concentration, lowering incubation time, or increasing the serum concentration in the media can lower toxicity.
Acknowledgments
This work was supported in part by the NIH (1R01EB016721). K.L.K. also thanks the NIH Cancer Nanotechnology Training Center (R25CA153952) at the JHU Institute for Nanobiotechnology for fellowship support.
References
- 1.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Wu W, Sun M, Zou GM, Chen J. MicroRNA and cancer: Current status and prospective. Int J Cancer. 2007;120(5):953–960. doi: 10.1002/ijc.22454. [DOI] [PubMed] [Google Scholar]
- 3.Yadav S, van Vlerken LE, Little SR, Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63(4):711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 5.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 6.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Accounts of chemical research. 2008;41(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynn DM, Langer R. Degradable poly (beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–10768. [Google Scholar]
- 8.Sunshine JC, Akanda MI, Li D, Kozielski KL, Green JJ. Effects of base polymer hydrophobicity and end-group modification on polymeric gene delivery. Biomacromolecules. 2011;12(10):3592–3600. doi: 10.1021/bm200807s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng SY, Guerrero-Cazares H, Martinez EE, Sunshine JC, Quiñones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on Poly (beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagerman PJ. Flexibility of RNA. Annu Rev Biophys Biomol Struct. 1997;26:139–156. doi: 10.1146/annurev.biophys.26.1.139. [DOI] [PubMed] [Google Scholar]
- 11.Kebbekus P, Draper DE, Hagerman P. Persistence length of RNA. Biochemistry. 1995;34(13):4354–4357. doi: 10.1021/bi00013a026. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki H, Taira K. Short hairpin type of dsRNAs that are controlled by tRNAVa1 promoter significantly induce RNAi-mediated gene silencing in the cytoplasm of human cells. Nucleic Acids Res. 2003;31(2):700–707. doi: 10.1093/nar/gkg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozielski KL, Tzeng SY, Green JJ. A bioreducible linear poly(beta-amino ester) for siRNA delivery. Chem Commun. 2013;49(46):5319–5321. doi: 10.1039/c3cc40718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozielski KL, Tzeng SY, Mendoza BAHd, Green JJ. Bioreducible Cationic Polymer-Based Nanoparticles For Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells. ACS Nano. 2014;8(4):3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero-Cázares H, Chaichana K, Quiñones-Hinojosa A. Neurosphere Culture and Human Organotypic Model to Evaluate Brain Tumor Stem Cells. In: Yu JS, editor. Cancer Stem Cells. Humana Press; 2009. pp. 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]