Abstract
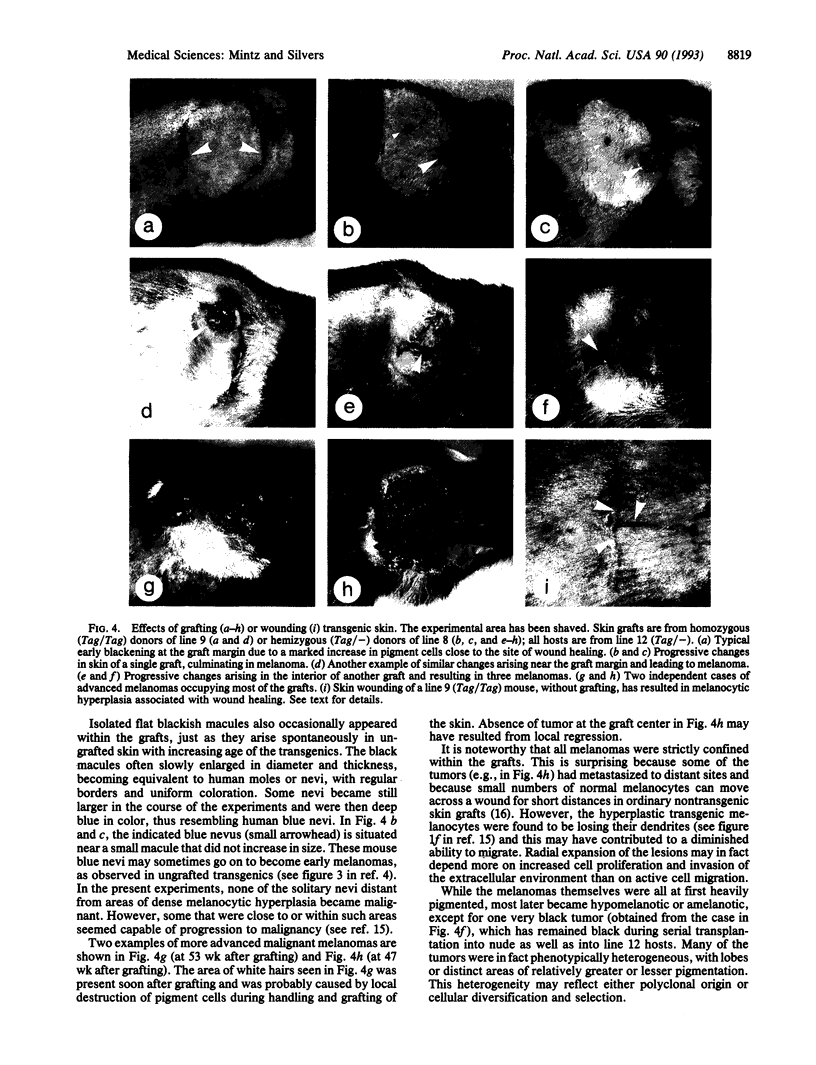
Tyr-SV40E transgenic mice are specifically susceptible to melanoma due to expression of the oncogene in pigment cells. Mice of the more susceptible lines die young of early-onset eye melanomas, when skin melanomas are still infrequent and benign. To surmount this obstacle, skin from donors of two high-susceptibility lines was grafted to Tyr-SV40E hosts of a low-susceptibility line of the same inbred strain, thereby enabling the skin to outlive the donors and continue to grow in immunocompetent but tolerant hosts. Unexpectedly, donor pigment cells in all the grafts soon selectively proliferated close to areas of greatest wound healing, forming a dense black tracery, especially at the outer rim of the grafts. These lesions slowly grew radially within the grafts, producing irregular greyish patches. Local vertical thickenings then appeared and developed into small melanomas, which soon ulcerated through the epidermis. The tumors rapidly enlarged and became deeply invasive. Discrete black nevi also arose, with many becoming larger and distinctly blue, but those not near areas of pronounced wound healing did not progress to malignancy. In this first series, malignant melanoma resulted in all the grafts from the more susceptible of two donor lines and in some grafts from the other line. Distant metastases occurred in some cases from each line. Most tumors were hypomelanotic and heterogeneous, with lobes or areas differing in melanization. The results strongly suggest that growth factors and cytokines--known to be produced in wound repair--are triggering the growth and malignant conversion of these genetically susceptible melanocytes and that in the graft situation we are merely witnessing a caricature--a usefully exaggerated manifestation of the true events underlying the genesis of melanomas. The striking resemblance to the human malignancy, the genetic uniformity and different susceptibilities of the transgenic lines, and the experimental possibilities in the grafted mice all make them an excellent model of the disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris T. S. Tumor promotion by abrasion induced epidermal hyperplasia in the skin of mice. J Invest Dermatol. 1980 Oct;75(4):360–362. doi: 10.1111/1523-1747.ep12531153. [DOI] [PubMed] [Google Scholar]
- Berkelhammer J., Oxenhandler R. W. Evaluation of premalignant and malignant lesions during the induction of mouse melanomas. Cancer Res. 1987 Mar 1;47(5):1251–1254. [PubMed] [Google Scholar]
- Bradl M., Klein-Szanto A., Porter S., Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M., Larue L., Mintz B. Clonal coat color variation due to a transforming gene expressed in melanocytes of transgenic mice. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6447–6451. doi: 10.1073/pnas.88.15.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986 Oct 28;865(2):171–195. doi: 10.1016/0304-419x(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Murray A. W. Tumor promotion and the induction of epidermal ornithine decarboxylase activity in mechanically stimulated mouse skin. Cancer Res. 1978 Mar;38(3):494–497. [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Epstein M. N., Greene M. H., Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984 Dec;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Epstein J. H., Epstein W. L., Nakai T. Production of melanomas from DMBA-induced "blue nevi" in hairless mice with ultraviolet light. J Natl Cancer Inst. 1967 Jan;38(1):19–30. [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason J. F., Bhartia P. K., Herman J. R., McPeters R., Newman P., Stolarski R. S., Flynn L., Labow G., Larko D., Seftor C., Wellemeyer C., Komhyr W. D., Miller A. J., Planet W. Record low global ozone in 1992. Science. 1993 Apr 23;260(5107):523–526. doi: 10.1126/science.260.5107.523. [DOI] [PubMed] [Google Scholar]
- Goerttler K., Loehrke H., Schweizer J., Hesse B. Two-stage tumorigenesis of dermal melanocytes in the back skin of the Syrian golden hamster using systemic initiation with 7,12-dimethylbenz(a)anthracene and topical promotion with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Jan;40(1):155–161. [PubMed] [Google Scholar]
- Hennings H., Boutwell R. K. Studies on the mechanism of skin tumor promotion. Cancer Res. 1970 Feb;30(2):312–320. [PubMed] [Google Scholar]
- Hook R. R., Jr, Berkelhammer J., Oxenhandler R. W. Melanoma: Sinclair swine melanoma. Am J Pathol. 1982 Jul;108(1):130–133. [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T., Takahashi M., Ito M., Hamatani K., Ohbayashi M., Wajjwalku W., Isobe K., Nakashima I. Aberrant melanogenesis and melanocytic tumour development in transgenic mice that carry a metallothionein/ret fusion gene. EMBO J. 1991 Nov;10(11):3167–3175. doi: 10.1002/j.1460-2075.1991.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Roth S. I., Fitzpatrick T. B., Szabo G. Mitotic activity in non-neoplastic melanocytes in vivo as determined by histochemical, autoradiographic, and electron microscope studies. J Cell Biol. 1975 Sep;66(3):663–670. doi: 10.1083/jcb.66.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Dougherty N., Bradl M., Mintz B. Melanocyte culture lines from Tyr-SV40E transgenic mice: models for the molecular genetic evolution of malignant melanoma. Oncogene. 1993 Mar;8(3):523–531. [PubMed] [Google Scholar]
- Larue L., Dougherty N., Mintz B. Genetic predisposition of transgenic mouse melanocytes to melanoma results in malignant melanoma after exposure to a low ultraviolet B intensity nontumorigenic for normal melanocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9534–9538. doi: 10.1073/pnas.89.20.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. D., Applegate L. A., Padilla R. S., Stuart T. D. Ultraviolet radiation--induced malignant melanoma in Monodelphis domestica. Photochem Photobiol. 1989 Jul;50(1):1–5. doi: 10.1111/j.1751-1097.1989.tb04123.x. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Halter S. A., Holt J. T., Hogan B. L., Coffey R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990 Jun 15;61(6):1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- Mintz B., Klein-Szanto A. J. Malignancy of eye melanomas originating in the retinal pigment epithelium of transgenic mice after genetic ablation of choroidal melanocytes. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11421–11425. doi: 10.1073/pnas.89.23.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K., Klein-Szanto A. J. Histopathogenesis of malignant skin melanoma induced in genetically susceptible transgenic mice. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8822–8826. doi: 10.1073/pnas.90.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski A., Haberman H. F., Menon I. A. Skin melanoma induced by 7,12-dimethylbenzanthracene in albino guinea pigs and its similarities to skin melanoma of humans. Cancer Res. 1980 Oct;40(10):3652–3660. [PubMed] [Google Scholar]
- Rigel D. S., Kopf A. W., Friedman R. J. The rate of malignant melanoma in the United States: are we making an impact? J Am Acad Dermatol. 1987 Dec;17(6):1050–1053. doi: 10.1016/s0190-9622(87)80487-5. [DOI] [PubMed] [Google Scholar]
- Romerdahl C. A., Stephens L. C., Bucana C., Kripke M. L. The role of ultraviolet radiation in the induction of melanocytic skin tumors in inbred mice. Cancer Commun. 1989;1(4):209–216. [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Qiu T. H., Palmiter R. D., Brinster R. L., Lee D. C. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol Cell Biol. 1993 Jan;13(1):320–330. doi: 10.1128/mcb.13.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih I. M., Herlyn M. Role of growth factors and their receptors in the development and progression of melanoma. J Invest Dermatol. 1993 Feb;100(2 Suppl):196S–203S. [PubMed] [Google Scholar]
- Sieweke M. H., Thompson N. L., Sporn M. B., Bissell M. J. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990 Jun 29;248(4963):1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Vassar R., Hutton M. E., Fuchs E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol Cell Biol. 1992 Oct;12(10):4643–4653. doi: 10.1128/mcb.12.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein P. J., Jewett L., Faas S., Brinster R. L., Knowles B. B. SV40 T-antigen is a histocompatibility antigen of SV40-transgenic mice. Immunogenetics. 1988;27(6):436–441. doi: 10.1007/BF00364430. [DOI] [PubMed] [Google Scholar]