Abstract
Endometriosis is a nonmalignant, but potentially metastatic, gynecological condition manifested by the extrauterine growth of inflammatory endometrial implants. Ten percent of reproductive-age women are affected and commonly suffer pelvic pain and/ or infertility. The theories of endometriosis histogenesis remain controversial, but retrograde menstruation and metaplasia each infer mechanisms that explain the immune cell responses observed around the ectopic lesions. Recent findings from our laboratories and others suggest that retinoic acid metabolism and action are fundamentally flawed in endometriotic tissues and even generically in women with endometriosis. The focus of our ongoing research is to develop medical therapies as adjuvants or alternatives to the surgical excision of these lesions. On the basis of concepts put forward in this review, we predict that the pharmacological actions and anticipated low side-effect profiles of retinoid supplementation might provide a new treatment option for the long-term management of this chronic and debilitating gynecological disease.
Keywords: chemokine, cytokine, metaplasia, retinoic acid, retrograde menstruation
Endometriosis is a common and ubiquitous gynecologic disorder, affecting up to 10% of reproductive-age women worldwide, which is defined by the presence of hormonally responsive, ectopic implants of endometrial mucosa dispersed in extrauterine locations. Based on data from the World Bank, it has been estimated that 176 million women affected by this condition suffer pelvic pain and/or infertility.1 The direct and indirect costs associated with these cardinal symptoms, including diagnostic tests, medical and surgical treatment expenses, and lost productivity, have been estimated to approach $12,000 per woman per year2 and represent a global health burden. Beyond its economic impact, the physical and psychological tolls of endometriosis are onerous.3 The immune system is intimately involved with mechanisms underlying the symptomatology of endometriosis.4,5 Our review focuses on the roles of inflammation and immune cell infiltration in the pathogenesis of this enigmatic disease process and summarizes evidence that supports fundamental defects in retinoid metabolism and action among women with endometriosis. Findings from our studies suggest a new line of investigation for developing potential endometriosis therapeutic or preventative agents.
The classical and neoclassical concepts of endometriosis etiology have been reviewed comprehensively6,7 and will not be reiterated exhaustively here. However, among all the postulated etiologies, we wish to emphasize two popular hypotheses—”retrograde menstruation and implantation” and “metaplasia”—that appear to be particularly relevant to the theme of inflammation as a pathogenetic mechanism of endometriosis.
“Retrograde menstruation and implantation” refer to the process of menstrual regurgitation through the fallopian tubes. John Sampson, a Johns Hopkins gynecologist at the turn of the 20th century, postulated that the ovarian endometriosis implant was “acquired from the implantation of epithelium escaping from the tube during menstruation and its subsequent invasion of the ovary.”8 This has become the dominant theory of peritoneal disease over the past century and has acquired more nuanced understanding with evidence that endometrial apoptosis is impeded in women with endometriosis,9 and invasiveness10 and neuroangiogenic properties are enhanced,11 predisposing this subset of women to the establishment of adherent and viable satellite lesions.
The second hypothesis, “metaplasia,” is the process by which one committed cell type is converted into an alternative cell type. Coelomic mucosa typically gives rise to the peritoneum, pleura, and surface epithelium of the gonads. In endometriosis, a metaplastic phenomenon is postulated to occur as a result of transdifferentiation of specialized peritoneal mesothelial cells into endometrial mucosa, as attributed to Robert Meyer of Berlin in the early 1920s.12
It is fair to say that cellular concepts of “inflammation” were rudimentary at the time these hypotheses were first promulgated, but the writings of these perceptive pioneers indicate that they recognized the peculiar significance of the stroma at the invading phalanx of endometriosis implants.13 The contributions of stromal cell–derived chemokines and the leukocytes they recruit have been a major focus of our studies in endometriosis.5,14 It is now accepted that within metaplastic foci, differentiated cells commonly arise in the setting of chronic inflammation and they may be predisposed to neoplastic transformation; all of these phenomena occur in endometriosis.15 Moreover, in contemporary views of metaplasia, the programming or recruitment and differentiation of intrinsic16 or bone marrow–derived17 stem cells, respectively, to ectopic sites is envisioned.
The presence of ectopic or metastatic rests of autologous, benign tissues is in fact quite rare in human biology, but two examples, not typically considered by students of endometriosis, may be informative. The first of these is Barrett esophagus, wherein gastroesophageal reflux of bile acids and other stomach contents triggers a progressive replacement of stratified squamous esophageal cells by ectopic foci of intestinal mucosa with mucin-containing goblet cells above the pyloric valve. Histological evidence of inflammation, particularly neutrophil infiltration, is commonly observed in these lesions.18 The degree of intestinal metaplasia and inflammation in human gastric mucosa specimens from 67 study participants were inversely correlated with the ability of the tissue to produce all-trans retinoic acid (RA).19
A second setting in which ectopic foci of autologous metaplastic “implants” occur is within the tracheobronchial mucosa, characteristically in response to chronic inflammation induced by cigarette smoking. These lesions also represent a clinically premalignant form of metaplasia, wherein reparative processes induce the substitution of respiratory epithelium by squamous cells. The density and cross-sectional area of microvessels within metaplastic lesions increase progressively as they manifest more neoplastic histological features.20 In respiratory tract biopsies from smokers, squamous metaplasia, immune cell (CD45 +) infiltration, and a profile of proinflammatory cytokines very similar to those reported in endometriosis (e.g., TNF-α, IL-1β, and IL-64) were all upregulated compared with biopsies from nonsmoking volunteers. In cell culture models, RA-deprived tracheobronchial epithelial cells also manifest squamous metaplasia.21,22 It has been established that RA can attenuate clinical and experimental airway inflammation.23,24
As with endometriosis, where odds ratios for developing clear cell or endometrioid carcinomas of the ovary may be as high as threefold,15 Barrett esophagus metaplasia25 and squamous metaplasia of the lung26 also are associated with increased carcinogenesis.
Endometriosis: An Inflammatory Paradigm
In each of the disorders described above, immune cell recruitment and infiltration into the ectopic lesions are a consistent theme. In endometriosis, intralesional accumulation of leukocytes was initially recognized around the turn of the 20th century, shortly after Paul Ehrlich and Ilya Mechnikov were jointly awarded the 1908 Nobel Prize in Physiology or Medicine for their discovery of what are now recognized to be the adaptive and innate immune systems, respectively.27 It was Meigs, then serving as director of gynecology at the Vincent Memorial Hospital in Massachusetts, who first described the microscopic infiltration of “endothelial leukocytes” into endometriosis implants, which he noted were associated with fibrosis and neoangiogenesis.28 These prescient observations are currently viewed as fundamental principles underlying the cell biology of endometriosis, but his progressive ideas lay dormant for nearly 60 years.
It was not until 1980 that the seminal publication of Weed and Arquembourg29 reinvigorated Meigs’ insights into leukocyte infiltration, suggesting there were multiple features of local autoimmune phenomena associated with ectopic endometriosis implants. In addition to intralesional lymphocyte accumulation, the authors provided immunohistochemical evidence of complement C3 deposition in ectopic and eutopic biopsies from affected women and postulated that endometriosis-associated infertility might be due to “the rejection of early implantation of embryos,”29 a hypothesis that continues to have advocates to this day.30
Indeed, autoimmune disorders31 and type 1 allergies, including immediate hypersensitivity,32 have been increasingly associated with endometriosis. Many of the chemokines and cytokines activated in lesions have been reviewed recently,33 and several of the genes that regulate these immunoactive proteins have single-nucleotide polymorphisms and copy variants affecting their expression.34
Abnormal innate cell-mediated immune responses, particularly those of macrophages and natural killer cells, appear to facilitate endometriotic lesion attachment and growth.35 Accumulation of activated macrophages within the pelvic fluid of women with endometriosis is well established36,37; however, the potency of their scavenger function and phagocytotic potential appears to be inhibited.5 As discussed in detail below, in a murine endometriosis model, peritoneal macrophage function can be partially rescued following RA supplementation.38
By the mid-1990s, investigators who questioned how these peritoneal macrophages were recruited into the pelvic fluid were rewarded by discovering chemokines that accumulate in the peritoneal fluid of subjects with endometriosis. Using bioassays39 and newly developed enzyme-linked immunosorbent assays (ELISAs),40 the concentrations of several of these activities were shown to be correlated directly with the extent of endometriosis as assessed by laparoscopic staging.4
In clinical endometriosis studies to date, misexpression of two major classes of chemokines has been identified; these are categorized based on their amino acid structure.33 The largest class consists of the CC chemokines, named for conserved adjacent cysteine residues in the proteins’ carboxyl termini. CC chemokines target monocytes, T cells, and eosinophils, and include MCP-1 (CCL2),41 MIP-1α (monocyte inflammatory protein-1α, CCL3),42 RANTES (CCL5),40 and eotaxin (CCL11).43 The second commonest class of chemokines is the CXC family, in which a single, variable amino acid is interposed between the two conserved cysteines. These chemokines predominantly attract monocytes and neutrophils and include growth regulated oncogene (GRO)-α (CXCL1),44 epithelial cell–derived neutrophil-activating peptide (ENA)-78 (CXCL5),45 IL-8 (CXCL8),46,47 and stromal cell–derived factor (SDF)-1 (CXCL12).48
Different chemokines have different sites of synthesis in endometrial and endometriosis tissues.33 RANTES protein and mRNA are mostly confined to the stromal compartment of endometriosis tissues.49 By contrast, eotaxin, ENA-78, MCP-1, and IL-8 are predominantly epithelial.43,45,50,51 As the highest concentrations of tissue-associated macrophages are found in the stromal compartment of endometriosis lesions, as well as endometrial hyperplasia and carcinoma,52 we have concentrated on the role of RANTES in immune cell recruitment to the stroma of these lesions. In vitro, stromal cell cultures derived from endometriosis implants robustly synthesize RANTES mRNA and secrete protein when stimulated by proinflammatory cytokines, whereas epithelial cells synthesize neither transcripts nor protein encoded by this gene.53,54 The transcription factor nuclear factor (NF)-κB is a critical regulator of RANTES gene and protein expression.4,55
Deficiency States of Anti-Inflammatory Hormones and Autacoids
Clinicians and investigators have suspected for over 60 years that the action of progesterone on uterine function was dysfunctional in cases of endometriosis. Since the early days of radioimmunoassay, the luteal rise in serum or pelvic fluid progesterone concentration was variably reported to be reduced56,57 or delayed.58,59 In more recent years, alterations in progesterone receptor isoform expression are increasingly recognized to modulate progesterone action.60 The original observation by Attia et al,61 that PR-B transcript levels were markedly reduced in endometriosis lesions, corroborated findings that progesterone-regulated endometrial genes were generally underexpressed in cases of endometriosis.62–64 This concept was further supported by evidence that the PR-B promoter was hypermethylated65 and other chromatin modifications occur that may account for reduced PR-B expression.66 Interaction of the PR with Hic-5 also is attenuated as a result of reduced expression of the latter in endometrial tissue and stromal cells derived from women with endometriosis.67 Moreover PR resistance also was shown to be manifested in baboons with surgically induced endometriosis.68
Excessive estrogen signaling has long been associated with endometriosis and constitutes a traditional target for medical therapies.69 Increased estrogenic action in these lesions appears to be a consequence of altered expression of both its receptors, estrogen receptor α (ERα) and ERβ,70,71 and increased local hormone biosynthesis by aromatase, the CYP19A1 gene product.72 Recent pharmaceutical developments have focused on the important role of estrogen receptor signaling in endometriosis. Two novel ER ligands, which bind preferentially to ERα and ERβ, respectively, are oxabicycloheptene sulfonate (OBHS) and chloroindazole (CLI). These compounds displayed dual suppression of proliferative and inflammatory activities and effectively prevented the establishment and progression of endometriotic lesions in a mouse model.73 The selective estrogen receptor modulators, bazedoxifene74 and ERB-041,75 also were shown to suppress endometriotic lesion growth in rodents.
Another family of nuclear receptor proteins with anti-inflammatory activities is the peroxisome proliferator-activated receptor (PPAR)-γ.76 The actions of PPAR-γ-ligand complexes affect endometriotic stromal cells,77–79 as well as infiltrating macrophages,5 and vascular endothelial cells.80,81 PPAR proteins are obligate heterodimer partners with retinoic X receptors (RXR) in target cell nuclei.82 Several naturally occurring, high-affinity ligands for PPAR-γ have been identified, including the eicosanoid (9S,10E,12Z)-9-hydroxy-octadeca-10,12-dienoic acid (9-(S)-HODE).82 To date, we are unaware of published evidence of reduced circulating concentrations of endogenous PPAR-γ ligands in cases of endometriosis. However, such findings have been described in the clinical setting of pregnancy complications.83
The salutary effects of synthetic PPAR-γ agonists have been shown in rodent84,85 and nonhuman primate86 models of endometriosis. Given the remarkable potential, but significant side-effect profiles, of current PPAR-γ pharmaceuticals (thiazolidinediones), a spectrum of natural compounds with high-affinity agonist activities have been screened. Among these are some familiar compounds—resveratrol, honokiol, and 6-hydroxydaidzein—but their safety and efficacy have not been tested rigorously in clinical trials.
Retinoid Metabolism in Endometriosis
In addition to critical regulation by steroid hormones and other known ligands for nuclear receptors as mentioned above, studies have shown that retinoids also play fundamental roles in the normal maintenance of the endometrium and particularly with respect to endometriosis.87–89 To this end, the action of RA, produced by metabolic conversion of retinol (ROL), has long been recognized as being necessary for endometrial cell differentiation and function.90,91 This activity is mediated by the expression of nuclear and cytoplasmic retinoid receptors and localized RA synthesis within endometrial and endometriotic stromal cells92,93 (Fig. 1). During the human menstrual cycle, expression of retinoid receptors and synthesis of RA are influenced by the changing patterns of ovarian steroid exposure. Among the numerous aspects of cell behavior pertinent to endometriosis and regulated by local RA production are matrix metalloproteinase (MMP) secretion, gap junctional intracellular communication, and the expression of a variety of cytokines involved in cell differentiation and immune regulation.94,95 Some examples of such RA-regulated genes are IL-6, MCP-1, TNF-α, VEGF, connexin 43, various integrins, and fas ligand,96–99 genes which are also known to be aberrantly expressed in endometriotic lesions.100 Thus, several seemingly discordant features of endometriosis, including repression of apoptosis, increased growth and migration, inflammation, and enhanced invasive properties of intraperitoneally seeded endometrial cells, could be accounted for by dysregulation of RA synthesis.
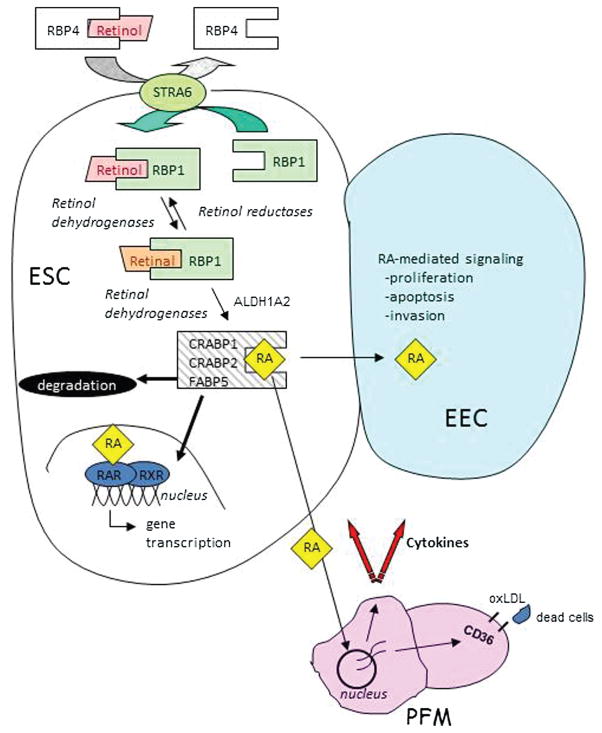
Fig. 1.
Endometrial vitamin A (retinol) metabolism and retinoic acid (RA) signaling. Uptake of retinol (ROL) bound with circulating plasma “retinol binding protein 4” (RBP4) is controlled by the membrane receptor, “stimulated by retinoic acid 6” (STRA6). Intracellular chaperone “cellular ROL-binding protein type 1” (RBP1) physically interacts with STRA6 to pick up ROL, protect ROL from nonspecific metabolism, and deliver it to retinol dehydrogenase enzymes which reversibly catalyze conversion of ROL to retinal. RBP1 then chaperones retinal-to-retinal dehydrogenases (ALDH1A2) which irreversibly convert retinal to RA. RA is then chaperoned by a distinct set of RA-binding proteins (CRABP1, CRABP2, FABP5), which are known to be expressed in endometrial stromal cells (ESC).101,103 Once formed, RA can be: (1) transported to the nucleus of the RA biosynthesizing ESC where it binds to nuclear receptors (RAR) and initiates gene transcription; (2) transported to adjacent epithelial cells (EEC) or secreted into the microenvironment to affect gene transcription in other cells such as peritoneal fluid macrophages (PFM); or (3) degraded. Genes known to be affected in PFM include various proinflammatory cytokines (IL-6, MCP-1, TNFα), which are downregulated by RA and the CD36 type-B scavenger receptor which is upregulated by RA. The consequence of this activity in PFM is a reduction in the inflammatory and oxidative status of the peritoneal environment and increased clearance of ectopic endometrial cells.
The group of Serdar Bulun was among the first to investigate the role of retinoid action in endometriosis.101 Those studies showed altered expression of several genes involved in retinoid biosynthesis and signaling in lesion cells from endometriosis patients, compared with normal eutopic endometrium from patients without endometriosis. Although their study did not measure RA levels directly, the results were consistent with decreased retinoid uptake, metabolism, and action within endometriotic lesions. Subsequent work by Pierzchalski et al102 utilizing matched samples from subjects with endometriosis (i.e., lesions vs eutopic tissue from the same person) directly quantified RA levels and metabolic conversion of ROL to RA in stromal cells derived from the corresponding biopsies; these cells are the primary source of RA biosynthesis in endometrial tissue.103 The studies confirmed that RA biosynthesis is impaired in ectopic endometriotic implants versus their normal eutopic counterpart. A major defect noted was the reduced expression of cellular retinol-binding protein type 1 (RBP1), an ROL chaperone protein that serves as the preferred substrate for retinol dehydrogenase enzymes and the rate-limiting step in RA biosynthesis.104 Thus, reduced RBP1 results in significantly less efficient metabolism of ROL to retinal and its subsequent oxidation to RA. In addition to endometriosis, RBP1 has been shown to be aberrantly expressed in certain mammary, cervical, and ovarian cancers,105–109 as well as some developmental diseases of the brain, bone, and skin.104,110 These studies suggest that defects in RBP1 gene expression in endometriotic stromal cells result in abnormal retinoid biosynthesis and could play a role in the etiology and/or progression of endometriosis.
Two possible scenarios to account for aberrant retinoid metabolism in ectopically growing endometrial cells have been suggested, based on the histogenic mechanisms we provided above. (1) Among the cells that reach the peritoneal cavity via retrograde menstruation, those with intrinsically defective RA synthesis preferentially populate the ectopic sites because of downstream effects of reduced RA levels (e.g., proliferative effects on cell cycle dynamics, increased MMP production, immune cell activation, and proinflammatory cytokine synthesis). (2) A hypothesis in keeping with the metaplastic theory of etiopathogenesis posits that the peritoneal milieu provides environmental cues that induce defects in RA synthesis in metaplastic foci, as opposed to impaired retinoid metabolism being an intrinsic characteristic of the cells. Evidence from studies showing alterations in cytokine and MMP profiles in eutopic endometrium from some women with endometriosis supports the former possibility.30,111 However, support for the latter hypothesis comes from the observation that oxidative stress and prostaglandin (PG)E2, known to be elevated in peritoneal fluid from patients with endometriosis,112 can inhibit biosynthesis of RA109,113 and induce transcriptional repression of RBP1.114,115 Indeed, quite a rich literature supports a critical role for PGE2 as a master regulator of endometriosis. It has been demonstrated to modify a variety of pathophysiological features of the disease, including cell proliferation, antiapoptosis, inflammation, and angiogenesis.116 Using direct, metabolic labeling of purified endometriotic stromal cells with [3H]arachidonic acid in vitro, we could demonstrate that PGE2, along with PGF2α, is a major prostanoid product of these cells (Fig. 2).
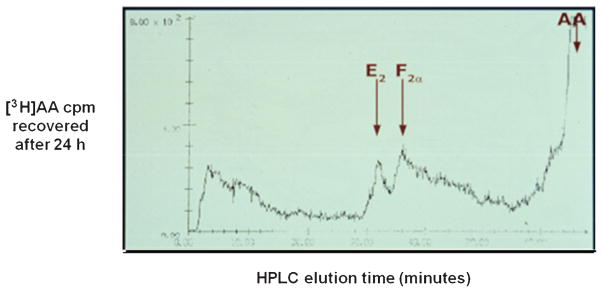
Fig. 2.
Endometriotic stromal cells effectively metabolize [3H]arachidonic acid to PGE2 and PGF2α in vitro. Metabolic labeling of endometriotic stromal cell prostanoid production was performed as described by de Groot et al.150 Briefly, the cultures were incubated with 3 nM [3H] arachidonic acid (AA) for 24 hours and the spent media were extracted with chloroform:methanol:acetic acid (180:20:1) and subjected to high-performance liquid chromatography using a 3.9 × 150 mm Nova-Pack C18 reverse phase column on a Waters Model 204 liquid chromatograph. Unlabeled PGE2 and PGF2α standards eluted at 32 and 38 minutes, respectively, under these conditions. Radioactive counts per minute (cpm) were detected with a Radiometer FLO/ONE-β Model A250 detector.
It has also been reported that cellular RA-binding protein 2 (CRABP2), which delivers RA to RA receptor α (RARα),117 is reduced in endometriosis, potentially as a consequence of progesterone resistance.30,101,103 Whereas it is unknown whether CRABP2 loss precedes RBP1 reduction, it is likely significant to the persistence of reduced RBP1 expression, because loss of CRABP2 function can result in heritable chromatin repression of multiple loci downstream of RARα, including RBP1.114
RA and Inflammation
The cause and effect relationship between impaired RA synthesis and the development of endometriosis is unknown. However, numerous papers indicate that the effects of RA on inflammatory processes suggest that reduced levels in endometriosis can promote some of the abnormal immunological changes that are thought to contribute to its etiology and/or progression. In model systems involving activated monocytes/macrophages, RA decreased proinflammatory cytokines while increasing anti-inflammatory proteins such as interleukin- (IL)10.118 In a variety of cell types, RA has been shown to profoundly affect IL-6-driven events through down-regulation of IL-6 ligand and/or IL-6 receptor production.119,120 One of the most striking in vivo demonstrations showing the ability of retinoids to alter IL-6 levels came from a trial of 13-cis RA (Accutane, Hoffmann-La Roche Ltd., Nutley, NJ) in patients with common variable immunodeficiency (CVI).119 These patients have elevated levels of circulating IL-6 thought to be due to reduced sensitivity and failure of CVI B cells to mature in an IL-6-dependent fashion. The IL-6 concentrations in four of five CVI patients fell to the normal range while on Accutane treatment. This change in circulating IL-6 levels was thought to have resulted from direct effects of the retinoid on monocytes/macrophages, the main identified source for the IL-6 produced in CVI.121 Endometriotic stromal cells also are a rich source of IL-6.122 Studies demonstrated that RA suppresses IL-6 from human endometrial cells through functional antagonism with the nuclear factor IL-6 binding site located in the IL-6 gene promoter.99 In addition to altering the cytokine profile of activated macrophages, RA has been shown to upregulate the CD36 type-B scavenger receptor in cells of the monocyte lineage.123,124 This receptor has been implicated in the uptake and degradation of apoptotic cells and other debris,125 and is regulated by RA in human monocytes/macrophages by a novel mechanism of action that does not require cell adherence.123,124 Thus, treatment of human monocytes/macrophages with RA results in markedly increased protein and mRNA levels of CD36 that occur in the absence of cellular adhesion and differentiation. This fact is important, as multiple studies have demonstrated that women with endometriosis show an increased number of nonadherent macrophages in their peritoneal cavity.36,37 In addition to increasing the ability of peritoneal macrophages to “clear” ectopic endometrial cells, upregulation of CD36 would also cause an increased scavenging of oxidized lipoproteins (e.g., oxLDL) in peritoneal fluid, effecting a net reduction of reactive oxygen species (ROS).126,127 ROS, and other consequences of oxidative stress in the peritoneal fluid of endometriosis patients, has been suggested to play an active role in exacerbating the growth of endometriotic lesions.128 Thus, the failure of adequate RA biosynthesis to selectively upregulate CD36 expression in the monocyte/ macrophage lineage would predict impaired scavenging function of peritoneal macrophages, allowing both the initiation and progressive growth of endometriotic implants. Furthermore, in primary murine astrocyte cultures, pretreatment with RA suppressed the production of chemokine (CCL2, CCL3, CCL5, CXCL1, and CXCL2) mRNAs and proteins in response to lipopolysaccharide endotoxin. Based on experiments using RAR and RXR inhibitors, it is hypothesized that both receptors are likely to be involved in RA’s anti-inflammatory effects.129
Therapeutic Implications
Although it has yet to be determined that the abnormal immune functions associated with endometriosis are caused directly by impaired retinoid action, there is mounting evidence that treatment modalities that target the retinoid metabolic pathway may have therapeutic utility. An example of this possibility was shown by the ability of statins to reduce the number and size of lesions in animal models of endometriosis.130–132 A mechanistic and genetic analysis of this effect indicated that statins modulate the expression of genes involved in the regulation of synthesis and actions of RA,133 suggesting that this action may play a role in their therapeutic efficacy. To directly test this possibility, studies by our group utilizing an immunocompetent mouse model of endometriosis demonstrated that in vivo RA treatment suppressed the establishment and growth of ectopic peritoneal implants along with inhibiting peritoneal fluid accumulation of IL-6 and MCP-1.38 In addition, RA treatment modulated the differentiated state of the murine peritoneal macrophages, as reflected by increased expression of CD38, CD11b, and F4/80. This observation may have important implications in terms of understanding the therapeutic mechanism of RA treatment. On a quantitative basis, both F4/80 and CD11b increase as monocytes differentiate to macrophages and with inflammatory reactions that are associated with mature macrophage function, such as phagocytosis.134–137 The type II transmembrane glycoprotein, CD38, is widely recognized as a marker of lymphocyte and macrophage activation and differentiation.138 RA transcriptionally activates CD38 expression in immune cells via an RA response element located in the first intron of the CD38 gene.139 In macrophages, RA induction of CD38 is associated with increased differentiated functions, including antigen presentation and cell adhesion.138,140 Together, these findings suggest that RA-induced inhibition of endometriotic implants in the mouse model was due, at least in part, to suppression of IL-6 and MCP-1, and promotion of peritoneal macrophage differentiation. In a rat model of endometriosis, where lesions were induced by autotransplantation of uterine pieces into the peritoneal cavity,141 the therapeutic effects of RA were compared with known antiangiogenic agents (bevacizumab and sorafenib). All three compounds induced a significant reduction in the size of the endometriotic implants. However, while the other two agents demonstrated antivascular effects that accompanied a reduction in endometriotic volumes (e.g., decreased VEGF and microvessel density), RA showed the most effective therapeutic benefits without affecting angiogenic parameters. The fact that host immune responses play a primary role on the growth of lesions in this model system142 suggests an immunologically mediated mechanism of RA action. Interestingly, RA was the only compound to concomitantly promote an increase in primordial follicle number, indicating a favorable effect also on ovarian reserve, which has been documented to be impaired in cases of endometriosis.143 Finally, a recent analysis of retinoid levels in the plasma and follicular fluid from women with endometriosis undergoing IVF showed a significantly lower mean concentration of RA, but not ROL, in both compartments compared with similar infertile subjects without endometriosis (women with unexplained, tubal, or male factor infertility).144 Although RA levels were within the reported normal concentration range,145,146 these data support the hypothesis that women whose overall retinoid metabolism is in the low normal range of activity are at higher risk for endometriosis. Such a possibility may help us gain insight into two quintessential questions: (1) Why do only some women develop endometriosis, in spite of the fact that retrograde menstruation seeds the peritoneal cavity with endometrial cells in almost all women?147 (2) What are the mechanisms that predispose women with endometriosis to peritoneal inflammation and immune cell dysfunction?
Conclusion and Future Directions
While the etiology of endometriosis remains obscure, scientific and clinical contributions over the past 95 years have progressively illuminated its pathophysiology. Surgery, particularly laparoscopic excision and bipolar electrocauterization of pelvic implants, remains a mainstay for the treatment of pain and infertility symptoms in affected women. With our advancing understanding of the cell biology of endometriosis, medical therapeutics are increasingly evolving from strategies that focus on the suppression of the hypothalamic–pituitary–ovarian endocrine axis148 to approaches that target alternative pathways, for example, oxidative stress and inflammation.149 As we have summarized in this review, activation of retinoid signaling in endometriosis tissues is predicted to have several salutary effects on the resident cells within these lesions. We propose that adjuvant medical therapies should be developed based on these concepts and predict that their pharmacological actions and anticipated low side-effect profiles will provide women with endometriosis more treatment options for the long-term management of a chronic and debilitating gynecologic disease.
Acknowledgments
This work was supported by Eunice Kennedy Shriver/ National Institute of Child Health grants U01HD66439 (N. S., R. N. T.), R01HD55379 (N. S.), U54HD55787 (R. N. T.), R01HD077260 (M. A. K.), the National Institute of Allergy and Infectious Diseases contract HHSN272202000046C (M. A. K.), and the University of Maryland School of Pharmacy Mass Spectrometry Center SOP1841-IQB2014 (M. A. K.). The authors thank Jean-Louis Vigne, PhD for his expert chromtography and preparation of Fig. 2.
References
- 1.Adamson GD, Kennedy SH, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J Endometriosis. 2010;2:3–6. [Google Scholar]
- 2.Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 3.De Graaff AA, D’Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, Simoens S WERF EndoCost Consortium. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685. doi: 10.1093/humrep/det284. [DOI] [PubMed] [Google Scholar]
- 4.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 5.Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955:159–173. doi: 10.1111/j.1749-6632.2002.tb02777.x. discussion 199–200, 396–406. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RN. In: Hedon B, Mettler L, Tinneberg H-R, editors. Classical and neo-classical concepts in the etiology of endometriosis; Proceedings of the IFFS 20th World Congress on Fertility and Sterility; Munich Lukon. 2010; pp. 70–73. [Google Scholar]
- 7.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–469. [Google Scholar]
- 9.Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod. 2001;16(9):1802–1808. doi: 10.1093/humrep/16.9.1802. [DOI] [PubMed] [Google Scholar]
- 10.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83(3):529–537. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–182. doi: 10.1146/annurev-physiol-012110-142158. [DOI] [PubMed] [Google Scholar]
- 12.Meyer R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in die Ovarien. Virch Arch Path Anat Phys. 1924;250:595–610. [Google Scholar]
- 13.Batt RE. A History of Endometriosis. London: Springer; 2011. p. 85. [Google Scholar]
- 14.Taylor RN, Yu J, Torres PB, et al. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16(2):140–146. doi: 10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce CL, Templeman C, Rossing MA, et al. Ovarian Cancer Association Consortium. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16(11):818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 17.Figueira PG, Abrão MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10–17. doi: 10.1111/j.1749-6632.2011.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberg S, Peters JH, DeMeester TR, et al. Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg. 1997;226(4):522–530. doi: 10.1097/00000658-199710000-00013. discussion 530–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto M, Yokoyama H, Suzuki H, Shiraishi-Yokoyama H, Hibi T. Retinoic acid formation from retinol in the human gastric mucosa: role of class IV alcohol dehydrogenase and its relevance to morphological changes. Am J Physiol Gastrointest Liver Physiol. 2005;289(3):G429–G433. doi: 10.1152/ajpgi.00502.2004. [DOI] [PubMed] [Google Scholar]
- 20.Fisseler-Eckhoff A, Rothstein D, Müller KM. Neovascularization in hyperplastic, metaplastic and potentially preneoplastic lesions of the bronchial mucosa. Virchows Arch. 1996;429(2–3):95–100. doi: 10.1007/BF00192431. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JH, Koo JS, Norford D, Guzman K, Gray T, Nettesheim P. Lysozyme expression during metaplastic squamous differentiation of retinoic acid-deficient human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20(4):573–581. doi: 10.1165/ajrcmb.20.4.3127. [DOI] [PubMed] [Google Scholar]
- 22.Jetten AM, Vollberg TM, Nervi C. Hyperplasia and squamous metaplasia in the tracheobronchial epithelium: alterations in the balance of growth and differentiation factors. Adv Exp Med Biol. 1992;320:89–93. doi: 10.1007/978-1-4615-3468-6_12. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. doi: 10.1186/1471-2172-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F, Marquez H, Kim YK, et al. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 2014;124(2):801–811. doi: 10.1172/JCI70291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64(5):700–706. doi: 10.1136/gutjnl-2014-307278. [DOI] [PubMed] [Google Scholar]
- 26.Herfs M, Hubert P, Poirrier AL, et al. Proinflammatory cytokines induce bronchial hyperplasia and squamous metaplasia in smokers: implications for chronic obstructive pulmonary disease therapy. Am J Respir Cell Mol Biol. 2012;47(1):67–79. doi: 10.1165/rcmb.2011-0353OC. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich P. The partial function of cells (Nobel Prize address given on 11 December 1908 at Stockholm) Int Arch Allergy Appl Immunol. 1954;5(2):67–86. [PubMed] [Google Scholar]
- 28.Meigs JV. Endometrial hematomas of the ovary. Boston Med Surg J. 1922;187:1–13. [Google Scholar]
- 29.Weed JC, Arquembourg PC. Endometriosis: can it produce an autoimmune response resulting in infertility? Clin Obstet Gynecol. 1980;23(3):885–893. [PubMed] [Google Scholar]
- 30.Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31(2):109–124. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- 31.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 32.Bungum HF, Vestergaard C, Knudsen UB. Endometriosis and type 1 allergies/immediate type hypersensitivity: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;179:209–215. doi: 10.1016/j.ejogrb.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19(4):406–418. doi: 10.1093/humupd/dmt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianco B, André GM, Vilarino FL, et al. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum Immunol. 2012;73(3):306–315. doi: 10.1016/j.humimm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- 36.Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35(6):696–698. doi: 10.1016/s0015-0282(16)45567-6. [DOI] [PubMed] [Google Scholar]
- 37.Halme J, Becker S, Hammond MG, Raj MH, Raj S. Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol. 1983;145(3):333–337. doi: 10.1016/0002-9378(83)90720-2. [DOI] [PubMed] [Google Scholar]
- 38.Wieser F, Wu J, Shen Z, Taylor RN, Sidell N. Retinoic acid suppresses growth of lesions, inhibits peritoneal cytokine secretion, and promotes macrophage differentiation in an immunocompetent mouse model of endometriosis. Fertil Steril. 2012;97(6):1430–1437. doi: 10.1016/j.fertnstert.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leiva MC, Hasty LA, Pfeifer S, Mastroianni L, Jr, Lyttle CR. Increased chemotactic activity of peritoneal fluid in patients with endometriosis. Am J Obstet Gynecol. 1993;168(2):592–598. doi: 10.1016/0002-9378(93)90500-i. [DOI] [PubMed] [Google Scholar]
- 40.Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169(6):1545–1549. doi: 10.1016/0002-9378(93)90433-j. [DOI] [PubMed] [Google Scholar]
- 41.Akoum A, Lemay A, Brunet C, Hébert J. Secretion of monocyte chemotactic protein-1 by cytokine-stimulated endometrial cells of women with endometriosis. Le groupe d’investigation en gynécologie. Fertil Steril. 1995;63(2):322–328. doi: 10.1016/s0015-0282(16)57363-4. [DOI] [PubMed] [Google Scholar]
- 42.Na YJ, Lee DH, Kim SC, et al. Effects of peritoneal fluid from endometriosis patients on the release of monocyte-specific chemokines by leukocytes. Arch Gynecol Obstet. 2011;283(6):1333–1341. doi: 10.1007/s00404-010-1583-1. [DOI] [PubMed] [Google Scholar]
- 43.Hornung D, Dohrn K, Sotlar K, et al. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Metab. 2000;85(7):2604–2608. doi: 10.1210/jcem.85.7.6665. [DOI] [PubMed] [Google Scholar]
- 44.Oral E, Seli E, Bahtiyar MO, Jones EE, Arici A. Growth-regulated alpha expression in human preovulatory follicles and ovarian cells. Am J Reprod Immunol. 1997;38(1):19–25. doi: 10.1111/j.1600-0897.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 45.Mueller MD, Mazzucchelli L, Buri C, Lebovic DI, Dreher E, Taylor RN. Epithelial neutrophil-activating peptide 78 concentrations are elevated in the peritoneal fluid of women with endometriosis. Fertil Steril. (79) 2003;(Suppl 1):815–820. doi: 10.1016/s0015-0282(02)04828-8. [DOI] [PubMed] [Google Scholar]
- 46.Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63(4):929–932. [PubMed] [Google Scholar]
- 47.Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2(1):40–45. doi: 10.1093/molehr/2.1.40. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz A, Salvo VA, Ruiz LA, Báez P, García M, Flores I. Basal and steroid hormone-regulated expression of CXCR4 in human endometrium and endometriosis. Reprod Sci. 2010;17(10):894–903. doi: 10.1177/1933719110379920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN. Regulated on activation, normal T-cell-expressed and -secreted mRNA expression in normal endometrium and endometriotic implants: assessment of autocrine/paracrine regulation by in situ hybridization. Am J Pathol. 2001;158(6):1949–1954. doi: 10.1016/S0002-9440(10)64664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuya M, Suyama T, Usui H, et al. Upregulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38(11):1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Ulukus M, Ulukus EC, Tavmergen Goker EN, Tavmergen E, Zheng W, Arici A. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil Steril. 2009;91(3):687–693. doi: 10.1016/j.fertnstert.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 52.Kelly MG, Francisco AM, Cimic A, et al. Type 2 endometrial cancer is associated with a high density of tumor-associated macrophages in the stromal compartment. Reprod Sci. 2015 doi: 10.1177/1933719115570912. In press. [DOI] [PubMed] [Google Scholar]
- 53.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82(5):1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 54.Wieser F, Vigne JL, Ryan I, Hornung D, Djalali S, Taylor RN. Sulindac suppresses nuclear factor-kappaB activation and RANTES gene and protein expression in endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 2005;90(12):6441–6447. doi: 10.1210/jc.2005-0972. [DOI] [PubMed] [Google Scholar]
- 55.Wang XQ, Yu J, Luo XZ, et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45(5):291–299. doi: 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- 56.Brosens IA, Koninckx PR, Corveleyn PA. A study of plasma progesterone, oestradiol-17beta, prolactin and LH levels, and of the luteal phase appearance of the ovaries in patients with endometriosis and infertility. Br J Obstet Gynaecol. 1978;85(4):246–250. doi: 10.1111/j.1471-0528.1978.tb10494.x. [DOI] [PubMed] [Google Scholar]
- 57.Barry-Kinsella C, Sharma SC, Cottell E, Harrison RF. Mid to late luteal phase steroids in minimal stage endometriosis and unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 1994;54(2):113–118. doi: 10.1016/0028-2243(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 58.Cheesman KL, Ben-NunChatterton RT, Jr, Cohen MR. Relationship of luteinizing hormone, pregnanediol-3-glucuronide, and estriol-16-glucuronide in urine of infertile women with endometriosis. Fertil Steril. 1982;38(5):542–548. doi: 10.1016/s0015-0282(16)46632-x. [DOI] [PubMed] [Google Scholar]
- 59.Pittaway DE, Maxson W, Daniell J, Herbert C, Wentz AC. Luteal phase defects in infertility patients with endometriosis. Fertil Steril. 1983;39(5):712–713. doi: 10.1016/s0015-0282(16)47072-x. [DOI] [PubMed] [Google Scholar]
- 60.Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 62.Taylor RN, Lundeen SG, Giudice LC. Emerging role of genomics in endometriosis research. Fertil Steril. 2002;78(4):694–698. doi: 10.1016/s0015-0282(02)03325-3. [DOI] [PubMed] [Google Scholar]
- 63.Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 64.Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- 66.Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358(2):208–215. doi: 10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 67.Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80(1):105–114. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28(1):75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- 69.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30(1):1–19. vii. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 70.Brandenberger AW, Lebovic DI, Tee MK, et al. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5(7):651–655. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- 71.Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Gong P, Chen Y, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7:271ra9. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulak J, Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152(8):3226–3232. doi: 10.1210/en.2010-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20(4):936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- 76.Hornung D, Waite LL, Ricke EA, Bentzien F, Wallwiener D, Taylor RN. Nuclear peroxisome proliferator-activated receptors alpha and gamma have opposing effects on monocyte chemotaxis in endometriosis. J Clin Endocrinol Metab. 2001;86(7):3108–3114. doi: 10.1210/jcem.86.7.7615. [DOI] [PubMed] [Google Scholar]
- 77.Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-gamma decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. 2002;87(4):1841–1844. doi: 10.1210/jcem.87.4.8409. [DOI] [PubMed] [Google Scholar]
- 78.Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N. Peroxisome proliferator-activated receptor-gamma ligand reduced tumor necrosis factor-alpha-induced interleukin-8 production and growth in endometriotic stromal cells. Fertil Steril. 2008;89(2):311–317. doi: 10.1016/j.fertnstert.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 79.McKinnon B, Bersinger NA, Mueller MD. Peroxisome proliferating activating receptor gamma-independent attenuation of interleukin 6 and interleukin 8 secretion from primary endometrial stromal cells by thiazolidinediones. Fertil Steril. 2012;97(3):657–664. doi: 10.1016/j.fertnstert.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPAR gamma represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2005;8(4):373–379. doi: 10.1007/s10456-005-9027-4. [DOI] [PubMed] [Google Scholar]
- 81.Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012;18(6):682–702. doi: 10.1093/humupd/dms026. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Waltenberger B, Pferschy-Wenzig EM, et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waite LL, Louie RE, Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in pre-eclamptic pregnancy. J Clin Endocrinol Metab. 2005;90(2):620–626. doi: 10.1210/jc.2004-0849. [DOI] [PubMed] [Google Scholar]
- 84.Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82(Suppl 3):1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- 85.Nenicu A, Körbel C, Gu Y, Menger MD, Laschke MW. Combined blockade of angiotensin II type 1 receptor and activation of peroxisome proliferator-activated receptor-γ by telmisartan effectively inhibits vascularization and growth of murine endometriosis-like lesions. Hum Reprod. 2014;29(5):1011–1024. doi: 10.1093/humrep/deu035. [DOI] [PubMed] [Google Scholar]
- 86.Lebovic DI, Mwenda JM, Chai DC, et al. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. 2007;88(4, Suppl):1108–1119. doi: 10.1016/j.fertnstert.2006.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loughney AD, Kumarendran MK, Thomas EJ, Redfern CP. Variation in the expression of cellular retinoid binding proteins in human endometrium throughout the menstrual cycle. Hum Reprod. 1995;10(5):1297–1304. doi: 10.1093/oxfordjournals.humrep.a136137. [DOI] [PubMed] [Google Scholar]
- 88.Kumarendran MK, Loughney AD, Prentice A, Thomas EJ, Redfern CP. Nuclear retinoid receptor expression in normal human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1996;2(2):123–129. doi: 10.1093/molehr/2.2.123. [DOI] [PubMed] [Google Scholar]
- 89.Osteen KG, Keller NR, Feltus FA, Melner MH. Paracrine regulation of matrix metalloproteinase expression in the normal human endometrium. Gynecol Obstet Invest. 1999;48(Suppl 1):2–13. doi: 10.1159/000052863. [DOI] [PubMed] [Google Scholar]
- 90.Bo WJ, Smith MS. The effect of retinol and retinoic acid on the morphology of the rat uterus. Anat Rec. 1966;156(1):5–9. doi: 10.1002/ar.1091560103. [DOI] [PubMed] [Google Scholar]
- 91.Thompson JN, Howell JM, Pitt GA. Vitamin A and reproduction in rats. Proc R Soc Lond B Biol Sci. 1964;159:510–535. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- 92.Li XH, Kakkad B, Ong DE. Estrogen directly induces expression of retinoic acid biosynthetic enzymes, compartmentalized between the epithelium and underlying stromal cells in rat uterus. Endocrinology. 2004;145(10):4756–4762. doi: 10.1210/en.2004-0514. [DOI] [PubMed] [Google Scholar]
- 93.Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE. Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology. 2000;141(2):802–808. doi: 10.1210/endo.141.2.7323. [DOI] [PubMed] [Google Scholar]
- 94.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 95.Wu J, Taylor RN, Sidell N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J Cell Physiol. 2013;228(4):903–910. doi: 10.1002/jcp.24241. [DOI] [PubMed] [Google Scholar]
- 96.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clin Immunol. 2006;119(3):272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 97.Sago K, Teitelbaum SL, Venstrom K, Reichardt LF, Ross FP. The integrin alphavbeta5 is expressed on avian osteoclast precursors and regulated by retinoic acid. J Bone Miner Res. 1999;14(1):32–38. doi: 10.1359/jbmr.1999.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sidell N, Feng Y, Hao L, et al. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24(1):148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sawatsri S, Desai N, Rock JA, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril. 2000;73(5):1012–1019. doi: 10.1016/s0015-0282(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 100.Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- 101.Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95(11):E300–E309. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pierzchalski K, Taylor RN, Nezhat C, et al. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod. 2014;91(4):84. doi: 10.1095/biolreprod.114.119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pavone ME, Dyson M, Reirstad S, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–2164. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821(1):152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cvetković D, Williams SJ, Hamilton TC. Loss of cellular retinol-binding protein 1 gene expression in microdissected human ovarian cancer. Clin Cancer Res. 2003;9(3):1013–1020. [PubMed] [Google Scholar]
- 106.Kuppumbatti YS, Bleiweiss IJ, Mandeli JP, Waxman S, Mira-Y-Lopez R. Cellular retinol-binding protein expression and breast cancer. J Natl Cancer Inst. 2000;92(6):475–480. doi: 10.1093/jnci/92.6.475. [DOI] [PubMed] [Google Scholar]
- 107.Esteller M, Guo M, Moreno V, et al. Hypermethylation-associated Inactivation of the Cellular Retinol-Binding-Protein 1 Gene in Human Cancer. Cancer Res. 2002;62(20):5902–5905. [PubMed] [Google Scholar]
- 108.Mendoza-Rodriguez M, Arreola H, Valdivia A, et al. Cellular retinol binding protein 1 could be a tumor suppressor gene in cervical cancer. Int J Clin Exp Pathol. 2013;6(9):1817–1825. [PMC free article] [PubMed] [Google Scholar]
- 109.Pierzchalski K, Yu J, Norman V, Kane MA. CrbpI regulates mammary retinoic acid homeostasis and the mammary microenvironment. FASEB J. 2013;27(5):1904–1916. doi: 10.1096/fj.12-219410. [DOI] [PubMed] [Google Scholar]
- 110.Xu L, Song C, Ni M, Meng F, Xie H, Li G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRα-induced β-catenin degradation. Int J Biochem Cell Biol. 2012;44(4):612–619. doi: 10.1016/j.biocel.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 111.Yu J, Boicea A, Barrett KL, et al. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod. 2014;20(3):260–270. doi: 10.1093/molehr/gat087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25(2):75–81. doi: 10.1080/09513590802485012. [DOI] [PubMed] [Google Scholar]
- 113.Stock A, Booth S, Cerundolo V. Prostaglandin E2 suppresses the differentiation of retinoic acid-producing dendritic cells in mice and humans. J Exp Med. 2011;208(4):761–773. doi: 10.1084/jem.20101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corlazzoli F, Rossetti S, Bistulfi G, Ren M, Sacchi N. Derangement of a factor upstream of RARalpha triggers the repression of a pleiotropic epigenetic network. PLoS ONE. 2009;4(1):e4305. doi: 10.1371/journal.pone.0004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tannous-Khuri L, Talmage DA. Decreased cellular retinol-binding protein expression coincides with the loss of retinol responsiveness in rat cervical epithelial cells. Exp Cell Res. 1997;230(1):38–44. doi: 10.1006/excr.1996.3399. [DOI] [PubMed] [Google Scholar]
- 116.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235(6):668–677. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 117.Budhu A, Gillilan R, Noy N. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J Mol Biol. 2001;305(4):939–949. doi: 10.1006/jmbi.2000.4340. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Allen C, Ballow M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol. 2007;27(2):193–200. doi: 10.1007/s10875-006-9068-5. [DOI] [PubMed] [Google Scholar]
- 119.Saxon A, Keld B, Braun J, Dotson A, Sidell N. Long-term administration of 13-cis retinoic acid in common variable immunodeficiency: circulating interleukin-6 levels, B-cell surface molecule display, and in vitro and in vivo B-cell antibody production. Immunology. 1993;80(3):477–487. [PMC free article] [PubMed] [Google Scholar]
- 120.Sidell N, Taga T, Hirano T, Kishimoto T, Saxon A. Retinoic acid-induced growth inhibition of a human myeloma cell line via down-regulation of IL-6 receptors. J Immunol. 1991;146(11):3809–3814. [PubMed] [Google Scholar]
- 121.Adelman DC, Matsuda TF, Hirano TF, Kishimoto TF, Saxon A. Elevated serum interleukin-6 associated with a failure in B cell differentiation in common variable immunodeficiency. J Allergy Clin Immunol. 1990;86(4):512–521. doi: 10.1016/s0091-6749(05)80207-6. [DOI] [PubMed] [Google Scholar]
- 122.Tseng JF, Ryan IP, Milam TD, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118–1122. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- 123.Han S, Sidell N. Peroxisome-proliferator-activated-receptor gamma (PPARgamma) independent induction of CD36 in THP-1 monocytes by retinoic acid. Immunology. 2002;106(1):53–59. doi: 10.1046/j.1365-2567.2002.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wuttge DM, Romert A, Eriksson U, Törmä H, Hansson GK, Sirsjö A. Induction of CD36 by all-trans retinoic acid: retinoic acid receptor signaling in the pathogenesis of atherosclerosis. FASEB J. 2001;15(7):1221–1223. doi: 10.1096/fj.00-0488fje. [DOI] [PubMed] [Google Scholar]
- 125.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161(11):6250–6257. [PubMed] [Google Scholar]
- 126.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268(16):11811–11816. [PubMed] [Google Scholar]
- 127.Nicholson AC, Frieda S, Pearce A, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995;15(2):269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 128.Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002;955:183–198. doi: 10.1111/j.1749-6632.2002.tb02779.x. discussion 19–200, 396–406. [DOI] [PubMed] [Google Scholar]
- 129.van Neerven S, Regen T, Wolf D, et al. Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid. J Neurochem. 2010;114(5):1511–1526. doi: 10.1111/j.1471-4159.2010.06867.x. [DOI] [PubMed] [Google Scholar]
- 130.Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94(7):2489–2494. doi: 10.1210/jc.2008-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oktem M, Esinler I, Eroglu D, Haberal N, Bayraktar N, Zeyneloglu HB. High-dose atorvastatin causes regression of endometriotic implants: a rat model. Hum Reprod. 2007;22(5):1474–1480. doi: 10.1093/humrep/del505. [DOI] [PubMed] [Google Scholar]
- 132.Yilmaz B, Ozat M, Kilic S, et al. Atorvastatin causes regression of endometriotic implants in a rat model. Reprod Biomed Online. 2010;20(2):291–299. doi: 10.1016/j.rbmo.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Sokalska A, Anderson M, Villanueva J, et al. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98(3):E463–E471. doi: 10.1210/jc.2012-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cui J, Zhu N, Wang Q, et al. p38 MAPK contributes to CD54 expression and the enhancement of phagocytic activity during macrophage development. Cell Immunol. 2009;256(1–2):6–11. doi: 10.1016/j.cellimm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 135.Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107(6):2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoffman SM, Fleming SD. Natural Helicobacter infection modulates mouse intestinal muscularis macrophage responses. Cell Biochem Funct. 2010;28(8):686–694. doi: 10.1002/cbf.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leukoc Biol. 2010;88(1):169–180. doi: 10.1189/jlb.0809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prus E, Fibach E. Retinoic acid induction of CD38 antigen expression on normal and leukemic human myeloid cells: relationship with cell differentiation. Leuk Lymphoma. 2003;44(4):691–698. doi: 10.1080/1042819031000060564. [DOI] [PubMed] [Google Scholar]
- 139.Kishimoto H, Hoshino S, Ohori M, et al. Molecular mechanism of human CD38 gene expression by retinoic acid. Identification of retinoic acid response element in the first intron. J Biol Chem. 1998;273(25):15429–15434. doi: 10.1074/jbc.273.25.15429. [DOI] [PubMed] [Google Scholar]
- 140.Musso T, Deaglio S, Franco L, et al. CD38 expression and functional activities are upregulated by IFN-gamma on human monocytes and monocytic cell lines. J Leukoc Biol. 2001;69(4):605–612. [PubMed] [Google Scholar]
- 141.Ozer H, Boztosun A, Açmaz G, Atilgan R, Akkar OB, Kosar MI. The efficacy of bevacizumab, sorafenib, and retinoic acid on rat endometriosis model. Reprod Sci. 2013;20(1):26–32. doi: 10.1177/1933719112452941. [DOI] [PubMed] [Google Scholar]
- 142.Pelch KE, Schroder AL, Kimball PA, Sharpe-Timms KL, Davis JW, Nagel SC. Aberrant gene expression profile in a mouse model of endometriosis mirrors that observed in women. Fertil Steril. 2010;93(5):1615–1627. e18. doi: 10.1016/j.fertnstert.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shah DK. Diminished ovarian reserve and endometriosis: insult upon injury. Semin Reprod Med. 2013;31(2):144–149. doi: 10.1055/s-0032-1333479. [DOI] [PubMed] [Google Scholar]
- 144.Pauli SA, Session DR, Shang W, et al. Analysis of follicular fluid retinoids in women undergoing in vitro fertilization: retinoic acid influences embryo quality and is reduced in women with endometriosis. Reprod Sci. 2013;20(9):1116–1124. doi: 10.1177/1933719113477487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moulas AN, Gerogianni IC, Papadopoulos D, Gourgoulianis KI. Serum retinoic acid, retinol and retinyl palmitate levels in patients with lung cancer. Respirology. 2006;11(2):169–174. doi: 10.1111/j.1440-1843.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 146.Sedjo RL, Ranger-Moore J, Foote J, et al. Circulating endogenous retinoic acid concentrations among participants enrolled in a randomized placebo-controlled clinical trial of retinyl palmitate. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1687–1692. [PubMed] [Google Scholar]
- 147.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 148.Dunselman GA, Vermeulen N, Becker C, et al. European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 149.Rogers PA, D’Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483–499. doi: 10.1177/1933719113477495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Groot CJ, Murai JT, Vigne JL, Taylor RN. Eicosanoid secretion by human endothelial cells exposed to normal pregnancy and preeclampsia plasma in vitro. Prostaglandins Leukot Essent Fatty Acids. 1998;58(2):91–97. doi: 10.1016/s0952-3278(98)90146-6. [DOI] [PubMed] [Google Scholar]