Abstract
Absent or severely diminished activity of ADAMTS13 (A Disintegrin And Metalloprotease with a ThromboSpondin type 1 motif, member 13) resulting in the intravascular persistence and accumulation of highly thrombogenic ultra large von Willebrand factor (UL-VWF) multimers is the pathophysiological mechanism underlying thrombotic thrombocytopenic purpura.
Reduced VWF-cleaving protease levels, however, are not uniquely restricted to primary thrombotic microangiopathy (TMA), e.g. thrombotic thrombocytopenic purpura, but also occur in other life-threatening thrombocytopenic conditions: severely decreased ADAMTS13 activity is seen in severe sepsis, disseminated intravascular coagulation (DIC) and complicated malarial infection.
The clinical relevance of these secondary thrombotic microangiopathies is increasingly recognized, but its therapeutic implications have not been determined yet. The presence of a secondary TMA in certain diseases may define patient groups which possibly could benefit from ADAMTS13 replacement or a VWF-targeting therapy.
This short-review focuses on the role of UL-VWF multimers in secondary TMA and discusses the potential of investigational therapies as candidates for the treatment of TTP.
Conclusion
Prospective clinical trials on the effectiveness of protease replacement in vivo seem reasonable. Carefully selected patients with secondary TMA may benefit from therapies primarily intended for the use in patients with TTP.
Keywords: ADAMTS13, VWF, TTP, DIC, sepsis, malaria
Background
Von Willebrand factor (VWF) is a multifunctional acute-phase glycoprotein with a central role in primary haemostasis (1, 2). Assembled as multimers in endothelial Weibel Palade bodies, VWF is exocytosed in response to various endogenous stimuli such as inflammatory cytokines, histamine, thrombin, fibrin, or to exogenous Desmopressin, tethering passing platelets to damaged endothelial sites in the presence of high shear rates (3-7).
Newly secreted ultra large VWF multimers (UL-VWF) have a high spontaneous platelet-binding potential and consequently must be enzymatically degraded into smaller, less thrombogenic units before entering the circulation. A specific metalloproteinase, ADAMTS13, cleaves VWF after binding to the VWF-A2 domain (8). Fluid shear stress is mandatory to stretch and unfold these multimers into scissile strings in order to facilitate enzymatic processing (9, 10). This cleavage process, which is accelerated by platelets and FVIII (11), prevents elongation and accumulation of hyper-reactive UL-VWF strands on activated endothelial surface (12).
The pathophysiology of thrombotic thrombocytopenic purpura, the prototypical TMA, is characterized by an abnormal persistence of UL-VWF multimers caused by insufficient or absent ADAMTS13 activity, which can be acquired through protease-directed autoantibodies in idiopathic TTP (13) or inherited due to homogeneous ADAMTS13 gene mutations in congenital TTP (14).
The giant strand-shaped multimers with a weight up to >1GDa and a length up to >1mm (10, 15, 16) spontaneously bind, activate and aggregate platelets and thereby provide the basic molecular substrate for thrombus formation in small blood vessels (16, 17). A widespread deposition of platelet-rich clots throughout the body may occur with occlusion of microvessels and subsequent organ failure. Erythrocytes passing through UL-VWF/platelet/fibrin meshwork in the microvasculature can become fragmented, leading to mechanical hemolysis with schistocyte formation, a diagnostic hallmark of thrombotic microangiopathies (18). Microvessel thrombosis in TMA predominantly affects organs with low or absent CD36-expression, especially the kidneys (19). This may suggest some (as yet unproven) restriction of the cleavage activity of ADAMTS13 to CD36-expressing cells.
In addition to platelet-activating properties, VWF promotes leucocyte adhesion to endothelial cells (20) and VWF multimers provide large surfaces for the activation of the complement cascade, thereby directly linking haemostasis to inflammation (21). Not only in haemolytic-uremic syndrome, but also in cases of TTP, complement-mediated inflammation may play a substantial role in disease pathogenesis (22-24). It has been hypothesized (21) that in those TTP patients with only slightly reduced enzyme levels an underlying heterozygous ADAMTS13 gene defect may be turned into clinically overt disease by a simultaneous defect in factor H, a circulating glycoprotein regulating the alternative complement pathway. The coincidence of insufficiently degraded multimers with aberrant complement regulation results in uncontrolled synthesis of anaphylatoxins and membrane attack complex, damaging host tissue and promoting thrombosis. This proposed mechanism could explain cases of TTP or TTP-like disorders refractory to extensive plasma exchange-based treatment and may provide a new therapeutic target.
Acquired TTP is a devastating and highly fatal condition if not treated immediately. The primary therapeutic intent is to replenish ADAMTS13 activity by urgent plasma exchange or, if this is not immediately available, by plasma transfusion. Plasma exchange therapy has dramatically decreased overall mortality in acquired TTP from nearly 90% to ≤25% (25, 26). Urgent replacement of ADAMTS13 facilitates cleavage of UL-VWF multimers into smaller, less thrombogenic forms and interrupts the vicious cycle of platelet entrapment and thrombus formation. Replacement therapy using recombinant ADAMTS13 is a reasonable therapeutic approach for TTP since it would specifically replenish the lacking enzyme (27-29). This is a promising approach for TTP due to congenital deficiency of ADAMTS13, but its usefulness in acquired forms of TTP is questionable, since even high-dose replacement may not be able to surmount the endogenous ADAMTS13 inhibitors present in those patients (30). One option to overcome this would be the use of recombinant enzyme variants with increased resistance to inhibition by autoantibodies, which have recently been tested in vitro with promising results (31). Alternatively, one could use anti-VWF agents specifically targeting the VWF-A1 domain to interrupt the process of binding and activating platelets. Two anti-VWF aptamers, ARC1779 (32-40) and ARC15105 (41), have been extensively investigated so far, although their clinical development is currently on hold (30). ALX-0081 (INN: caplacizumab, Ablynx) is a selective and highly potent bivalent humanized nanobody specifically targeting the GPIb-binding site of VWF (42, 43). Recent results from a phase II trial indicate that caplacizumab is effective in patients with acquired TTP (44).
However, the problem of excessive UL-VWF is not exclusively restricted to primary TMAs, but also seems to be a major determinant of prognosis in other life-threatening inflammatory disorders (45-52). Reduced ADAMTS13 activity strongly correlates with the severity of coagulopathy and in-hospital mortality in patients with disseminated intravascular coagulation (DIC) (53) and is further associated with a poor outcome in septic organ failure (54). Thrombotic microangiopathy can complicate sepsis and may even persist after resolution of DIC, prolonging organ dysfunction and influencing prognosis (55).
Although the pathophysiology of ADAMTS13 deficiency in sepsis differs from that in TTP, in a subset of patients with severe sepsis, clinical and laboratory features closely resemble those seen in TTP (50), suggesting the potential for therapeutic utility of enzyme replacement or VWF-A1 targeting therapeutic strategies in patients with severely depressed ADAMTS13 activity unrelated to primary TTP.
This review focuses on the role of UL-VWF multimers in severe sepsis and other secondarily acquired thrombotic microangiopathies and discusses the potentials of ADAMTS13 replacement and VWF-targeting agents as future therapeutics.
Thrombotic microangiopathies in systemic inflammation
Often TTP follows an acute episode of inflammation, which possibly triggers autoantibody formation (56, 57).
The unifying pathology linking both systemic inflammation and primary TMAs is acute dysfunctional endothelial cell activation, indicated by high VWF antigen (VWFAg) and VWF propeptide (VWFpp) in both sepsis and TTP (58, 59).
As an acute phase reactant VWF is up-regulated and secreted in inflammatory conditions (30, 56, 60). Its plasma levels usually correlate with the extent of systemic inflammation. Its simultaneously released VWFpp is considered a suitable marker of endothelial cell activation since it is not consumed in (patho-)physiologic haemostatic processes.
An inflammation-related imbalance between overwhelming VWF-release and inhibited and/or exhausted cleaving capacity of ADAMTS13 may finally result in a substantial accumulation of highly adhesive UL-VWF strings compromising the microcirculation. Accordingly, the inverse relation between high VWF antigen levels and low ADAMTS13 activity is associated with the extent of inflammation and the severity of organ failure (61). This is in line with the finding that the enzymatic processing of VWF multimers after endothelial release is predictive for mortality in systemic inflammatory conditions (62).
In a large cohort of a heterogeneous patient population with systemic inflammatory response syndrome (SIRS), VWF levels were significantly higher in non-survivors with an independent (APACHE Score-adjusted) 2-fold increased hazard of death for those in the highest VWF-level-tertile (62). In contrast, patients with the highest ADAMTS13 levels showed a 4-fold lower mortality rate compared to those with lowest ADAMTS13 levels (HR=0.22; CI 0.07-0.74). This is supported by another study which found that VWF levels continuously increase with growing severity of inflammation while ADAMTS13 levels simultaneously decline, culminating in the accumulation of uncleaved VWF molecules (63). Mounting VWF/protease- imbalance therefore was proportional to the risk of developing TMA and consequently organ failure. Accordingly, the ratio between high VWFAg/VWFpp and low ADAMTS13 levels (median 25%±8.5) may be a sensitive marker of disease severity and a useful tool to stratify patients at risk of developing inflammatory TMA (63).
Similar findings were observed in patients with severe sepsis, both in adults (54) and infants (52, 64). Consistently, reduced ADAMTS13 activity determined disease severity (52, 65), organ failure and mortality (64, 65). A causal relationship to sepsis is indicated by the observation that ADAMTS13 levels discriminated between differences in inflammation, endothelial dysfunction, renal failure, hemodynamic compromise and mortality only in patients with severe sepsis, but not in those with organ failure unrelated to sepsis (54). Severely septic patients with ADAMTS13 levels below 43% showed a significantly lower survival rate, independent of the presence of DIC.
However, two studies, a septic mouse model (66) and a clinical study in septic adults (67) yielded different results; although VWF parameters inversely correlated with ADAMTS13 activity there was no relation between either of these parameters and disease severity or outcome. This apparently negative finding reported by Kremer Hovinga et al. may be explained by fact that their patients’ median ADAMTS13 level was 60% of normal, which is rather high compared to the levels found in other studies yielding positive results, and thus is likely to have been well above a critical threshold value. In contrast, in the study reported by Ono et al (50) showing a positive correlation between ADAMTS13 level and clinical outcome ADAMTS13 levels were markedly lower, possibly due to a genetic polymorphism in the ADAMTS13 gene which is frequently found in Japan but which has not been reported in Caucasians. Another potential cause for the discrepancy may be the presence or absence of DIC. While Ono et al (50), who found a clear inverse association between outcome and median ADAMTS13 activity (43%), only included septic patients with DIC (calculated by the LMHW-DIC score) Kremer Hovinga and colleagues (67) provide no information on DIC prevalence in their septic study population (20% with septic shock, median ADAMTS13 activity 60%). Supporting a possible role of DIC, Habe and colleagues (68) investigated 69 patients with DIC (36% with septic DIC; median ADAMTS13 activity 35%) and found an inverse correlation between the ISTH-DIC score and ADAMTS13 activity with a trend of lower enzyme activity in non-survivors (25 vs 38%). However, the patient population was very heterogeneous including only 36% with septic DIC, but 40% with malignancy and 14% with various conditions. Finally, similarly to Ono et al, Martin and colleagues (54) described a significant association between outcome and ADAMTS13 activity (median 43%), but however, this finding was independent of disease severity (APACHE II score) and the presence of DIC (assessed by ISTH DIC score). However, the application of different DIC scoring systems (JMHW vs. ISTH DIC score) and the inclusion of patients with different underlying pathologies causing DIC makes it difficult to compare these studies.
Several molecular mediators of endothelial activation with consequent VWF liberation have been investigated so far. The insufficient cleavage capacity of ADAMTS13 likely is also a multifactorial event and may be caused by exhaustion, direct inhibition, reduced synthesis, proteolytic cleavage and enhanced clearance (66, 69-71) (Table 1).
Table 1. Potential mechanisms of ADAMTS13 deficiency and VWF release.
| Mediator | Mode of action | References | |
|---|---|---|---|
|
ADAMTS13 deficiency
| |||
| Inhibition | IL6 | Delays the rate of UL-VWF cleavage under flow | (5) |
| Free hemoglobin | Competitive binding to VWF A2 domain | (126), (127) | |
| Thrombospondin-1 | Competitive binding to VWF A3 domain | (83) | |
| Leukocyte elastase | Proteolysis of ADAMTS13 | (50), (128) | |
| Bacterial enzymes | Proteolysis of ADAMTS13 | (80) | |
| Plasmin | Proteolysis of ADAMTS13 | (129) | |
| Thrombin | Proteolysis of ADAMTS13 | (129) | |
| FXa | (96) | ||
| FVIII deficiency | FVIII accelerates VWF cleavage | (11) | |
| Exhaustion | ULVWF multimers consuming ADAMTS13 molecules | (9), (41), (61) | |
| Increased Clearance | Non-neutralizing antibodies | (130) | |
| Reduced synthesis | Liver failure | (50) | |
| Indirect Inhibition | Reactive oxygen species | Oxidation of VWF impairs its cleavage by ADAMTS13 | (70), (78), (79) |
|
VWF release by
| |||
| Cytokines | TNFα, interleukin-8 | (5) | |
| Interleukin-11 | (131) | ||
| Endotoxin | LPS | (107), (132) | |
| Biogenic amine | Histamine | (7) | |
| Enzyme | Thrombin | (133) | |
| Catecholamines | Epinephrine | (134) | |
| Reactive oxygen species | (135) | ||
| Clotting factor | FVIIa | (136) | |
Exhaustion
A consumptive ADAMTS13 deficiency occurs when inflammation-triggered substrate supply exceeds the enzyme’s proteolytic capacity. The reciprocal correlation between VWF and ADAMTS13 was observed in several in vitro and in vivo studies ranging from an experimental endotoxinema model (72) to prospective clinical trials. Lipopolysaccharide exposure results in a significant reduction of ADAMTS13- activity, hepatic ADAMTS13-mRNA expression, and platelet counts, whereas both VWF antigen and neutrophil counts increase. The opposing changes in VWF and ADAMTS13 blood levels also correspond to respective changes in hepatic mRNA expression patterns (73). Finally, in a septic mouse model, ADAMTS13 activity was mainly determined by VWF levels and preserved in VWF-gene-deleted septic animals (66). However, even without accompanying inflammation, stimulating secretion of VWF by administration of Desmopressin decreases ADAMTS13 levels (69, 74).
Inhibition
In vitro experiments on platelet rich plasma showed that inflammatory cytokines released from activated leukocytes and endothelial cells at an early stage of inflammation stimulate the synthesis of UL-VWF multimers-strings (both interleukin-8 and tumor necrosis factor (TNF) α) and concurrently inhibit their cleavage (interleukin-6 alone) (5). This corresponds to the finding that both reduced ADAMTS13 activity (54) and high interleukin-6 predict poor prognosis in TTP (5) and septic shock (75, 76). Finally, DIC is associated with the consumption of circulating clotting factors, including FVIII, which normally accelerates VWF proteolysis by ADAMTS13 (11).
Proteolytic cleavage
A moderate correlation between ADAMTS13 levels and granulocyte elastase-mediated fibrin degradation products in septic adults (50) suggests a contributory role of proteolytic cleavage by neutrophils to diminished ADAMTS13 activity in inflammation. This would be in line with the observation that neutrophil elastase levels are significantly elevated not only in sepsis but also in acute TTP, where both neutrophil extracellular traps (77) and the plasma elastase concentration (71) were found to contribute to disease severity. Accordingly, elastase inhibitors abolished the ADAMTS13 decrease after LPS challenge in mice (73).
Thrombin, plasmin and reactive oxygen species, all commonly elevated in sepsis and SIRS, as well as specific microbial proteases may also impair the catalytic activity of ADAMTS13 (70, 78, 79). The latter has been shown for Bacillus anthracis (80) and conceivably might apply to other bacteria more frequently causing bloodstream infections.
Competition
Thrombospondin-1 (TSP-1) is a matrix-glycoprotein which is elevated in inflammatory conditions including human sepsis and which is considered an early marker of sepsis-related DIC (81). It regulates the inflammatory response by activating leukocytes and enhancing chemotaxis and was linked to mortality in a murine sepsis model (82). Thrombospondin-1 is known to be essentially involved in haemostatic processes but its role regarding the development of secondary TMA has not been evaluated yet. By competing with ADAMTS13 for the interaction with the VWF-A3 domain (83), thrombospondin-1 protects newly secreted haemostatically reactive UL-VWF multimers against degradation and slows the rate of their proteolysis (84, 85). Whether increased thrombospondin-1 levels are of clinical relevance regarding the emergence of TMAs in sepsis and SIRS is unclear.
However, patients with sepsis represent a heterogeneous population who suffer from various comorbidities. Host characteristics and risk factors finally determining the development of secondary TMA in inflammation are not well-defined. Coagulopathy in sepsis is a complex process characterized not only by tissue factor related thrombin generation but also by an impaired fibrinolysis, referred to as “fibrinolytic shut down” (86, 87). This may also play a role regarding the occurrence of UL-VWF strings, since plasminogen activation on endothelial cells acts as a natural backup for ADAMTS13 to degrade obstructive platelet-VWF complexes (88). Septic patients lacking both pathways of multimer degradation (ADAMTS13 and fibrinolytic enzymes) may be at high risk of additionally developing TMA. The relative contribution of fibrinolytic enzymes to VWF cleavage, however, is questionable, given that patients suffering from congenital TTP are a priori not expected to have a defect in fibrinolysis.
Also, the contributing role of complicating DIC regarding the development of TMA in sepsis is uncertain. One recent study specifically addressed this issue and found that the occurrence of ADAMTS13 deficiency (median 30%; IQR 9 - 45) and its predictive value for mortality in severe sepsis are independent of the presence of DIC (76). Indeed, TMA associated organ failure can outlast DIC and perpetuate microvascular compromise despite the resolution of plasmatic coagulopathy (55).
Thrombotic microangiopathy in DIC
The scientific subcommittee on DIC of the International Society on Thrombosis and Haemostasis (ISTH) defines DIC as an acquired syndrome characterized by a systemic intravascular activation of coagulation with loss of localization, which causes damage to the microvasculature, and if sufficiently severe, can produce organ dysfunction (89). Disseminated intravascular coagulation is the worst manifestation of a globally activated and deteriorated coagulation system caused by heterogeneous disorders of which sepsis may be one of the most common. It is not a primary disorder but can be superimposed upon and can complicate various medical conditions with high morbidity and mortality.
In contrast to TMAs, where there is little activation of the coagulation cascade, tissue factor-driven coagulopathy in DIC results in excessive thrombin generation with activation and subsequent exhaustion of all coagulation components and platelets, which finally culminates in disseminated depositions of fibrin-rich (rather than platelet-rich) thrombi, organ damage and bleeding. Table 2 shows laboratory features of TTP, DIC and coagulopathy in malaria.
Table 2. Laboratory features of TTP, DIC and coagulopathy in malaria.
| TTP | DIC | Malaria | |
|---|---|---|---|
| PLT | ↓ ↓ ↓ * | ↓↓(↓) | ↓(↓↓) |
| Anemia | MHA~ | MHA~ | § |
| PT | ↔ | ↑ | # |
| Fibrinogen | ↔,↑ | ↓ ↓ | # |
| D-dimer | ↔, ↑(↑) | ↑ ↑ | # |
| FRC | ↑ ↑ ↑ ** | ↑(↑) | # |
| LDH | ↑ ↑ ↑ | ↑(↑↑) | ↑(↑↑)# |
| Thrombus | platelet-rich | fibrin-rich | variable |
| ADAMTS13 | absent | variable | variable |
Median platelet count was 16×109/L (range:5-120) (118) and 25.5 ×109/L (range:2-124) (119), respectively.
Microangiopathic haemolytic anemia
Prevalence of thrombocytopenia in malaria ranges from 60%-80% (97).
Degree of coagulation derangement varies from mild to severe and correlates with parasitemia and disease severity (120).
In a study on patients (18-40y) with acute, uncomplicated plasmodium falciparum malaria the mean serum lactate dehydrogenase (LDH) activity was 789 (± 35) IU in males (n=76) and 634 ± 35 IU in females (n=76), considered to result from both red cell lysis and hepatic injury (121).
Anemia is common in malarial infection, typically presenting with normal erythrocyte indices and a low reticulocyte count (122). Its pathogenesis is multifactorial including intravascular destruction of parasited RBCs, extravascular clearance of erythrocytes and bone marrow suppression with dyserythropoiesis (123). Black water fever is a very rare form of anemia and typically associated with a preceding use of quinine and the presence of glucose-6- phosphatase deficiency.
No routine laboratory finding is, however, highly specific for malaria. Platelets <150×109/L along with fever, enlarged spleen and serum bilirubin level >1.3 mg/dL was most predictive of malaria in a prospective study on 1900 European febrile travelers (124). In a retrospective analysis the cumulative presence of fever, thrombocytopenia and splenomegaly had a sensitivity of 71% and specificity of 88% (125). DIC occurs in up to 30% of previously naïve patients with complicated falciparum malaria (92). Elevated plasminogen activator inhibitor-1 (PAI-1) and reduced tissue plasminogen activator (t-PA) levels indicate a thrombotic phenotype of DIC.
Fragmented red blood cells (schistocytes and helmet cells). Schistocytes >1% is highly suggestive for TTP (18).
A recently published study on patients with various thrombocytopenic disorders found low ADAMTS13 levels to occur in a substantial subset of these patients. Low protease levels were significantly associated with high VWF Ag levels and low platelet counts (51). The most pronounced decrease in VWF proteolytic activity was found in individuals with severe thrombocytopenia. The abnormalities in VWF proteolysis were indistinguishable from that of TTP patients compared to patients with other thrombocytopenic disorders (51).
Another trial investigating patients with sepsis-related DIC yielded similar results: 16% of DIC patients had severely reduced ADAMTS13 activity levels (<5%) with a clinical and chemical profile indistinguishable from that of patients with primary TMA. DIC patients with severely reduced protease activity fulfilled all clinical and laboratory criteria for TTP, indicated by marked non-immune hemolysis (mean LDH: 2481 U/L, mean haemoglobin: 8.3 g/dL), severe thrombocytopenia (mean platelet count 7×109/L), impaired renal function (mean creatinine: 1.9 mg/dL) and altered consciousness in 47% (50). The incidence of renal failure, but not that of other organs, was significantly higher in those patients with ADAMTS13 activity <20%, with a strong correlation between UL-VWF multimers and creatinine levels (50). Furthermore, other studies confirmed a higher incidence of renal failure in septic patients with deficient ADAMTS13 (90) and/or high VWFpp levels (67, 90), irrespective of the presence or absence of DIC (54). The higher incidence of kidney damage in septic patients with low enzyme activity might be explained by the suspected, but not yet proven, restriction of ADAMTS13 on CD36-expressing cells, as mentioned above.
Considering that survival in septic shock is substantially lower in those patients with early renal failure (91), therapeutic interventions targeting UL-VWF multimers may be expected to reduce mortality.
Patients with DIC have significantly higher VWFAg and VWFpp levels and lower ADAMTS13 activity than those with related underlying diseases but without DIC. Patients with VWFpp levels >500U/dL have a significantly lower survival rate (39 vs. 61%; p<0.01) (68). The negative impact of high VWFpp on survival is also characteristic for primary TMAs. Furthermore, in DIC a low activity of ADAMTS13 strongly correlates with the severity of coagulopathy and in-hospital mortality in adults and is a predictor of poor survival (53).
Thrombotic microangiopathy in severe falciparum malaria
Microvessel obstruction by infected red cells is a crucial event in malarial coagulopathy, the extent of which is proportional to the disease severity (92). Mechanisms of microvascular compromise in malaria are complex and have been the focus of recent investigations (92-96). As in sepsis and SIRS, disease-related early endothelial cell activation with stimulated Weibel Palade exocytosis is considered a hallmark of malarial disease (94).
Parasite-induced endothelial tissue factor expression, activation of the complement cascade, impairment of coagulation inhibitors and dysfunctional fibrinolysis are contributing and intertwining mechanisms of coagulopathy in severe malaria, finally leading to DIC in 33-50% (92).
However, a common feature of patients infected with plasmodium falciparum is a drop in platelet count (60-80%) (97), which occurs already in the earliest phase of blood stage infection (even before clinical symptoms appear) and becomes progressively more severe in later stages (98).
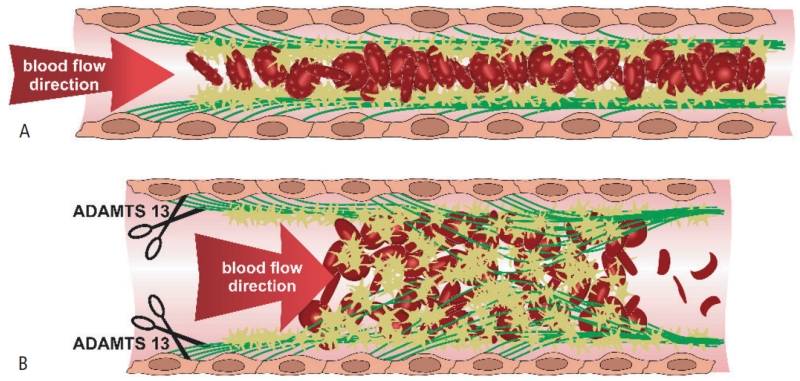
Early thrombocytopenia is not due to an incipient consumptive coagulopathy (99) but rather to the first obvious sign of incipient VWF-mediated microvessel occlusion. Acutely exocytosed UL-VWF multimers are a critical component linking plasmodium-infected erythrocytes to the endothelial surface and platelets, thereby providing the basis for microthrombus formation with early platelet consumption and ensuing microangiopathic organ damage (Figure 1). This would be in line with the observation that patients with blood group 0, who constitutively express significantly lower VWF levels, are relatively resistant to develop severe malaria (100).
Figure1. Entrapment of platelets and erythrocytes by uncleaved von Willebrand factor multimers under shear flow causing microvessel occlusion in (A) malarial infection and (B) TTP.
(A) Microvessel obstruction by infected red cells is a crucial event in malarial coagulopathy and determines disease severity. Acutely exocytosed ultra large VWF strings attached to the endothelium thereby play a critical role by spontaneously binding large numbers of platelets, which finally facilitate endothelial adhesion and sequestration of red blood cells infected with plasmodia by providing the essential CD36-expressing surfaces. (B) In TTP, strand-shaped hyper-reactive VWF-multimers with a length exceeding 0,2mm spontaneously bind, activate and aggregate platelets leading to widespread depositions of microvessel-occlusive platelet-rich clots. Red blood cells passing through this UL-VWF-platelet meshwork get fragmented into schistocytes and helmet cells. Detaching and cleaving of UL-VWF strings through ADAMTS13 replenishment may interrupt microthrombus formation, re-establish vessel patency and improve organ function. Freely adapted from José A. López (101).
However, since infected red blood cells (RBCs) cannot directly bind to the vascular surface without appropriate receptors, the exact mechanism underlying cytoadherence remained unclear until recently. Platelets have already been recognized as bridging structures between activated brain endothelial cells lacking CD36 and parasited erythrocytes. However, Bridges and colleagues have demonstrated the critical role of VWF to accomplish red cell adherence from results of a laminar flow model. Accordingly, endothelial adhesion and sequestration of infected RBC is mediated by UL-VWF strings, which are littered with platelets abounding in CD36 molecules (93, 101, 102). Platelet microparticles transferring antigens to the RBC surface also increase cytoadherence, at least to cerebral endothelial cells, and may have a triggering role (103).
This proposed microangiopathic mechanism of VWF-mediated microvascular disease in malaria resembling TTP is emphasized by studies showing that both VWFAg and VWFpp levels are markedly elevated early (94, 96), especially in severe and cerebral malaria, indicating massive endothelial perturbation. Both continuously increase with ongoing malarial infection - while platelets proportionally decrease (98) - and strongly correlate with disease severity (94, 104). Due to the short half-life of VWFpp, its levels rapidly fall with disease recovery (94), indicating the acuity of endothelial activation in malaria. These observations could also be reproduced in an experimental human malarial infection model in healthy volunteers, where VWF levels started to rise immediately after plasmodium inoculation with activated VWF reaching levels usually only found in TTP (98). The significant correlation between VWF and low platelet count was even stronger with the activated, GPIbα-binding-domain expressing form of VWF, suggesting the latter to be the important mediator of thrombocytopenia during early malaria (98).
Studies investigating ADAMTS13 activity in both uncomplicated and complicated malaria further highlight the parallels between TTP and malarial microvascular disease (96). As observed in severe sepsis and DIC, the presence of procoagulant UL-VWF multimers in malaria is accompanied by a persistently reduced ADAMTS13 level and strongly correlates with increased VWF collagen binding activity, which indicates the multimers’ hyper-reactive phenotype (95). Using recombinant ADAMTS13, malaria-associated UL-VWF strings that capture RBCs could rapidly be cleaved from the endothelium in vitro, a finding which emphasizes a disease-contributing role of enzyme deficiency in malaria. Underlying mechanisms are likely similar to those suspected in other conditions with secondary TMA. In addition to consumptive deficiency due to high substrate level, inhibition by inflammatory cytokines, acquired autoantibodies and non-immune mechanisms (microbial proteases, thrombin, plasmin, hemoglobin) including genetic polymorphisms (105) may play a role (Table 1).
To date, four clinical studies specifically investigated ADAMTS13 activity in the context of malaria and demonstrated that severe and cerebral, but not uncomplicated malaria, are associated with ADAMTS13 deficiency. However no study yet exists demonstrating a definitive impact of protease-deficiency on outcome in malaria, and it is still unclear if low ADAMTS13 levels really represent a causal pathology mediating malarial disease or rather constitute an epiphenomenal consequence; also, the cut-off value of ADAMTS13 activity which is predictive of a complicated course of disease remains to be defined. More research is required to identify a causal relationship between reduced ADAMTS13 activity and mortality.
Potential future therapeutic approaches in secondary TMAs
Inhibition of VWF release
Anti-inflammatory agents such as steroids (106) or TNFα-inhibitors (107) block the release of acute phase reactants including VWF. However, their usefulness in sepsis related TMA has not been established, given that the evidence-level of recommendation regarding the use of steroids in sepsis has been downgraded by the Surviving Sepsis Campaign already in 2008 in view of inconsistent trial results.
Inhibition of VWF activity
The VWF-GPIb interaction is thought to be „un-druggable“ by conventional small molecule medicinal chemistry, since the area of contact between the A1 domain and the GPIb receptor is considered far too extensive for a small molecular weight antagonist to block (108). However, several small molecule inhibitors have been investigated so far.
Anti-VWF aptamers
Aptamers are oligonucleotides with drug-like properties that share some of the attributes of monoclonal antibodies, and were designed to block VWF-platelet binding in patients with TTP (29). Two aptamers have been investigated in more detail to date. The aptamer ARC1779 (32-40) is a first generation, nuclease resistant aptamer, binding to the activated VWF A1-domain with high potency and specificity. It has, however, a rapid elimination half-life and low subcutaneous bioavailability, which properties limit its therapeutic potential. ARC15105 (41) is a chemically advanced follower with a higher affinity to VWF and better pharmacokinetic profile than ARC1779, but it was never advanced into clinical development.
Anti-VWF nanobody
ALX-0081 (INN: caplacizumab) is a bivalent, and therefore highly potent humanized nanobody specifically targeting the GPIb binding site of VWF. It directly inhibits platelet binding to UL-VWF multimers.
In phase I studies ALX-0081 proved to be a potent and safe inhibitor of VWF mediated platelet aggregation over a wide range of doses, even when administered in combination with aspirin, clopidogrel and heparin. The international Phase II TITAN trial, a randomized controlled trial including 75 patients with acquired TTP was recently completed and yielded promising results. Caplacizumab significantly decreased time to platelet count normalization by 39%, with finally 81% of patients achieving complete remission. The treatment was generally well tolerated and associated with fewer exacerbations as compared to placebo, with a manageable increase in bleeding tendency (44).
VWF/GPIb inhibitor
Agkisacucetin (Anfibatide), a non-enzymatic lectin-like protein purified from snake venom, is a first-in-class antagonist of the platelet GP1b receptor, which mediates binding of platelets to VWF at high shear flow. Considered as a novel antithrombotic agent, Anfibatide has been tested in vitro and in vivo, where it showed good antithrombotic effects without increasing the risk of bleedings in a phase I study in healthy volunteers (109). Anfibatide is currently evaluated in a randomized multi-center phase II trial on patients with acute coronary syndrome [ClinicalTrials.gov NCT01585259] (110).
ADAMTS13 replacement
Plasma exchange
Case series and observational studies suggest that plasma exchange may be an effective adjunct therapy and may contribute to a better outcome in patients with severe sepsis and septic shock (111-114). In one case of TMA complicating septic DIC treatment with plasma exchange resulted in an improvement of TMA-related laboratory abnormalities along with a marked increase in consciousness. Nguyen et al. also reported beneficial effects of plasma exchange in critically ill children with secondary multiple organ failure-associated ADAMTS13 deficiency (115).
However, although plasma exchange is the gold standard of care in TTP, its value in the therapy of septic patients and its effectiveness in sepsis-related TMA remain to be established.
Recombinant ADAMTS13 (rADAMTS13) and autoantibody-resistant ADAMTS13 variants
The administration of recombinant human ADAMTS13 completely prevented the emergence of thrombotic microangiopathy in ADAMTS13-deficient mice challenged with UL-VWF multimers (27). Accordingly, Plaimauer and colleagues found a linear relation between the inhibitor titer and the effective concentration of rADAMTS13 required for the reconstitution of ADAMTS13 activity in the presence of neutralizing autoantibodies (28). However, the usefulness of rADAMTS13 in acquired TTP may be limited by an overwhelming amount of autoantibodies counteracting its effects. This drawback might be overcome by using gain-of-function ADAMTS13 variants, generated by site-directed mutagenesis, with enhanced cleaving activity and increased resistance to binding and subsequent inhibition by autoantibodies (31).
Given that ADAMTS13 deficiency is considered multifactorial in secondary TMAs, where it is at least partly caused by exhaustion and reduced synthesis, enzyme replacing strategies may have some therapeutic value there.
Complement inhibitor
Though the contributing role of the complement cascade regarding the pathogenesis and disease severity in TTP remains unknown, it is well conceivable that complement-targeting treatments could be of some benefit in selected patients with secondary TMA. Eculizumab, a terminal complement inhibitor, significantly improved renal function in patients with atypical haemolytic–uremic syndrome (116), the prototype of complement-mediated TMA, and has also demonstrated therapeutic efficacy in a case of TTP recalcitrant to daily plasma exchange therapy (117). One advantage of eculizumab in the treatment of septic TMA may be its high specificity to the complement component 5 (C5) resulting in preserved functions of the up-stream complement system. Considering the importance of humoral host defence in sepsis, eculizumab may accomplish both a sufficient suppression of excessive complement activation and the maintenance of essential immunologic function in sepsis related TMA. However, further studies on the prevalence and implications of complement defects in secondary TMAs are needed.
Conclusion
ADAMTS13 proteolytically controls and prevents the occurrence of microvascular obstruction by VWF-platelet complexes under physiologic conditions. Absent or severely reduced enzyme activity causes the accumulation of ULVWF multimers, giant molecules with high spontaneous platelet-activating capacity. The longer the VWF multimers the higher is their procoagulant potential.
Recent evidence suggests that a pathomechanism formerly considered unique to TTP may take place also in other thrombotic and inflammatory diseases where it contributes to mortality. Whether these findings can finally be translated into therapeutic practice needs further investigation. To date, it is unclear whether VWF-directed therapy in patients with severely reduced ADAMTS13 levels or a high VWFpp/ADAMTS13 ratio may improve or even prevent sepsis/DIC related organ failure. VWF-targeted and enzyme replacement therapies would possibly help to eliminate microvascular occlusion and restore vital organ function. Since the use of recombinant ADAMTS13 significantly reduces the number of UL-VWF strings in vitro, prospective clinical trials of the effectiveness of protease replacement in vivo seem reasonable. It may be that carefully selected patients with secondary TMA could benefit from therapeutic approaches primarily intended for the use in TTP.
Acknowledgement
The authors thank Dr. Jim Gilbert for critical revision of the manuscript.
This study was supported by a grant from the Austrian Science Fund (FWF SFB-54).
Footnotes
Conflict of interest disclosures:
Dr. Bernd Jilma has served as the head of the data safety monitoring board of the TITAN trial [ALX-0081 (INN: caplacizumab)] sponsored by Ablynx and has been an investigator in TTP trials [ARC1779] sponsored by Archemix and a trial of ADAMTS13 in congenital TTP.
Dr. Michael Schwameis has no conflicts of interest or financial ties to disclose.
Dr. Christian Schörgenhofer has no conflicts of interest or financial ties to disclose.
Dr. Alice Assinger has no conflicts of interest or financial ties to disclose.
Cand. med. Margarete Maria Steiner has no conflicts of interest or financial ties to disclose.
To the best of our knowledge, no other direct or indirect conflicts of interest or financial relationships with industry (through investments, employment, consultancies, stock ownership, honoraria) exist.
REFERENCES
- 1.Ruggeri ZM, Ware J. von Willebrand factor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1993;7(2):308–16. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- 2.Lenting PJ, Casari C, Christophe OD, et al. von Willebrand factor: the old, the new and the unknown. Journal of thrombosis and haemostasis : JTH. 2012;10(12):2428–37. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 3.Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(3):883–91. doi: 10.1161/01.atv.20.3.883. [DOI] [PubMed] [Google Scholar]
- 4.Ribes JA, Francis CW, Wagner DD. Fibrin induces release of von Willebrand factor from endothelial cells. The Journal of clinical investigation. 1987;79(1):117–23. doi: 10.1172/JCI112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardo A, Ball C, Nolasco L, et al. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(1):100–6. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. The Journal of clinical investigation. 1987;79(2):600–8. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jilma B, Pernerstorfer T, Dirnberger E, et al. Effects of histamine and nitric oxide synthase inhibition on plasma levels of von Willebrand factor antigen. The Journal of laboratory and clinical medicine. 1998;131(2):151–6. doi: 10.1016/s0022-2143(98)90157-3. [DOI] [PubMed] [Google Scholar]
- 8.Dong JF. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. Journal of thrombosis and haemostasis : JTH. 2005;3(8):1710–6. doi: 10.1111/j.1538-7836.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- 9.Dong JF, Moake JL, Bernardo A, et al. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. The Journal of biological chemistry. 2003;278(32):29633–9. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 10.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(2):270–7. doi: 10.1182/blood-2012-07-442285. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Krishnaswamy S, Camire RM, et al. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(21):7416–21. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng XL. Structure-function and regulation of ADAMTS-13 protease. Journal of thrombosis and haemostasis : JTH. 2013;11(Suppl 1):11–23. doi: 10.1111/jth.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. The New England journal of medicine. 1998;339(22):1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 15.Huck V, Schneider MF, Gorzelanny C, et al. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thrombosis and haemostasis. 2014;111(4):598–609. doi: 10.1160/TH13-09-0800. [DOI] [PubMed] [Google Scholar]
- 16.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Li CQ, Moake JL, et al. Shear stress-induced binding of large and unusually large von Willebrand factor to human platelet glycoprotein Ibalpha. Annals of biomedical engineering. 2004;32(7):961–9. doi: 10.1023/b:abme.0000032458.88212.54. [DOI] [PubMed] [Google Scholar]
- 18.Burns ER, Lou Y, Pathak A. Morphologic diagnosis of thrombotic thrombocytopenic purpura. American journal of hematology. 2004;75(1):18–21. doi: 10.1002/ajh.10450. [DOI] [PubMed] [Google Scholar]
- 19.Davis AK, Makar RS, Stowell CP, et al. ADAMTS13 binds to CD36: a potential mechanism for platelet and endothelial localization of ADAMTS13. Transfusion. 2009;49(2):206–13. doi: 10.1111/j.1537-2995.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 20.Grassle S, Huck V, Pappelbaum KI, et al. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(7):1382–9. doi: 10.1161/ATVBAHA.113.303016. [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Nolasco L, Nolasco J, et al. Thrombotic microangiopathies and the linkage between von Willebrand factor and the alternative complement pathway. Seminars in thrombosis and hemostasis. 2014;40(5):544–50. doi: 10.1055/s-0034-1383547. [DOI] [PubMed] [Google Scholar]
- 22.Turner NA, Moake J. Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasis-thrombosis. PloS one. 2013;8(3):e59372. doi: 10.1371/journal.pone.0059372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reti M, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. Journal of thrombosis and haemostasis : JTH. 2012;10(5):791–8. doi: 10.1111/j.1538-7836.2012.04674.x. [DOI] [PubMed] [Google Scholar]
- 24.Westwood JP, Langley K, Heelas E, et al. Complement and cytokine response in acute Thrombotic Thrombocytopenic Purpura. British journal of haematology. 2014;164(6):858–66. doi: 10.1111/bjh.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock GA, Shumak KH, Buskard NA, et al. Canadian Apheresis Study Group Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. The New England journal of medicine. 1991;325(6):393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 26.von Baeyer H. Plasmapheresis in thrombotic microangiopathy-associated syndromes: review of outcome data derived from clinical trials and open studies. Therapeutic apheresis : official journal of the International Society for Apheresis and the Japanese Society for Apheresis. 2002;6(4):320–8. doi: 10.1046/j.1526-0968.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 27.Schiviz A, Wuersch K, Piskernik C, et al. A new mouse model mimicking thrombotic thrombocytopenic purpura: correction of symptoms by recombinant human ADAMTS13. Blood. 2012;119(25):6128–35. doi: 10.1182/blood-2011-09-380535. [DOI] [PubMed] [Google Scholar]
- 28.Plaimauer B, Kremer Hovinga JA, Juno C, et al. Recombinant ADAMTS13 normalizes von Willebrand factor-cleaving activity in plasma of acquired TTP patients by overriding inhibitory antibodies. Journal of thrombosis and haemostasis : JTH. 2011;9(5):936–44. doi: 10.1111/j.1538-7836.2011.04224.x. [DOI] [PubMed] [Google Scholar]
- 29.Firbas C, Siller-Matula JM, Jilma B. Targeting von Willebrand factor and platelet glycoprotein Ib receptor. Expert review of cardiovascular therapy. 2010;8(12):1689–701. doi: 10.1586/erc.10.154. [DOI] [PubMed] [Google Scholar]
- 30.Knobl P. Inherited and Acquired Thrombotic Thrombocytopenic Purpura (TTP) in Adults. Seminars in thrombosis and hemostasis. 2014;40(4):493–502. doi: 10.1055/s-0034-1376883. [DOI] [PubMed] [Google Scholar]
- 31.Jian C, Xiao J, Gong L, et al. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119(16):3836–43. doi: 10.1182/blood-2011-12-399501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayr FB, Knobl P, Jilma B, et al. The aptamer ARC1779 blocks von Willebrand factor-dependent platelet function in patients with thrombotic thrombocytopenic purpura ex vivo. Transfusion. 2010;50(5):1079–87. doi: 10.1111/j.1537-2995.2009.02554.x. [DOI] [PubMed] [Google Scholar]
- 33.Knobl P, Jilma B, Gilbert JC, et al. Anti-von Willebrand factor aptamer ARC1779 for refractory thrombotic thrombocytopenic purpura. Transfusion. 2009;49(10):2181–5. doi: 10.1111/j.1537-2995.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 34.Jilma-Stohlawetz P, Gorczyca ME, Jilma B, et al. Inhibition of von Willebrand factor by ARC1779 in patients with acute thrombotic thrombocytopenic purpura. Thrombosis and haemostasis. 2011;105(3):545–52. doi: 10.1160/TH10-08-0520. [DOI] [PubMed] [Google Scholar]
- 35.Jilma B, Paulinska P, Jilma-Stohlawetz P, et al. A randomised pilot trial of the anti-von Willebrand factor aptamer ARC1779 in patients with type 2b von Willebrand disease. Thrombosis and haemostasis. 2010;104(3):563–70. doi: 10.1160/TH10-01-0027. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation. 2007;116(23):2678–86. doi: 10.1161/CIRCULATIONAHA.107.724864. [DOI] [PubMed] [Google Scholar]
- 37.Diener JL, Daniel Lagasse HA, Duerschmied D, et al. Inhibition of von Willebrand factor-mediated platelet activation and thrombosis by the anti-von Willebrand factor A1-domain aptamer ARC1779. Journal of thrombosis and haemostasis : JTH. 2009;7(7):1155–62. doi: 10.1111/j.1538-7836.2009.03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jilma-Stohlawetz P, Gilbert JC, Gorczyca ME, et al. A dose ranging phase I/II trial of the von Willebrand factor inhibiting aptamer ARC1779 in patients with congenital thrombotic thrombocytopenic purpura. Thrombosis and haemostasis. 2011;106(3):539–47. doi: 10.1160/TH11-02-0069. [DOI] [PubMed] [Google Scholar]
- 39.Cataland SR, Peyvandi F, Mannucci PM, et al. Initial experience from a double-blind, placebo-controlled, clinical outcome study of ARC1779 in patients with thrombotic thrombocytopenic purpura. American journal of hematology. 2012;87(4):430–2. doi: 10.1002/ajh.23106. [DOI] [PubMed] [Google Scholar]
- 40.Jilma-Stohlawetz P, Knobl P, Gilbert JC, et al. The anti-von Willebrand factor aptamer ARC1779 increases von Willebrand factor levels and platelet counts in patients with type 2B von Willebrand disease. Thrombosis and haemostasis. 2012;108(2):284–90. doi: 10.1160/TH11-12-0889. [DOI] [PubMed] [Google Scholar]
- 41.Siller-Matula JM, Merhi Y, Tanguay JF, et al. ARC15105 is a potent antagonist of von Willebrand factor mediated platelet activation and adhesion. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(4):902–9. doi: 10.1161/ATVBAHA.111.237529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartunek J, Barbato E, Heyndrickx G, et al. Novel antiplatelet agents: ALX-0081, a Nanobody directed towards von Willebrand factor. Journal of cardiovascular translational research. 2013;6(3):355–63. doi: 10.1007/s12265-012-9435-y. [DOI] [PubMed] [Google Scholar]
- 43.Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood. 2011;118(3):757–65. doi: 10.1182/blood-2010-11-317859. [DOI] [PubMed] [Google Scholar]
- 44.Press-release_Phase-II-results-caplacizumab. ABLYNX’S ANTI-vWF NANOBODY, CAPLACIZUMAB, ACHIEVES CLINICAL PROOF-OF-CONCEPT IN PHASE II TITAN STUDY. 2014. p. 4. [Google Scholar]
- 45.Musio F, Bohen EM, Yuan CM, et al. Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus. Seminars in arthritis and rheumatism. 1998;28(1):1–19. doi: 10.1016/s0049-0172(98)80023-1. [DOI] [PubMed] [Google Scholar]
- 46.Matsuyama T, Kuwana M, Matsumoto M, et al. Heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thrombosis and haemostasis. 2009;102(2):371–8. doi: 10.1160/TH08-12-0825. [DOI] [PubMed] [Google Scholar]
- 47.Franchini M, Montagnana M, Targher G, et al. Reduced von Willebrand factor-cleaving protease levels in secondary thrombotic microangiopathies and other diseases. Seminars in thrombosis and hemostasis. 2007;33(8):787–97. doi: 10.1055/s-2007-1000365. [DOI] [PubMed] [Google Scholar]
- 48.Dold S, Singh R, Sarwar H, et al. Frequency of microangiopathic hemolytic anemia in patients with systemic lupus erythematosus exacerbation: Distinction from thrombotic thrombocytopenic purpura, prognosis, and outcome. Arthritis and rheumatism. 2005;53(6):982–5. doi: 10.1002/art.21583. [DOI] [PubMed] [Google Scholar]
- 49.Shah AA, Higgins JP, Chakravarty EF. Thrombotic microangiopathic hemolytic anemia in a patient with SLE: diagnostic difficulties. Nature clinical practice Rheumatology. 2007;3(6):357–62. doi: 10.1038/ncprheum0511. [DOI] [PubMed] [Google Scholar]
- 50.Ono T, Mimuro J, Madoiwa S, et al. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107(2):528–34. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 51.Moore JC, Hayward CP, Warkentin TE, et al. Decreased von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood. 2001;98(6):1842–6. doi: 10.1182/blood.v98.6.1842. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen TC, Liu A, Liu L, et al. Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica. 2007;92(1):121–4. doi: 10.3324/haematol.10262. [DOI] [PubMed] [Google Scholar]
- 53.Hyun J, Kim HK, Kim JE, et al. Correlation between plasma activity of ADAMTS-13 and coagulopathy, and prognosis in disseminated intravascular coagulation. Thrombosis research. 2009;124(1):75–9. doi: 10.1016/j.thromres.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Martin K, Borgel D, Lerolle N, et al. Decreased ADAMTS-13 (A disintegrin-like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis-induced organ failure. Critical care medicine. 2007;35(10):2375–82. doi: 10.1097/01.ccm.0000284508.05247.b3. [DOI] [PubMed] [Google Scholar]
- 55.Sakamaki Y, Konishi K, Hayashi K, et al. Renal thrombotic microangiopathy in a patient with septic disseminated intravascular coagulation. BMC nephrology. 2013;14:260. doi: 10.1186/1471-2369-14-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cserti CM, Landaw S, Uhl L. Do infections provoke exacerbations and relapses of thrombotic thrombocytopenic purpura? Journal of clinical apheresis. 2007;22(1):21–5. doi: 10.1002/jca.20114. [DOI] [PubMed] [Google Scholar]
- 57.Swisher KK, Doan JT, Vesely SK, et al. Pancreatitis preceding acute episodes of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: report of five patients with a systematic review of published reports. Haematologica. 2007;92(7):936–43. doi: 10.3324/haematol.10963. [DOI] [PubMed] [Google Scholar]
- 58.van Mourik JA, Boertjes R, Huisveld IA, et al. von Willebrand factor propeptide in vascular disorders: A tool to distinguish between acute and chronic endothelial cell perturbation. Blood. 1999;94(1):179–85. [PubMed] [Google Scholar]
- 59.Brott DA, Katein A, Thomas H, et al. Evaluation of von Willebrand Factor and von Willebrand Factor Propeptide in Models of Vascular Endothelial Cell Activation, Perturbation, and/or Injury. Toxicologic pathology. 2014;42(4):672–83. doi: 10.1177/0192623313518664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bockmeyer CL, Claus RA, Budde U, et al. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica. 2008;93(1):137–40. doi: 10.3324/haematol.11677. [DOI] [PubMed] [Google Scholar]
- 61.Claus RA, Bockmeyer CL, Sossdorf M, et al. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Current molecular medicine. 2010;10(2):236–48. doi: 10.2174/156652410790963367. [DOI] [PubMed] [Google Scholar]
- 62.Hyseni A, Kemperman H, de Lange DW, et al. Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood. 2014;123(14):2153–6. doi: 10.1182/blood-2013-08-508093. [DOI] [PubMed] [Google Scholar]
- 63.Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thrombosis and haemostasis. 2009;101(2):239–47. [PubMed] [Google Scholar]
- 64.Karim F, Adil SN, Afaq B, et al. Deficiency of ADAMTS-13 in pediatric patients with severe sepsis and impact on in-hospital mortality. BMC pediatrics. 2013;13(1):44. doi: 10.1186/1471-2431-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bongers TN, Emonts M, de Maat MP, et al. Reduced ADAMTS13 in children with severe meningococcal sepsis is associated with severity and outcome. Thrombosis and haemostasis. 2010;103(6):1181–7. doi: 10.1160/TH09-06-0376. [DOI] [PubMed] [Google Scholar]
- 66.Lerolle N, Dunois-Larde C, Badirou I, et al. von Willebrand factor is a major determinant of ADAMTS-13 decrease during mouse sepsis induced by cecum ligation and puncture. Journal of thrombosis and haemostasis : JTH. 2009;7(5):843–50. doi: 10.1111/j.1538-7836.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 67.Kremer Hovinga JA, Zeerleder S, Kessler P, et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. Journal of thrombosis and haemostasis : JTH. 2007;5(11):2284–90. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 68.Habe K, Wada H, Ito-Habe N, et al. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thrombosis research. 2012;129(5):598–602. doi: 10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 69.Reiter RA, Knobl P, Varadi K, et al. Changes in von Willebrand factor-cleaving protease (ADAMTS13) activity after infusion of desmopressin. Blood. 2003;101(3):946–8. doi: 10.1182/blood-2002-03-0814. [DOI] [PubMed] [Google Scholar]
- 70.Lancellotti S, De Filippis V, Pozzi N, et al. Oxidized von Willebrand factor is efficiently cleaved by serine proteases from primary granules of leukocytes: divergence from ADAMTS-13. Journal of thrombosis and haemostasis : JTH. 2011;9(8):1620–7. doi: 10.1111/j.1538-7836.2011.04367.x. [DOI] [PubMed] [Google Scholar]
- 71.Mikes B, Sinkovits G, Farkas P, et al. Elevated plasma neutrophil elastase concentration is associated with disease activity in patients with thrombotic thrombocytopenic purpura. Thrombosis research. 2014;133(4):616–21. doi: 10.1016/j.thromres.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 72.Zheng GJ, Wu ZX, Zhu HY, et al. The effects of activated protein C on the von Willebrand factor and von Willebrand factor cleaving protease of rat aortic endothelial cell induced by lipopolysaccharide. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2012;24(8):487–9. [PubMed] [Google Scholar]
- 73.Mimuro J, Niimura M, Kashiwakura Y, et al. Unbalanced expression of ADAMTS13 and von Willebrand factor in mouse endotoxinemia. Thrombosis research. 2008;122(1):91–7. doi: 10.1016/j.thromres.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Vischer UM, Ingerslev J, Wollheim CB, et al. Acute von Willebrand factor secretion from the endothelium in vivo: assessment through plasma propeptide (vWf:AgII) Levels. Thrombosis and haemostasis. 1997;77(2):387–93. [PubMed] [Google Scholar]
- 75.Pinsky MR, Vincent JL, Deviere J, et al. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103(2):565–75. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 76.Peigne V, Azoulay E, Coquet I, et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin-6 and is not dependent on disseminated intravascular coagulation. Critical care. 2013;17(6):R273. doi: 10.1186/cc13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuchs TA, Kremer Hovinga JA, Schatzberg D, et al. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120(6):1157–64. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lancellotti S, De Filippis V, Pozzi N, et al. Formation of methionine sulfoxide by peroxynitrite at position 1606 of von Willebrand factor inhibits its cleavage by ADAMTS-13: A new prothrombotic mechanism in diseases associated with oxidative stress. Free radical biology & medicine. 2010;48(3):446–56. doi: 10.1016/j.freeradbiomed.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Fu X, Wang Y, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115(3):706–12. doi: 10.1182/blood-2009-03-213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung MC, Popova TG, Jorgensen SC, et al. Degradation of circulating von Willebrand factor and its regulator ADAMTS13 implicates secreted Bacillus anthracis metalloproteases in anthrax consumptive coagulopathy. The Journal of biological chemistry. 2008;283(15):9531–42. doi: 10.1074/jbc.M705871200. [DOI] [PubMed] [Google Scholar]
- 81.Song J, Hu D, He C, et al. Novel biomarkers for early prediction of sepsis-induced disseminated intravascular coagulation in a mouse cecal ligation and puncture model. Journal of inflammation. 2013;10(1):7. doi: 10.1186/1476-9255-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMaken S, Exline MC, Mehta P, et al. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PloS one. 2011;6(5):e19654. doi: 10.1371/journal.pone.0019654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie L, Chesterman CN, Hogg PJ. Control of von Willebrand factor multimer size by thrombospondin-1. The Journal of experimental medicine. 2001;193(12):1341–9. doi: 10.1084/jem.193.12.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107(3):955–64. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators of inflammation. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayakawa M, Sawamura A, Gando S, et al. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thrombosis research. 2012;130(6):906–13. doi: 10.1016/j.thromres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Gando S, Hayakawa M, Sawamura A, et al. The activation of neutrophil elastase-mediated fibrinolysis is not sufficient to overcome the fibrinolytic shutdown of disseminated intravascular coagulation associated with systemic inflammation. Thrombosis research. 2007;121(1):67–73. doi: 10.1016/j.thromres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Tersteeg C, de Maat S, De Meyer SF, et al. Plasmin cleavage of von Willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation. 2014;129(12):1320–31. doi: 10.1161/CIRCULATIONAHA.113.006727. [DOI] [PubMed] [Google Scholar]
- 89.Taylor FB, Jr., Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thrombosis and haemostasis. 2001;86(5):1327–30. [PubMed] [Google Scholar]
- 90.Fukushima H, Nishio K, Asai H, et al. Ratio of von Willebrand factor propeptide to ADAMTS13 is associated with severity of sepsis. Shock. 2013;39(5):409–14. doi: 10.1097/SHK.0b013e3182908ea7. [DOI] [PubMed] [Google Scholar]
- 91.Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive care medicine. 2009;35(5):871–81. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 92.Angchaisuksiri P. Coagulopathy in malaria. Thrombosis research. 2014;133(1):5–9. doi: 10.1016/j.thromres.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Bridges DJ, Bunn J, van Mourik JA, et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood. 2010;115(7):1472–4. doi: 10.1182/blood-2009-07-235150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollestelle MJ, Donkor C, Mantey EA, et al. von Willebrand factor propeptide in malaria: evidence of acute endothelial cell activation. British journal of haematology. 2006;133(5):562–9. doi: 10.1111/j.1365-2141.2006.06067.x. [DOI] [PubMed] [Google Scholar]
- 95.Larkin D, de Laat B, Jenkins PV, et al. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS pathogens. 2009;5(3):e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lowenberg EC, Charunwatthana P, Cohen S, et al. Severe malaria is associated with a deficiency of von Willebrand factor cleaving protease, ADAMTS13. Thrombosis and haemostasis. 2010;103(1):181–7. doi: 10.1160/TH09-04-0223. [DOI] [PubMed] [Google Scholar]
- 97.Srichaikul T. Hemostatic alterations in malaria. The Southeast Asian journal of tropical medicine and public health. 1993;24(Suppl 1):86–91. [PubMed] [Google Scholar]
- 98.de Mast Q, Groot E, Lenting PJ, et al. Thrombocytopenia and release of activated von Willebrand Factor during early Plasmodium falciparum malaria. The Journal of infectious diseases. 2007;196(4):622–8. doi: 10.1086/519844. [DOI] [PubMed] [Google Scholar]
- 99.de Mast Q, de Groot PG, van Heerde WL, et al. Thrombocytopenia in early malaria is associated with GP1b shedding in absence of systemic platelet activation and consumptive coagulopathy. British journal of haematology. 2010;151(5):495–503. doi: 10.1111/j.1365-2141.2010.08399.x. [DOI] [PubMed] [Google Scholar]
- 100.Fischer PR, Boone P. Short report: severe malaria associated with blood group. The American journal of tropical medicine and hygiene. 1998;58(1):122–3. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- 101.Lopez JA. Malignant malaria and microangiopathies: merging mechanisms. Blood. 2010;115(7):1317–8. doi: 10.1182/blood-2009-12-254060. [DOI] [PubMed] [Google Scholar]
- 102.Wassmer SC, Lepolard C, Traore B, et al. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. The Journal of infectious diseases. 2004;189(2):180–9. doi: 10.1086/380761. [DOI] [PubMed] [Google Scholar]
- 103.Faille D, Combes V, Mitchell AJ, et al. Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23(10):3449–58. doi: 10.1096/fj.09-135822. [DOI] [PubMed] [Google Scholar]
- 104.Phiri HT, Bridges DJ, Glover SJ, et al. Elevated plasma von Willebrand factor and propeptide levels in Malawian children with malaria. PloS one. 2011;6(11):e25626. doi: 10.1371/journal.pone.0025626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kraisin S, Naka I, Patarapotikul J, et al. Association of ADAMTS13 polymorphism with cerebral malaria. Malaria journal. 2011;10:366. doi: 10.1186/1475-2875-10-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Kruif MD, Lemaire LC, Giebelen IA, et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. Journal of immunology. 2007;178(3):1845–51. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 107.DeLa Cadena RA, Majluf-Cruz A, Stadnicki A, et al. Recombinant tumor necrosis factor receptor p75 fusion protein (TNFR:Fc) alters endotoxin-induced activation of the kinin, fibrinolytic, and coagulation systems in normal humans. Thrombosis and haemostasis. 1998;80(1):114–8. [PubMed] [Google Scholar]
- 108.Huizinga EG, Tsuji S, Romijn RA, et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science. 2002;297(5584):1176–9. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 109.Lei X, Reheman A, Hou Y, et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thrombosis and haemostasis. 2014;111(2):279–89. doi: 10.1160/TH13-06-0490. [DOI] [PubMed] [Google Scholar]
- 110. http://clinicaltrials.gov/show/NCT01585259. Available at: http://clinicaltrials.gov/show/NCT01585259.
- 111.Soltysiak J, Bartkowska-Sniatkowska A, Rosada-Kurasinska J, et al. Efficacy of plasma exchange in septic shock: a case report. Anaesthesiology intensive therapy. 2014;46(2):92–5. doi: 10.5603/AIT.2014.0018. [DOI] [PubMed] [Google Scholar]
- 112.De Simone N, Racsa L, Bevan S, et al. Therapeutic plasma exchange in the management of sepsis and multiple organ dysfunction syndrome: A report of three cases. Journal of clinical apheresis. 2014;29(2):127–31. doi: 10.1002/jca.21296. [DOI] [PubMed] [Google Scholar]
- 113.Qu L, Kiss JE, Dargo G, et al. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. Journal of clinical apheresis. 2011;26(4):208–13. doi: 10.1002/jca.20296. [DOI] [PubMed] [Google Scholar]
- 114.Hadem J, Hafer C, Schneider AS, et al. Therapeutic plasma exchange as rescue therapy in severe sepsis and septic shock: retrospective observational single-centre study of 23 patients. BMC anesthesiology. 2014;14(1):24. doi: 10.1186/1471-2253-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Critical care medicine. 2008;36(10):2878–87. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Legendre CM, Licht C, Loirat C. Eculizumab in atypical hemolytic-uremic syndrome. The New England journal of medicine. 2013;369(14):1379–80. doi: 10.1056/NEJMc1308826. [DOI] [PubMed] [Google Scholar]
- 117.Tsai E, Chapin J, Laurence JC, et al. Use of eculizumab in the treatment of a case of refractory, ADAMTS13-deficient thrombotic thrombocytopenic purpura: additional data and clinical follow-up. British journal of haematology. 2013;162(4):558–9. doi: 10.1111/bjh.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rock G, Kelton JG, Shumak KH, et al. Canadian Apheresis Group Laboratory abnormalities in thrombotic thrombocytopenic purpura. British journal of haematology. 1998;103(4):1031–6. doi: 10.1046/j.1365-2141.1998.01080.x. [DOI] [PubMed] [Google Scholar]
- 119.George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060–9. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 120.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15(2):81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garba IH, Ubom GA. Total serum lactate dehydrogenase activity in acute Plasmodium falciparum malaria infection. Singapore medical journal. 2005;46(11):632–4. [PubMed] [Google Scholar]
- 122.Roberts DJ, Casals-Pascual C, Weatherall DJ. The clinical and pathophysiological features of malarial anaemia. Current topics in microbiology and immunology. 2005;295:137–67. doi: 10.1007/3-540-29088-5_6. [DOI] [PubMed] [Google Scholar]
- 123.Fendel R, Brandts C, Rudat A, et al. Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PloS one. 2010;5(4):e10038. doi: 10.1371/journal.pone.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bottieau E, Clerinx J, Van den Enden E, et al. Fever after a stay in the tropics: diagnostic predictors of the leading tropical conditions. Medicine. 2007;86(1):18–25. doi: 10.1097/MD.0b013e3180305c48. [DOI] [PubMed] [Google Scholar]
- 125.Maroushek SR, Aguilar EF, Stauffer W, et al. Malaria among refugee children at arrival in the United States. The Pediatric infectious disease journal. 2005;24(5):450–2. doi: 10.1097/01.inf.0000160948.22407.0d. [DOI] [PubMed] [Google Scholar]
- 126.Studt JD, Kremer Hovinga JA, Antoine G, et al. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: in vitro inhibition of ADAMTS13 activity by hemoglobin. Blood. 2005;105(2):542–4. doi: 10.1182/blood-2004-06-2096. [DOI] [PubMed] [Google Scholar]
- 127.Zhou Z, Han H, Cruz MA, et al. Haemoglobin blocks von Willebrand factor proteolysis by ADAMTS-13: a mechanism associated with sickle cell disease. Thrombosis and haemostasis. 2009;101(6):1070–7. [PubMed] [Google Scholar]
- 128.Galbusera M, Ruggenenti P, Noris M, et al. alpha 1-Antitrypsin therapy in a case of thrombotic thrombocytopenic purpura. Lancet. 1995;345(8944):224–5. doi: 10.1016/s0140-6736(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 129.Crawley JT, Lam JK, Rance JB, et al. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105(3):1085–93. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 130.Scheiflinger F, Knobl P, Trattner B, et al. Nonneutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood. 2003;102(9):3241–3. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- 131.Ragni MV, Novelli EM, Murshed A, et al. Phase II prospective open-label trial of recombinant interleukin-11 in desmopressin-unresponsive von Willebrand disease and mild or moderate haemophilia A. Thrombosis and haemostasis. 2013;109(2):248–54. doi: 10.1160/TH12-06-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jilma B, Blann A, Pernerstorfer T, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. American journal of respiratory and critical care medicine. 1999;159(3):857–63. doi: 10.1164/ajrccm.159.3.9805087. [DOI] [PubMed] [Google Scholar]
- 133.de Groot PG, Gonsalves MD, Loesberg C, et al. Thrombin-induced release of von Willebrand factor from endothelial cells is mediated by phospholipid methylation. Prostacyclin synthesis is independent of phospholipid methylation. The Journal of biological chemistry. 1984;259(21):13329–33. [PubMed] [Google Scholar]
- 134.Rickles FR, Hoyer LW, Rick ME, et al. The effects of epinephrine infusion in patients with von Willebrand’s disease. The Journal of clinical investigation. 1976;57(6):1618–25. doi: 10.1172/JCI108432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vischer UM, Jornot L, Wollheim CB, et al. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995;85(11):3164–72. [PubMed] [Google Scholar]
- 136.Brown SA, Bowen DJ, Hallett MB, et al. Factor VIIa induced release of von Willebrand factor from human umbilical vein endothelial cells by a tyrosine kinase dependent pathway. Thrombosis and haemostasis. 2002;87(6):1057–61. [PubMed] [Google Scholar]