Abstract
Renal lymphangiectasia is a disorder of the lymphatic system of the kidneys, which can be congenital or acquired. Although the exact etiology remains unknown, an obstructive process resulting from several causes, including infection, inflammation or malignant infiltration, has been suggested to be responsible for the acquired form. This disorder may be associated with several pathologies. We report a case of a 24-year-old man with renal lymphangiectasia presenting with polycythemia, ascites and pleural effusion associated with hepatitis C virus (HCV) infection in an intravenous (IV) drug user. Our case is the first in the literature that shows an association between HCV infection and IV drug use.
Keywords: Pleural effusion, Lymphangiectasia, Kidney
INTRODUCTION
Renal lymphangiectasia is a rare benign etiology consisting of bilateral nephromegaly associated with perinephric and/or retroperitoneal fluid collection, ascites, and rarely, pleural effusion. It is also called renal lymphangiomatosis, renal lymphangioma, peripelvic lymphangiectasia and peripelvic multicystic lymphangiectasia. The preferred nomenclature is renal lymphangiectasia, which describes ectatic lymphatic vessels in renal parenchyma and perirenal and peripelvic regions (1). The exact etiology remains unknown. The neonatal form, essentially congenital, has also been described alongside the acquired type. It may be encountered at any age equally in men and women (2,3). A familial association has been reported in some cases (4).
The most common hypothesis for the etiology of lymphoceles is the occlusion of draining lymphatic ducts secondary to trauma, scarring, infection, inflammation or malignant cells (4,2), all of which usually result in the events that lead to perinephric renal cyst formation and/or fluid collection around the kidneys. Progression during pregnancy has also been noted (1,3), and a total of 43 cases were reported between 1890 and 1993 (5). Only 13 cases have been described since 1993 in the literature. Two of them had pleural effusion (2,6) and two others had polycythemia (2,7). We present a case of renal lymphangiectasia with polycythemia and pleural effusion associated with HCV infection in a male IV drug user.
CASE SUMMARIES
A 24-year-old man presented with abdominal swelling and dyspnea on exertion. His symptoms first appeared one month earlier. He was an intravenous (IV) heroin user and a 10-pack/year smoker. There was no other medical or surgical history and other family members were healthy. He had no recent weight loss, anorexia, abdominal pain, jaundice or edema.
Physical examination showed a blood pressure, respiratory rate, body temperature, heart rate and SaO2 of 120/80 mmHg, 15 breaths/minute, 37°C, 82 bpm and 96%, respectively. The only abnormalities recorded were a reduction in the intensity of breath sounds on auscultation, mild localized abdominal swelling and scrotal edema. Lymphadenopathy was absent, heart sounds were regular, and a focal swelling was palpable in the left lower quadrant of the abdomen. Abdominal ultrasonography revealed moderate ascites and enlarged kidneys (right kidney: 136×65 mm, left kidney: 134×61 mm) with increased corticomedullary differentiation. Multiple hypoechogenic foci in the cortex of the kidneys were observed. Computed tomography (CT) of the lung revealed left-sided pleural effusion (Figure 1A), and the abdominopelvic CT with intravenous and oral contrasts revealed ascites, fluid in the perinephric, retroperitoneal and extraperitoneal pelvis and presacral areas and soft tissue edema of the anterior lower abdominal wall (Figure 2). In addition, bilateral enlarged kidneys were noted (approx. 150×70 mm each) with prominent medulla and low attenuated pyramids, along with the thickening of the posterior perirenal fascia. The two-dimensional transthoracic echocardiography was normal.
Figure 1.
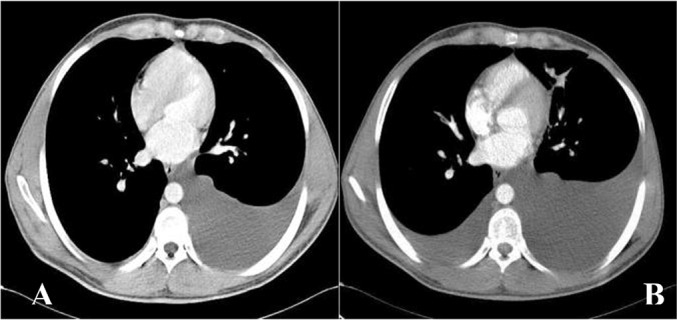
(A) Chest computed tomography on admission revealed left-sided pleural effusion. (B) Bilateral pleural effusion three months later
Figure 2.
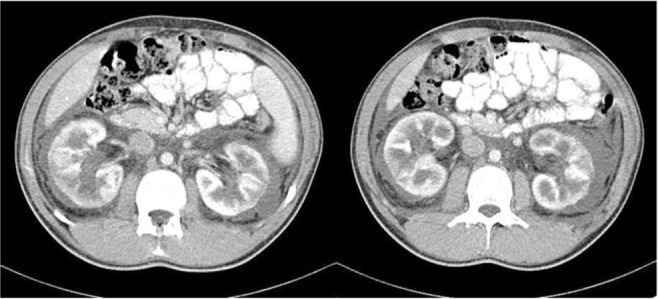
Abdominal computed tomography with intravenous and oral contrasts revealed bilateral perinephric fluid.
Abnormal laboratory tests included a hemoglobin of 19.7g/dL [197 g/L], hematocrit of 55.4%, alanine transaminase (ALT) of 105 IU/L [1.78 μkat/L], and erythrocyte sedimentation rate (ESR) and quantitative C reactive protein (CRP) of 2 mm/h [mm/h] and 3 mg/L [mg/L], respectively. Urea and serum creatinine levels were within the normal ranges. The 24-h urine collection demonstrated neither proteinuria nor hematuria, with normal levels of the complement components and a normal serum protein electrophoresis. Immunologic markers including the anti-nuclear antibody (ANA), rheumatoid factor (RF) and anti-neutrophil cytoplasmic antibody (ANCA) were normal. The diagnosis of HCV infection was confirmed by reactive serum HCV Ab followed by positivity of the reverse transcriptase polymerase chain reaction (RT-PCR) for HCV genotype 1. The other viral markers for the diagnosis of hepatitis B and human immunodeficiency virus (HIV) were negative. Other hematologic and biochemical tests were within their normal ranges.
In order to assess the nature of the excess free fluid (pleural and perinephric) and polycythemia, samples were obtained by puncture, and the level of serum erythropoietin was analyzed. Cytology of the fluids was negative for malignancy and unremarkable for Gram stain and cultures. Plasma erythropoietin level was 18.6 IU/L [(normal range: 3.22–31.9) mIU/mL] ( Table 1 ). The arterial blood gas showed a pH of 7.37, PaO2 of 90.6 mmHg and SaO2 of 96.4%.
Table 1.
Analysis of body fluids
| Pleural fluid | Perinephric fluid | Blood | |
|---|---|---|---|
| Appearance | Colorless, Non turbid | Colorless, Non turbid | - |
| Red blood cell (cells/μL) [/μL] | 2580 | 3330 | - |
| White blood cell (cells/μL) [×109/L] | 110 [0.11] | 280 [0.28] | - |
| PMN (%) | 3 | 4 | - |
| Lymph. (%) | 97 | 96 | - |
| Sugar (mg/dl) | 108 [5.99] | 106 [5.88] | 81 [4.50] |
| Protein (g/dl) [g/L] | <2 [<20] | <2 [<20] | 8 [80] |
| LDH (U/L) [μkat/L] | 31 [53] | 71 [1.2] | 440 [7.5] |
| ADA (U/L) nkat/L | 15 [250] | - | - |
| Creatinine (mg/dL) [μmol/L] | 1.2 [106] | - | 1.3 [114] |
| Amylase (U/L) [μkat/L] | - | 17 [0.28] | 86 [1.43] |
| Triglyceride (mg/dL) [mmol/L] | - | 4 [0.04] | - |
| Cholesterol (mg/dL) [mmol/L] | - | 5 [0.1295] | - |
On the basis of clinical, imaging and laboratory investigations, the diagnosis of renal lymphangiectasia was made. The treatment plan included initiation of diuretic treatment in order to prevent fluid sequestration in the third space. However, the risk of thrombosis due to associated severe polycythemia deterred us from using minimal doses of diuretics and the patient was discharged with close follow-ups and conservative management. Three months later, 800 mL of pleural fluid was drained secondary to symptoms such as increased dyspnea (Figure 1B).
DISCUSSION
Fluid collection around the kidneys may occur under various conditions in relation to different etiologies. The nature of fluid may be urine, blood, pus, lymph or plasma. Computed tomography is the diagnostic tool of choice for detecting such collections but it cannot demonstrate the nature of the fluid (3). Renal lymphangiectasia is regarded as one of the rare etiologies for which CT is characteristic, showing the typical dilation of renal, perirenal and peripelvic lymphatic ducts (8,9). The etiology of this disorder remains unknown, but a common hypothesis concerning the acquired type is the occlusion of the lymphatic ducts by infection, inflammation, malignant processes, etc. (4). In more than 90% of the cases, it is usually bilateral in nature (2), yet it may be unilateral, focal or diffuse (8). It may be asymptomatic or it may present with bulging or flank pain, erythrocytosis, hypertension, hematuria, proteinuria or impaired renal function (2). However, even if renal lymphangiectasia is benign in nature, it can lead to serious complications such as hypertension and renal failure (3,10). Fortunately, the renal function is usually preserved (4). The differential diagnoses for this condition are adult polycystic kidney disease, nephroblastomatosis, lymphoma and other causes of renal masses (1). Differential diagnosis is done by imaging features, characterized by fluid attenuation (0–10 HU) of the perinephric collections, which favors the hypothesis of renal lymphangiectasia (1). Definite diagnosis of renal lymphangiectasia is based on imaging findings (especially CT) characterized by presence of a uni- or multilocular cystic lesion with or without peripheral or septal enhancement (8). In the circumstance that imaging studies confirm the diagnosis, other investigations, including invasive procedures, are unnecessary (9). However, drained fluid usually reveals a chylous fluid with lymphocytic predominance (≥90%) (3), while occasionally cell staining may prove colorless at gross examination (2). Treatment is usually conservative, with diuretics or symptomatic drainage of fluid collections. Occasionally, in rare cases, marsupialization or nephrectomy is necessary (2). Some associations have been reported with renal lymphangiectasia, such as monoclonal gammopathy (2), chronic myeloid leukemia (4), renal venous thrombosis and hypertension (1), but association with HCV infection and IV drug use has not been previously reported.
Our patient presented with pleural effusion, ascites and polycythemia, but without hypertension or kidney failure. Also, he did not have any serious complications. Pleural effusion was initially left-sided, then it progressed to a bilateral involvement. There are two previous reports of pleural effusion, one bilateral and the other unilateral (2,8). Pleural effusion, in these two cases, was not the main finding at presentation; whereas the perinephric fluid and ascites, as with all previous cases in the literature, were superior in quantity compared to our patient. In contrast, in our case, pleural effusion was the most important finding at presentation. The renal function in our case was unaffected in contrast to some of the previous cases in which renal failure occurred as a consequence of perinephric fluid compression on the kidneys. Imaging studies, including CT and ultrasonography, definitely confirmed our diagnosis. Although the diagnosis of renal lymphangiectasia is based on imaging results, perinephric fluid draining and kidney biopsy had been performed in four and one former case, respectively (2,3, 9). Furthermore, we performed these two procedures to further confirm the diagnosis. The results of the perinephric and pleural fluid analyses were similar to the previously reported cases and kidney biopsy revealed no glomerular or tubular disturbances. Infectious fluid collection and malignancy were ruled out in all previous cases and we excluded them in our patient as well. The renin concentration in the perinephric fluid in a previous report was elevated, which is in favor of a renal origin. Unfortunately, we could not determine the level of renin in the perinephric fluid because the sample was quantitatively inadequate for this analysis. Our patient had polycythemia, which is a finding similar to two other cases in the past. Also, since detection of carboxyhemoglobin level could not be done in our center, we were unable to determine whether the erythrocytosis was primary or secondary.
Our case is the first in the literature to report association of HCV infection and IV drug use with renal lymphangiectasia. After a heroin self-injection by the patient, dyspnea was aggravated and repeated imaging demonstrated bilateral pleural effusion (Figure 1). It is unclear whether re-initiation of heroin or the HCV infection deteriorated the pleural effusion. Also, some other unanswered questions regarding this case include whether erythrocytosis was primary or secondary to smoking or renal lymphangiectasia, as well as the causal relationship between HCV infection, heroin use and renal lymphangiectasia.
REFERENCES
- 1. Ramseyer LT. Case 34: renal lymphangiectasia. Radiology 2001; 219 (2): 442– 4. [DOI] [PubMed] [Google Scholar]
- 2. Bazari H, Attar EC, Dahl DM, Uppot RN, Colvin RB. Case records of the Massachusetts General Hospital. Case 23-2010. A 49-year-old man with erythrocytosis, perinephric fluid collections, and renal failure. N Engl J Med 2010; 363 (5): 463– 75. [DOI] [PubMed] [Google Scholar]
- 3. Wani NA, Kosar T, Gojwari T, Qureshi UA. Perinephric fluid collections due to renal lymphangiectasia. Am J Kidney Dis 2011; 57 (2): 347– 51. [DOI] [PubMed] [Google Scholar]
- 4. Rastogi R, Rastogi UC, Sarikwal A, Rastogi V. Renal lymphangiectasia associated with chronic myeloid leukemia. Saudi J Kidney Dis Transpl 2010; 21 (4): 724– 7. [PubMed] [Google Scholar]
- 5. Schwarz A, Lenz T, Klaen R, Offermann G, Fiedler U, Nussberger J. Hygroma renale: pararenal lymphatic cysts associated with renin-dependent hypertension (Page kidney). Case report on bilateral cysts and successful therapy by marsupialization. J Urol 1993; 150 (3): 953– 7. [DOI] [PubMed] [Google Scholar]
- 6. Al-Dofri SA. Renal lymphangiectasia presented by pleural effusion and ascites. J Radiol Case Rep 2009; 3 (10): 5– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton IE, Sambrook P, McWilliam LJ. Secondary polycythaemia associated with bilateral renal lymphocoeles. Postgrad Med J 1994; 70 (825): 515– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics 2008; 28 (4): 1005– 17. [DOI] [PubMed] [Google Scholar]
- 9. Pianezza ML, Mokhtassi A, Wu L, D’A Honey RJ. Case report: renal lymphangiectasia. Can J Urol 2006; 13 (4): 3204– 7. [PubMed] [Google Scholar]
- 10. Rastogi R, Rastogi V. Computed tomographic scan in the diagnosis of bilateral renal lymphangiectasia. Saudi J Kidney Dis Transpl 2008; 19 (6): 976– 9. [PubMed] [Google Scholar]


