Abstract
Objectives:
To assess the proportion and grades of retinopathy and its risk factors in diabetes type 2 patients.
Materials and Methods:
This was a cross-sectional study of 401 type 2 diabetic patients. A questionnaire and checklist were used to collect the data. Retinopathy was diagnosed and graded by fundus photographs and slit lamp examination. The duration of diabetes, age of patients, age at onset of diabetes, body mass index, hemoglobin A1c level, blood pressure, and complications were noted.
Results:
The mean age of male and female patients was 54.93 and 54.25 years; 57.6% were males. The mean age of onset and mean duration of diabetes were 43.91 and 13.4 years, respectively. The proportion of retinopathy was 36.4%. Grades of retinopathy were: Mild 57.5%, moderate 19.9%, severe nonproliferative 11%, and proliferative retinopathy 11.6%; 7.2% of patients had maculopathy. Retinopathy was significantly associated with older age, younger age at onset, longer duration of disease, poorly controlled blood sugar, hypertension, insulin use; the presence of neuropathy and nephropathy appeared as a significant risk. Younger age at onset, longer duration, and insulin use appeared as the strongest predictors for diabetic retinopathy.
Conclusions:
More than a third (36.4%) of the diabetic patients attending a diabetic center had retinopathy. The control of the risk factors may reduce both prevalence and consequences of retinopathy.
Keywords: Diabetic retinopathy, grades of retinopathy, risk factor for retinopathy, type 2 diabetes
INTRODUCTION
Diabetes mellitus (DM) has been known as a potentially disabling chronic disease with multiple complications.[1] In the Kingdom of Saudi Arabia (KSA), diabetes has emerged as a major public health problem that has reached an epidemic stage.[2] The crude prevalence of diabetes has been documented as 23.7%, accounting for 37.8% of Saudis aged between 30 and 70 years.[3] With the advances in the healthcare facilities in the Kingdom, the life expectancy of diabetic patients has increased. Therefore, the complications associated with longer duration of the disease have become one of the challenges faced by health care institutions.
Of these complications, a retinal vascular disorder, retinopathy is considered the leading cause of blindness in the working age population[4] and accounts for considerable adult work disability.[5] Its presence may also indicate and predict other diabetic complications.[6] It is documented that more than 77% of patients who survive for over 20 years with DM are affected by retinopathy.[7] Untreated diabetic retinopathy (DR) not only leads to blindness, which is a personal catastrophe for the individual but also increases the economic burden of health care servicesin the community.[8]
DR is characterized by signs of retinal ischemia (retinal microvascular abnormalities, microaneurysms, hemorrhages, intra-venous caliber abnormalities, cotton-wool spots, and neovascularization) and/or signs of increased retinal vascular permeability. Vision loss can result from several mechanisms, including neovascularization leading to vitreous hemorrhage and/or retinal detachment, macular edema, and retinal capillary nonperfusion.[4] Depending on these signs, retinopathy is classified into nonproliferative diabetic retinopathy (NPDR) and proliferative retinopathy (PDR). The NPDR is further divided into mild, moderate, and severe.
A number of studies show marked differences in the prevalence of retinopathy[9,10,11,12,13] in patients with type 2 diabetes. Of the estimated 10.2 million US adults 40 years and older known to have DM, the estimated crude prevalence rates for retinopathy was 40.3%,[4] while an urban population-based study from India documented the prevalence of DR to be 18%.[10] The prevalence in the UK has been reported as 50%[9] and 26.11% in Spain.[14] Similar to global prevalence differences the prevalence of DR reported in studies from the Middle East also shows varied figures: United Arab Emirates (UAE) (19%),[15] Kuwait (8–12%),[15,16] Oman (42.4%),[11] Egypt (42%),[17] and Jordan (64%).[18] The studies from different regions of Saudi Arabia also show variable prevalence: Al-Hassa (30%),[19] Madinah (36.8%),[20] Aseer region (11.3%),[21] Riyadh (31%),[19] and Taif (36.1%).[22]
The risk factors associated with this complication are also not uniform in all the studies from different geographical regions. The risk factors and epidemiological determinants mostly documented in various studies are age, gender, obesity, duration of disease, presence of hypertension, dyslipidemia, uncontrolled diabetes, and geographical area.[19]
Considering the variability of the prevalence of DR in different geographical regions and it is importance as a complication due to the fact that it not only results in serious consequences, it is a key indicator of systemic diabetic microvascular complications. It is, therefore, considered as a sentinel indicator of the impact of diabetes. This study has the principal aim of describing the most recent prevalence of DR and the associated risk factors in the type 2 diabetic patients attending the diabetes center at Abha, KSA for follow-up. The objective of the present study was to assess the proportion and grades of retinopathy and its risk factors in diabetes type 2 patients.
MATERIALS AND METHODS
The study was conducted at a diabetic center in Abha, Aseer region. The diagnosed diabetic patients are referred to this center from different hospitals and primary health care centers (PHCCs) in this region.
The sample size for this study was calculated according to Swinscow,[23] as 350 with the estimated prevalence of DR = 35.24 (average of DR prevalence reported from different geographical regions of Saudi Arabia). To compensate for the missing patients, we increased the sample size to 401.
A total of 10,576 type 2 diabetic patients were registered in this center from January 2008 to December 2013. Systematic random sampling was used to select 401 cases from the records. Every 26th patient's medical record was selected, and the patients were then contacted for their permission and to provide the relevant data in the questionnaire. The last medical investigation results shown in the records were noted in the checklist.
To maintain confidentiality, data were collected anonymously with the approval of the Research Ethical Committee of the College of Medicine, King Khalid University.
Data collected included demographic and clinical parameters. The demographic parameters were age, gender, occupation, and family history of diabetes. Clinical parameters noted were: Duration of diabetes, age at onset of diabetes, control of blood sugar (hemoglobin A1c [HbA1c] ≥7% was considered as controlled, 7-9% as uncontrolled and >9% poorly controlled), use of anti-diabetic drugs, dyslipidemia, obesity (classified as overweight body mass index [BMI] 25.0–29.9 kg/m2, obese 30.0–39.9 kg/m2 and morbidly obese 40.0 kg/m2), blood pressure (grouped as normotensive and hypertensive receiving antihypertensive drugs). Complications of diabetes included ischemic heart disease, peripheral vascular disease, cerebrovascular accident, cataract, neuropathies (diagnosed on clinical findings), nephropathy (presence of microalbuminuria/gross albuminuria or high creatinine level).
The diagnosis and grading of DR were done by slit lamp (with a Volk 90 D lens) examination and colored fundus photographs using a Topcon TRC-NW6 nonmydriatic fundus camera by a trained ophthalmologist in the diabetic clinic and findings were recorded in patients' files. Retinopathy was classified into NPDR and PDR, NPDR was further subdivided into mild (microaneurysms confined mainly to the area temporal to the fovea), moderate (vascular changes seen in one to two quadrants of the retina), and severe (vascular changes seen in more than two quadrants).
The Statistical package for social studies (SPSS) (SPSS version 17.0. In: Cary NC, editor.: SAS Institute; 2002, USA) was used for data analysis. Descriptive statistics (number, the percentage for categorical variables, mean, standard deviation [SD], and range for continuous variables) and Chi-square tests (χ2) was used to test for the association. P ≤ 0.05 was considered statistically significant. Individual risk factors were identified by using univariable analysis. Odds ratio (OR) with 95% confidence interval (95% CI) were generated to quantify relationships with each risk factor. Multiple logistic regression was applied to identify the predictor of DR.
RESULTS
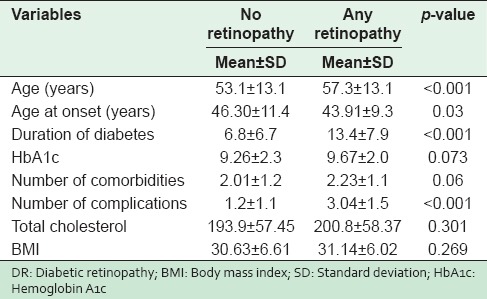
In total, 401 randomly selected type 2 diabetic patients were included. Their ages ranged from 20 to 90 years, with a mean SD of 54.6 (12.3) years and a median of 54.0 years. The mean age of male and female diabetic patients were 54.93 years and 54.25 years. The mean age of patients with DR was significantly higher (P < 0.001) in comparison to patients without DR (57.3 vs. 53.1 years). The mean age of onset for DM was 43.91 years with DR while it was 46.30 years for diabetics (P = 0.03). A significant difference was observed in the mean duration of diabetes (13.4 ± 7.9 years vs. 6.8 ± 6.7 respectively, P < 0.001) between patients with DR and diabetics without retinopathy [Table 1].
Table 1.
Baseline characteristics of participants with and without Diabetic Retinopathy
The overall prevalence of DR was 146 (36.4%). Mild NPDR was in 57.5% of the patients, moderate NPDR in 19.9% and severe NPDR in 11.0% while 11.6% of diabetic patients had PDR. Exudative and focal maculopathy were found in only 7.2% of DR patients. Maculopathy was associated significantly with the severe NPDR and PDR [Table 2]. Analysis of baseline characteristics and different risk factors with different grades of retinopathy showed a significant difference in mean duration among the four grades of retinopathy; (P = 0.001). Post-hoc test revealed that the duration of DM was significantly different for PDR (P = 0.003) in comparison to NPDR. Similarly, the total mean cholesterol was different among the four grades (P = 0.019). This difference was found to be with the severe grade of NPDR (post-hoc test) however, no significant difference was observed in the levels of high-density, low-density lipoprotein and other risk factors (data not shown).
Table 2.
Grades of retinopathy and association with maculopathy
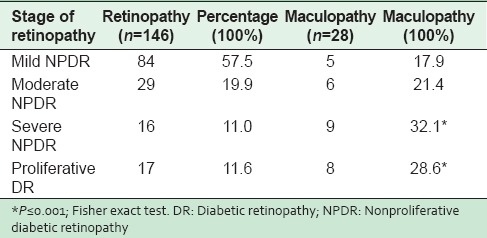
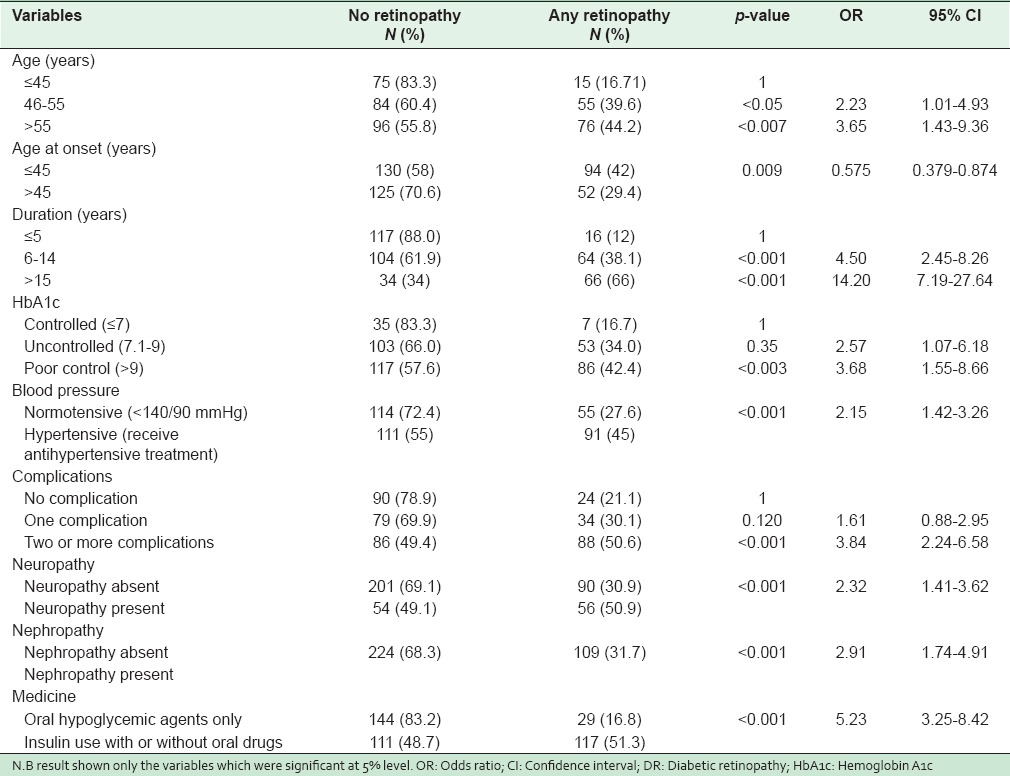
Table 3 presents the potential risk factors of DR. In univariable analysis, the rate of retinopathy was significantly associated with older age group, younger age at onset, longer duration of disease, poorly controlled blood sugar, the presence of hypertension (receiving drug treatment), insulin use, and the presence of multiple complications. The presence of neuropathy and nephropathy in DM appeared as a significant risk. Gender, higher BMI and dyslipidemia including total cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL) levels and smoking did not appear as significant risk factors.
Table 3.
Unadjusted OR and 95% CI for risk factors of Diabetic Retinopathy
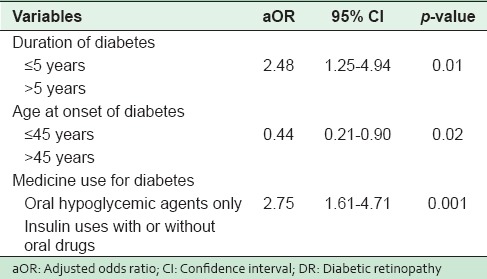
In multivariable logistic regression analysis [Table 4], longer duration of diabetes, younger age of onset and the use of insulin appeared as the strongest predictors of DR. Odds of having retinopathy were higher among patients who had developed diabetes at a younger age (≤45 years) compared with patients who had developed diabetes aged more than 45 years (adjusted OR [aOR] = 0.44, 95%; CI: 0.21, 0.90). Patients with a lengthy duration of diabetes (5 years or more) were more than twice as likely to develop retinopathy than the patients with shorter duration of diabetes (aOR = 2.48, 95%; CI: 1.25 – 4.94). The use of insulin with or without oral hypoglycemic drugs for the treatment of diabetes was almost 3 times likely to result in retinopathy.
Table 4.
Logistic regression analysis results - aOR and 95% CI for factors that might be associated with the DR in Abha, Saudi Arabia
DISCUSSION
Abnormal glucose metabolism has reached epidemic proportions in the Kingdom, with the prevalence of diabetes at 23.7%.[1] This has led to a considerable increase in the burden of diabetic complications including DR, which appears to be highly prevalent in the general adult Saudi population.[24]
The overall prevalence of DR in our study was 36.4%, which is nearly the same as the global documented estimated prevalence of 34.6% in individuals with diabetes.[25] It is also close to what was found in Taiwan 35.0%,[26] Southern India[27] and the reports from studies in different regions of Kingdom of Saudi Arabia: Al-Hassa 30%, Madinah 36.8%, Taif 36.1%, and Riyadh 31%[19,20,22,24] Our result shows a lower prevalence of DR than what is reported in the UK 50%, USA 40.3%, Oman 42.4%, Egypt 42%, and Jordan 64%.[9,11,17,18,28] The prevalence of DR in our study is higher than those documented in many other studies; 21.9% in Australia, 26.11% in Spain, 19% in UAE, 8–12% in two different studies in Kuwait and 18% in India.[10,14,15,26] The difference of prevalence could be explained by the fact that most of these studies were population based and also that different tools of measurement were used.
The prevalence of DR reported by Al-Khaldi from Aseer region was 11.3%,[21] which is much lower than the results of our study in the same region. The variation could be due to the difference in study location and difference in the method of diagnosis. Their study was conducted in a single PHCC while our study was conducted at a diabetic clinic to which all patients from the PHCCs in the region are referred for annual screening, Their diagnosis of retinopathy was based on fundoscopic examination, while in our study the diagnosis was made by colored photographs and slit lamp examination as it was the standard technique used in other studies.[19,20,22,24]
Regarding the grades of retinopathy in our study, the proportion of mild grade of retinopathy is higher than that reported from Oman and Madina (KSA)[11,20] but our finding is in agreement with that documented from Hassa (KSA).[19] The present study showed a lower prevalence of PDR than that of Madina (KSA)[20] but higher than that reported from UAE and Oman,[11,15] and similar to the study in Hassa (KSA). Our study is in agreement with a review article which indicated that in patients with DR, severe vision impairment was not as common as mild vision impairment.[29]
The results of our study indicate that retinopathy increases with younger age at onset of diabetes and showed a significant association between DR and duration of diabetes, which is consistent with most of the previous studies.[11,15,16,19,20,22,24] A study from Sweden documents that prevalence of DR reached 100% after 30 years of diabetes.[30]
Our study found no significant gender difference in the development of DR, which is in accord with multiple studies mostly from the Middle East and Saudi Arabia[4,7,11,28] but it is in contrast to a study from Sweden,[30] which documents higher rates for women than men;[20] and studies from Madina, India, and UAE where DR was observed to be more prevalent in male diabetics.[10,15]
Many studies on DR have documented the close association of chronic hyperglycemia (with high HbA1c) and the development of the condition.[7,16,18,19,20,21,22,23,24] However, a longitudinal study by Aiello et al.[31] reported that the prevalence of DR in long-standing diabetes is not dependent on the control of the disease. Similarly, the degree of glycemic control did not show any significant association with DR in the study sample in Madina (KSA)[20] and from UAE.[15] Our finding showed significant association in univariable analysis but failed to reveal the same in multiple logistic regression.
Hypertension has been documented as a risk factor in studies from Jordan,[18] Oman,[11] and also by a longitudinal UK prospective diabetes study group.[32] Report from UAE reveals that DR is marginally significantly associated with hypertension.[15] While in contrast, many studies including those from Hassa (KSA), Riyadh (KSA) and from South India[27] were not able to find any significant role of hypertension in the development of DR. In our study, the univarent analysis showed hypertension as a risk factor for DR, but this was not significant after adjusting for confounders.
BMI and smoking did not appear as significant risk factors as reported by the studies in UAE[15] and Tehran;[33] the latter finding is in contrast to a report from Madina[20] and South India[27] that documented smoking to be associated with an increased prospect of DR. This finding may be due to the fewer number of smokers in our study.
Similar to van Leiden's study[34] and the study in Riyadh,[24] the present study found that high total cholesterol, abnormal LDL, and HDL levels did not have a positive association with the development of DR, but contrasting results were observed in studies in Hassa (KSA) Jordan and Oman.[11,18,19] In our study, the total cholesterol level was significantly higher in the severe grade of DR, which may indicate that it could be a risk of the progression of DR to severe retinopathy, a finding that emphasizes the importance of good lipid control as a preventive measure for the progression of retinopathy.
The univariable analysis revealed that the age of the patient, the number of other complications and presence of neuropathy and nephropathy were significant risks for DR, but these were not supported by multiple regression analysis. This finding is consistent with the study in Oman in which similarly aged patients, poor control of diabetes (with HbA1c ≥9), high systolic blood pressure and complications were documented as insignificant after multiple regression analysis.[11]
In the present study study, the strongest determinants for the development of DR were Younger age at onset, longer duration of diabetes and the use of insulin, which may be due to the fact that most of the type 2 DM patients with longer duration ultimately end up using insulin. All these three risk factors have been documented in most of the studies related to DR.[11,15,16,19,20,22,24]
The limitation of our study is that a predictive inference cannot be drawn from our observational data since a more extensive study is required. Despite this limitation, our study provides a picture of the prevailing situation in this region, and the data is sufficient to exhibit the enormity of this complication in diabetic patients and emphasize the main priorities of attention in regional programmes for screening retinopathy in all patients with diabetes type 2 at an early stage in the PHCC with recommended tools (presently not available in the PHCC) and prevent its progression.
CONCLUSIONS
Our findings clearly demonstrate that retinopathy is a common complication of diabetes in diabetic patients at Abha, Aseer region and that the situation is no different from other regions of Saudi Arabia. This is in contrast to a previous study from the same region that documented the prevalence as much lower in this region. We support the idea that the retina of a diabetic patient provides a summary measure of lifetime exposure to the effects of hyperglycemia, and which emphasizes the fact that screening for DR at the initial stage of diabetes may prevent disability from blindness caused by DR. Small investments in prevention, awareness and care can dramatically improve the quality of life of patients with long-standing diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to extend our thanks to Dr. Raouf, the ophthalmologist, other doctors, nurses and all the employees at the diabetic clinic, Abha, for their invaluable support and co-operation in making this study possible.
REFERENCES
- 1.Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, Al-Harthi SS, Arafah MR, Khalil MZ, et al. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004;25:1603–10. [PubMed] [Google Scholar]
- 2.Elhadd TA, Al-Amoudi AA, Alzahrani AS. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: A review. Ann Saudi Med. 2007;27:241–50. doi: 10.5144/0256-4947.2007.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Rubeaan K, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM, et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: A Saudi National Diabetes Registry-based study. PLoS One. 2014;9:e88956. doi: 10.1371/journal.pone.0088956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteves J, da Rosa CM, Kramer CK, Osowski LE, Milano S, Canani LH. Absence of diabetic retinopathy in a patient who has had diabetes mellitus for 69 years, and inadequate glycemic control: Case presentation. Diabetol Metab Syndr. 2009;1:13. doi: 10.1186/1758-5996-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27:161–76. doi: 10.1016/j.preteyeres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 8.Alhowaish AK. Economic costs of diabetes in Saudi Arabia. J Family Community Med. 2013;20:1–7. doi: 10.4103/2230-8229.108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparrow JM, McLeod BK, Smith TD, Birch MK, Rosenthal AR. The prevalence of diabetic retinopathy and maculopathy and their risk factors in the non-insulin-treated diabetic patients of an English town. Eye (Lond) 1993;7(Pt 1):158–63. doi: 10.1038/eye.1993.34. [DOI] [PubMed] [Google Scholar]
- 10.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.el Haddad OA, Saad MK. Prevalence and risk factors for diabetic retinopathy among Omani diabetics. Br J Ophthalmol. 1998;82:901–6. doi: 10.1136/bjo.82.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González Villalpando ME, González Villalpando C, Arredondo Pérez B, Stern MP. Diabetic retinopathy in Mexico. Prevalence and clinical characteristics. Arch Med Res. 1994;25:355–60. [PubMed] [Google Scholar]
- 13.Fernando DJ, Siribaddana S, De Silva, Subasinge Z. Prevalence of retinopathy in a Sri Lankan diabetes clinic. Ceylon Med J. 1993;38:120–3. [PubMed] [Google Scholar]
- 14.Pedro RA, Ramon SA, Marc BB, Juan FB, Isabel MM. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010;17:251–65. doi: 10.3109/09286586.2010.498661. [DOI] [PubMed] [Google Scholar]
- 15.Al-Maskari F, El-Sadig M. Prevalence of diabetic retinopathy in the United Arab Emirates: A cross-sectional survey. BMC Ophthalmol. 2007;7:11. doi: 10.1186/1471-2415-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Shammari KH, Al-Meraghi O, Nasif A, Al-Otaibi S. The prevalence of diabetic retinopathy and associated risk factors in type 2 diabetes mellitus in Al-Naeem area (Kuwait) Dr. Middle East J Fam Med. 2005;3: 1–8. [Google Scholar]
- 17.Herman WH, Aubert RE, Engelgau MM, Thompson TJ, Ali MA, Sous ES, et al. Diabetes mellitus in Egypt: Glycaemic control and microvascular and neuropathic complications. Diabet Med. 1998;15:1045–51. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Al-Bdour MD, Al-Till MI, Abu Samra KM. Risk Factors for diabetic retinopathy among Jordanian diabetics. Middle East Afr J Ophthalmol. 2008;15:77–80. doi: 10.4103/0974-9233.51997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AR, Wiseberg JA, Lateef ZA, Khan SA. Prevalence and determinants of diabetic retinopathy in Al Hasa Region of Saudi Arabia: Primary health care centre based cross-sectional survey, 2007-2009. Middle East Afr J Ophthalmol. 2010;17: 257–63. doi: 10.4103/0974-9233.65502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Bab MF, Shawky N, Al-Sisi A, Akhtar M. Retinopathy and risk factors in diabetic patients from Al-Madinah Al-Munawarah in the Kingdom of Saudi Arabia. Clin Ophthalmol. 2012;6:269–76. doi: 10.2147/OPTH.S27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Khaldi YM, Khan MY, Khairallah SH. Audit of referral of diabetic patients. Saudi Med J. 2002;23:177–81. [PubMed] [Google Scholar]
- 22.Al Ghamdi AH, Rabiu M, Hajar S, Yorston D, Kuper H, Polack S. Rapid assessment of avoidable blindness and diabetic retinopathy in Taif, Saudi Arabia. Br J Ophthalmol. 2012;96:1168–72. doi: 10.1136/bjophthalmol-2012-301874. [DOI] [PubMed] [Google Scholar]
- 23.Swinscow TD. Statistics at Square One. 9th ed. U.K: BMJ Publishing Group; 1997. [Google Scholar]
- 24.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Kangave D, Moharram OA. Risk factors for diabetic retinopathy among Saudi diabetics. Int Ophthalmol. 1998-1999;22:155–61. doi: 10.1023/a:1006240928938. [DOI] [PubMed] [Google Scholar]
- 25.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tapp RJ, Shaw JE, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26:1731–7. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 27.Rema M, Ponnaiya M, Mohan V. Prevalence of retinopathy in non insulin dependent diabetes mellitus at a diabetes centre in Southern India. Diabetes Res Clin Pract. 1996;34:29–36. doi: 10.1016/s0168-8227(96)01327-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen MS, Kao CS, Chang CJ, Wu TJ, Fu CC, Chen CJ, et al. Prevalence and risk factors of diabetic retinopathy among noninsulin-dependent diabetic subjects. Am J Ophthalmol. 1992;114:723–30. doi: 10.1016/s0002-9394(14)74051-6. [DOI] [PubMed] [Google Scholar]
- 29.Bloomgarden ZT. Screening for and managing diabetic retinopathy: Current approaches. Am J Health Syst Pharm. 2007;64(17 Suppl 12):S8–14. doi: 10.2146/ajhp070331. [DOI] [PubMed] [Google Scholar]
- 30.Jerneld B, Algvere P. Relationship of duration and onset of diabetes to prevalence of diabetic retinopathy. Am J Ophthalmol. 1986;102:431–7. doi: 10.1016/0002-9394(86)90069-3. [DOI] [PubMed] [Google Scholar]
- 31.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 32.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Maghbooli Z, Pasalar P, Keshtkar A, Farzadfar F, Larijani B. Predictive factors of diabetic complications: A possible link between family history of diabetes and diabetic retinopathy. J Diabetes Metab Disord. 2014;13:55. doi: 10.1186/2251-6581-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: The Hoorn study. Arch Ophthalmol. 2003;121:245–51. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]


