Abstract
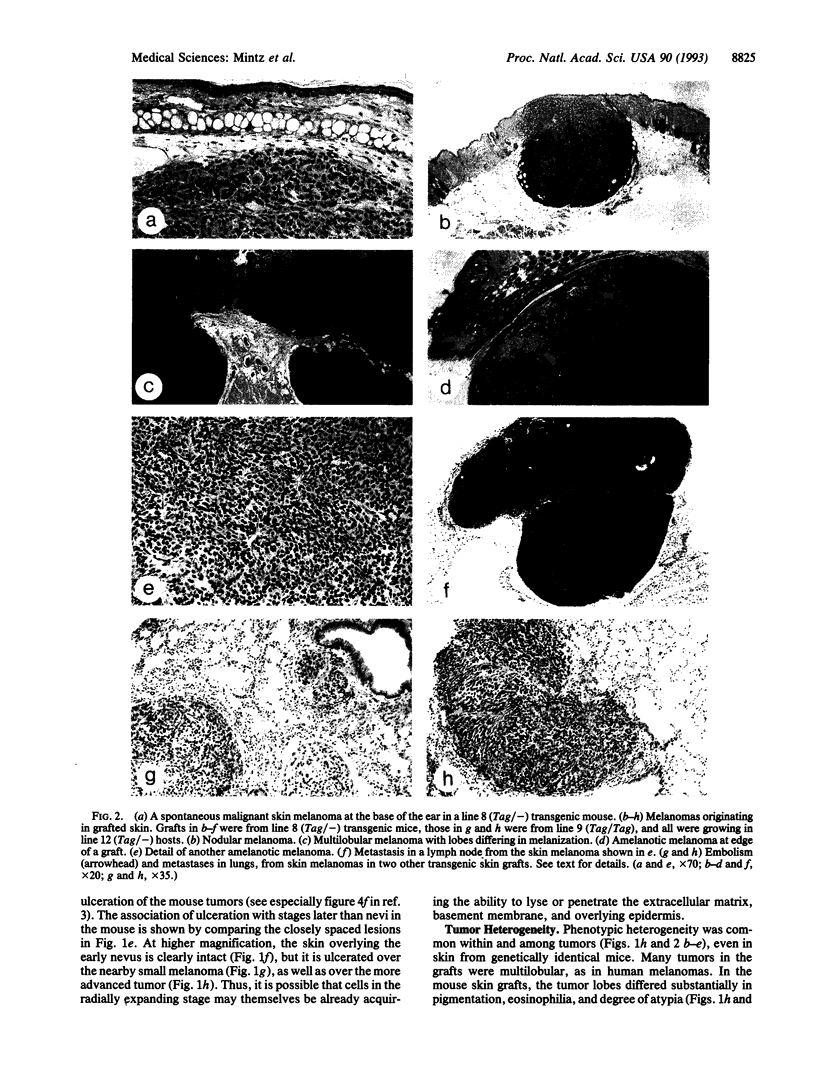
Animal models of human malignant skin melanoma were created in melanoma-susceptible inbred-strain transgenic mice by grafting skin from donors of high-susceptibility lines to hosts of a low-susceptibility line, thereby overcoming the problem of early death of the more susceptible animals from eye melanomas. As already described [Mintz, B. & Silvers, W. K. (1993) Proc. Natl. Acad. Sci. USA 90, 8817-8821], melanocytes within the grafts selectively proliferated in close proximity to areas of greatest wound healing, presumably in response to mitogenic factors from cells contributing to wound repair. An orderly sequence of externally visible events culminated in malignant melanoma. We examine here the histogenetic concomitants of these changes and find that they define a stepwise sequence strikingly comparable to that leading to human cutaneous melanoma. Moreover, the histological details suggest some of the underlying mechanisms. While the early lesions are first seen in the superficial dermis in the mouse, and in the basal layer of the epidermis in the human, both progress by radial growth followed by vertical growth. Melanocytic hyperplasia resulted in nests of densely melanized fusiform cells which were losing their dendrites. Some discrete lesions in the deep dermis appeared as blue nevi. As radial proliferation advanced, cellular atypia increased and the previously independent melanocytes cohered closely and formed a small solid tumor; the cells were usually then hypomelanotic or amelanotic. Ulceration of tumor through the epidermis occurred early. The tumor mass grew rapidly in the deep dermis and invaded and destroyed subcutaneous tissue and muscle. Primary tumors in the skin were often heterogeneous, with lobules or regions differing in pigmentation or atypia. However, the cells in circulating emboli, or in metastases in lymph nodes and lungs, appeared relatively homogeneous. These genetically uniform transgenic mouse models provide experimental access to the multistage genesis of melanoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., SILVERS W. K. The melanocytes of mammals. Q Rev Biol. 1960 Mar;35:1–40. doi: 10.1086/402951. [DOI] [PubMed] [Google Scholar]
- Berkelhammer J., Oxenhandler R. W. Evaluation of premalignant and malignant lesions during the induction of mouse melanomas. Cancer Res. 1987 Mar 1;47(5):1251–1254. [PubMed] [Google Scholar]
- Bradl M., Klein-Szanto A., Porter S., Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Epstein M. N., Greene M. H., Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984 Dec;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Van Horn M. The biologic forms of malignant melanoma. Hum Pathol. 1986 May;17(5):443–450. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- Connelly J., Smith J. L., Jr Malignant blue nevus. Cancer. 1991 May 15;67(10):2653–2657. doi: 10.1002/1097-0142(19910515)67:10<2653::aid-cncr2820671041>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gordon P. R., Mansur C. P., Gilchrest B. A. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989 Apr;92(4):565–572. doi: 10.1111/1523-1747.ep12709595. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Markert C L, Silvers W K. The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics. 1956 May;41(3):429–450. doi: 10.1093/genetics/41.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. A., Seperack P. K., Strobel M. C., Copeland N. G., Jenkins N. A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991 Feb 21;349(6311):709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. Transgenic mouse model of malignant skin melanoma. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8817–8821. doi: 10.1073/pnas.90.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Romerdahl C. A., Stephens L. C., Bucana C., Kripke M. L. The role of ultraviolet radiation in the induction of melanocytic skin tumors in inbred mice. Cancer Commun. 1989;1(4):209–216. [PubMed] [Google Scholar]





