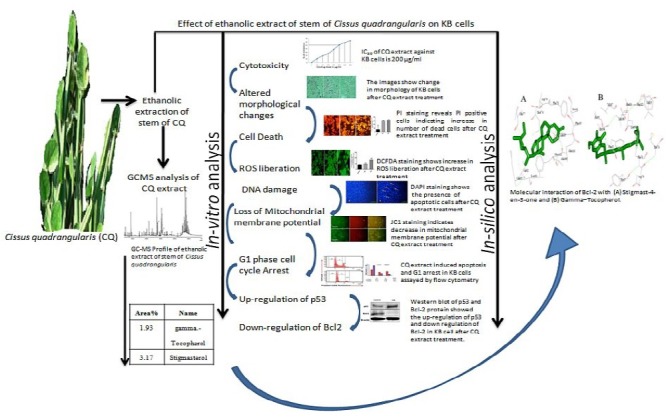
Abstract
Background:
Cissus quadrangularis Linn. (CQ) commonly known as Hadjod (Family: Vitaceae) is usually distributed in India and Sri Lanka and contains several bioactive compounds responsible for various metabolic and physiologic effects.
Objective:
In this study, the biological effects of CQ ethanolic extract were evaluated by in vitro and supported by in silico analysis on KB oral epidermoid cancer cell line.
Materials and Methods:
Anti-cancer potential of ethanolic extract of CQ stem against KB oral epidermoid cancer cells was evaluated in terms of morphological analysis, nuclei staining, liberation of reactive oxygen species (ROS), cell cycle arrest, mitochondrial membrane potential (MMP) and p53 and Bcl-2 protein expression which reveal the induction of apoptosis along with supporting in silico analysis.
Results:
Ethanolic extract of CQ stem contains various bioactive compounds responsible for cancer cell morphological alterations, liberation of ROS, G1 phase cell cycle arrest and decreased MMP along with up-regulation of p53 and down-regulation of Bcl-2. By employing in silico approach, we have also postulated that the CQ extract active constituents sequester Bcl-2 with higher affinity as compared to p53, which may be the reason for induction of growth arrest and apoptosis in KB cells.
Conclusion:
Our data indicate that the CQ extract has a remarkable apoptotic effect that suggests that it could be a viable treatment option for specific types of cancers.
SUMMARY
Cissus quadrangularis stem ethanolic extract induces apoptosis and cell cycle arrest at G1 phase
It liberates (ROS) and mitochondria mediated apoptosis
It upregulates p53 and down-regulates Bcl-2 protein expression
In silico studies indicates that the active constituents of CQ binds Bcl-2 with higher affinity as compared to p53.
Keywords: Anti-cancer, apoptosis, Bcl-2, cell cycle KB cells, reactive oxygen species
INTRODUCTION
Oral cancer incidence rate is about 1–10/100,000 people which make it the third most common cancer in Asia and the sixth most common cancer in the world. The treatment of oral cancer has been highlighted because of the poor prognosis accompanied with poor response to surgery and/or radiotherapy or chemotherapy associated with low survival rate, disfigurement and abridged quality of life.[1,2] Because of being expensive and therapy-associated side effects of the modern modality of treatment, the search persists for a superlative treatment which is safe, effective and cost-efficient. Medicinal plants have played a significant role in various ancient traditional systems of medicine because of the presence of rich sources of bioactive compounds which make them as important raw materials and a target for the search of new drugs. Therefore, it is thus considered important to screen apoptotic inducers from plants, either in the form of crude extracts or as active components isolated from them. These compounds are recognized as one of the most effective classes of cancer preventive agents; because they exhibit little or no systemic toxicity. Most of these substances exert their chemotherapeutic activity by blocking cell cycle progression and triggering apoptotic cell death. However, the development of OSCC is a multi-step process involving genes related to cell cycle, growth control, apoptosis, DNA damage response and other cellular regulators.[3,4,5] Therefore, the need of the hour is to search for natural products which involve signalling pathway for inducing cell cycle arrest and apoptosis in tumor cells. Cissus quadrangularis Linn. (CQ) commonly known as Hadjod (Family: Vitaceae) is usually distributed throughout the hotter parts of India and Sri Lanka. The stem of CQ is reputed in Ayurveda as alternative, anti-helmintic, dyspeptic, digestive, tonic, analgesic in eye and ear diseases, in the treatment of irregular menstruation and asthma, in complaints of the back and spine. Scientific studies have revealed that the CQ extract possesses cardiotonic and androgenic property.[6] Phytochemical analysis revealed the presence of ascorbic acid, carotene, anabolic steroidal substances and calcium in CQ. The CQ stem contains two asymmetric tetracyclic triterpenoids and two steroidal principles. The presence of β-sitosterol, δ-amyrin, δ-amyrone, and flavonoids (quercetin) has also been reported.[7] All of these components have potentially different metabolic and physiologic effects. Accordingly, the anti-cancer effects of CQ ethanolic extract were evaluated on KB cell line (oral epidermoid cancer cell line) in this study, and the results were validated by in silico analysis. We implemented molecular docking simulation methods, followed by searching the best conformation of Protein receptors and all chief chemical compound complexes, of the plant extract on the basis of molecular binding energy. From the present findings, we propose that CQ stem ethanolic extract has the potential to modulate cell proliferation and induce apoptosis in KB cells.
MATERIALS AND METHODS
In vitro experiments
Chemicals and reagents
Dulbecco's modified eagle medium (DMEM), fetal calf serum, penicillin, streptomycin, and trypsin/ethylenediamine tetraacetic acid (EDTA) were purchased from HiMedia. Dimethyl sulfoxide and 3-(4, 5-dimethyl thizol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Plant material
The stem of CQ was taken from the Department of Botany, Lucknow University, Lucknow. The plant material was authenticated by the Department of Botany, Lucknow University where a voucher specimen was submitted. The plant material was shade dried and powdered.
Preparation of plant extract
The powder of CQ stem (20 g) was extracted with 250 ml ethanol by soxhlet extraction for 8 h. The extract was concentrated on a water bath at 60°C. The obtained dark brown thick liquid was stored in a glass vial in the refrigerator.[8]
Gas chromatograph interfaced to a mass spectrometer analysis
GC/MS analysis was carried by employing 2 μl of the CQ extract. Gas chromatograph interfaced to a mass spectrometer (GC-MS) analysis was performed on a GC clarus 500 Perkin Elmer system comprising a AOC-20i autosampler and GC-MS instrument employing the following conditions: Column elite-1 fused silica capillary column (30 × 0.25 mm ID × 1 EM df, composed of 100% dimethyl poly siloxane), operating in electron impact mode at 70 eV, helium (99.999%) was used as carrier gas at a constant flow of 1 ml/min and an injection volume of 0.5 EI was employed (split ratio of 10:1) injector temperature 270°C, ion-source temperature 230°C. The oven temperature was programmed from 110°C (isothermal for 2 min), with an increase of 10°C/min, to 200°C/min, then 5°C/min to 280°C/min, ending with a 9 min isothermal at 280°C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 550 Da.
Cell culture and treatment
The oral epidermoid carcinoma cell line (KB) was procured from the National Centre for Cell Science, Pune, India. The cells were maintained in a CO2 incubator with 5% CO2 and 95% humidity, and supplemented with DMEM and 10% fetal bovine serum. Penicillin and streptomycin were also added to the medium to ×1 final concentration from a ×100 stock. Once the cells had attained confluent growth, the cells were trypsinized using trypsin-EDTA and the number of cells needed for carrying out various assays was seeded into sterile six-well and 96-well plate. Then, the plates were incubated in a CO2 incubator with 5% CO2 and 95% humidity.
Cell viability assay by 3-(4, 5-dimethyl thizol-2-yl)-2, 5-diphenyl tetrazolium bromide reducing activity
The effect of CQ extract was assessed in KB cells by MTT assay. Briefly, cells were seeded at a number of 2 × 104 per well onto 96-well plates in triplicates, allowed to attach and grow for 24 h and subsequently exposed to 25–500 µg/ml dose of CQ extract for 24 h. At the end of the treatment, the medium was removed and cells were incubated with 20 μl of MTT (5 mg/ml in phosphate buffered saline [PBS]) in fresh medium for 4 h at 37°C. After 4 h, formazan crystals formed by mitochondrial reduction of MTT, were solubilized in DMSO (150 μl/well) and the absorbance was read at 570 nm after 10 min incubation on the iMark Microplate Reader (BioRad, USA). Cell viability was calculated as a fraction of control and the cytotoxicity of CQ extract was expressed as IC50.[9]
Analysis of morphological changes by haematoxylin/eosin staining
For hematoxylin/eosin (H/E) staining, cells (20 × 103 cells per well) were placed in DMEM by using 24-well plates. After treating with the CQ extracts at different concentrations for 24 h period, the medium was removed, the cells washed with distilled water and fixed in ethanol, and stained with hematoxylin/eosin. After staining, the cells were observed by a light inverted microscope (Nikon).[10] By this way, cellular and nuclear morphology are shown in cultured cells stained with hematoxylin/eosin.
Cytotoxicity of Cissus quadrangularis on KB cells detected by propidium iodide staining
The CQ treated and un-treated control KB cancer cells (20 × 103 cells per well on 24 well plate) were washed in ice-cold PBS, spun at 1400 rpm for 5 min at 4°C and fixed in 75% methanol and 25% glacial acetic acid for 30 min in ice. Fixed cells were washed twice in PBS and stained with propidium iodide (PI; 30 mg/ml; sigma) containing DNase-free RNase A (1 mg/ml) for 30 min at 37°C. Stained cells were washed in PBS and observed under a fluorescence microscope (Nikon).[11]
Evaluation of nuclear changes by 4’, 6-diamidino-2-phenylindole staining
A fluorescent stain, 4’,6-diamidino-2-phenylindole (DAPI), was used to determine the number of nuclei and to assess gross cell morphology. (DAPI) staining was performed according to the procedure described by Ansil et al.[12] with minor modifications. The studies related to chemoprevention, DAPI staining is used to observe the apoptotic changes at DNA level as a reliable apoptotic assay.[13] Cells were cultured in a 24-well tissue culture grade plate for 24 h. After incubation with IC50 concentrations (µg/ml) of CQ extract for 24 h, KB cells were washed in PBS, fixed with 2% paraformaldehyde for 15 min and were treated with 0.2% triton X-100 in PBS for 15 min at room temperature. Cells after washing with PBS were stained with DAPI (2 µg/ml) and incubated in dark for 30 min. The cells were then examined and photographed using a fluorescence microscope (Nikon ECLIPSE Ti-S, Japan).
Assay of mitochondrial membrane potential changes in vitro
JC-1 probe was employed to measure mitochondrial depolarization in KB cancer cells. After treatment with CQ for 24 h, KB cells were washed with PBS and incubated for 30 min in 10% RPMI medium without phenol red containing JC-1 at a concentration of 2.5 µg/ml then washed twice with PBS and placed in 2 ml culture medium. Green fluorescence (JC-1 as a monomer at low membrane potentials) and red fluorescence (JC-1 as “J-aggregates” at higher membrane potentials) were monitored under a fluorescence microscope. The mitochondrial depolarization was indicated by a decrease in the red/green fluorescence intensity ratio and images were taken by (Nikon) fluorescent microscope.[12]
Detection of intracellular reactive oxygen species liberation
The effect of the application of the extract on reactive oxygen species (ROS) production was measured in CQ treated KB cancer cells using 2’, 7’-dischloro fluorescein diacetate (DCFDA). Briefly, cells were exposed to the IC50 dose of CQ extract for 24 h. Cells were then trypsinized and washed with PBS and were resuspended in PBS containing 10 μM DCFDA for 30 min at 37°C in the incubator. The relative amount of intracellular ROS was subjected to observation by fluorescence microscopy. The pictures were taken by blue FITC filters of Fluorescence Inverted Microscope (Nikon, Japan) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm.[14]
Cell cycle analysis by flow cytometry
Cells after 24 h with or without CQ extract were harvested by centrifugation for determining the cell cycle distribution analyzed by flow cytometry (Becton-Dickinson, USA) as previously described. For cell cycle distribution, the adherent cells were washed with PBS, and 300 µl trypsin was added for 5 min at room temperature to collect and harvested the cells by centrifugation. Cells were fixed gently in 70% ethanol at 4°C overnight and then re-suspended in PBS containing 40 µg/ml PI and 0.1 mg/ml RNase and 0.1% triton X-100 in a dark room for 30 min at 37°C. Cells were analyzed with a flow cytometer.[15] The results were analyzed using ModFit LT 3.0 software.
Western blotting
Western blotting was done by resolving proteins on 15% sodium dodecyl sulphate-polyacrylamide gel gels, transferred onto a polyvinylidene fluoride membrane (Bio-RAD, USA) and subjected to Western blot analysis using anti-Bcl-2 and anti-p53 anti-bodies (Abcam, UK). Proteins were visualized with a chemiluminescence kit (Amersham Bioscience,). The levels of beta-actin (anti-beta-actin; Abcam) was detected and were monitored as a loading control.[16]
In silico experiments
Preparation of ligand structures
Ligand files of all chief chemical compounds, of the plant extract, were downloaded in. Mol format from ChemSpider and PubChem Chemical Database. These files could not be directly used by AutoDock version 4.0 tools[17] thus they were converted it into.pdb files using Discovery Studio Visualizer version 2.5.5. Further, the ligands were energy minimized using Chimera version 1.5.3 using genetic algorithm steps 2000 and 0.5 grid units optimized.[18]
Preparation of protein structures
The three-dimensional structures of proteins were obtained from RCSB protein data bank, and all structures were edited to remove HETATM using Discovery Studio Visualizer. Chimera was used for energy minimization, removal of steric collision with the steepest descent steps 1000, steepest descent size 0.02 Å, conjugated gradient steps 1000 and the conjugate gradient step size 0.02 Å for the conjugate gradient minimization.[19]
Molecular docking studies
Docking studies were performed by AutoDock version 4.0 suites[20] and Cygwin interface was used in the Microsoft Windows 7 professional Version 2002, Intel (R) i5, CPU 3.30 GHz and 8.0 GB of RAM of Intex Machine. We implemented molecular docking simulation methods, followed by searching the best conformation of protein receptors and all chief chemical compound complexes, of the plant extract on the basis of molecular binding energy. Water molecules were deleted from the protein structures before docking simulation and hydrogen atoms were added to all target proteins. Kollman united charges, and salvation parameters were added to the proteins. Gasteiger charge was added to the chemical compounds. Grid box was set to cover the maximum part of protein and chemical compounds. The values were set to 60 × 60 × 60 Å in X, Y, and Z axis of the grid point.
The default grid points spacing was 0.375 Å. Lamarckian genetic algorithm (LGA)[21] was used for proteins and chemical compounds, flexible docking calculations. The LGA parameters such as population size (ga_pop_size), energy evaluations (ga_num_generation), mutation rate, crossover rate and step size were set to 150, 2,500,000, 27,000, 0.02, 0.8, and 0.2 Å, respectively. The LGA runs were set to 10 runs. All obtained 10 conformations of proteins and all chief chemical compounds complexes, of the plant extract were analyzed for the interactions and binding energy of the docked structure using Discovery Studio Visualizer version 2.5.5.
Protein-protein interaction analysis
The most favorable functional partner of Bcl-2 was found using STRING version 9.0 database (known and predicted protein-protein interactions that predict, interacting proteins against our query; http://string-db.org/). The Hex software[22] was used for protein-protein docking. Hex scores and best protein orientation obtained from both protein-protein interactions (Bcl-2 with theirs functional partner TP53) as well as from Bcl-2 complex (Bcl-2 protein + all chief chemical compounds, of the plant extract) with theirs functional partner TP53 interaction.
Statistical analysis
All the experiments were done in triplicates and results are expressed as the mean ± standard deviation statistical comparisons were made by means of one-way ANOVA test followed and P ≤ 0.05 were considered as significant. GraphPad version 6 prism software was used for the statistical analysis.
RESULTS
Gas chromatograph interfaced to a mass spectrometer analysis
GC-MS chromatogram of the CQ extract shows 67 peaks [Figure 1] and have been identified after comparison of the mass spectra with Wiley and NIST libraries, indicating the presence of several phytocomponents [Table 1]. From the results, it was observed that the presence of many anti-cancer phyto-compounds in CQ ethanolic extract justifies the observed anti-cancer activity.
Figure 1.
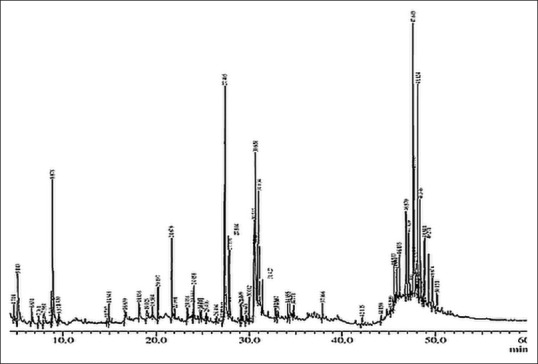
Gas chromatograph interfaced to a mass spectrometer Profile of ethanolic extract of Cissus quadrangularis Linn.
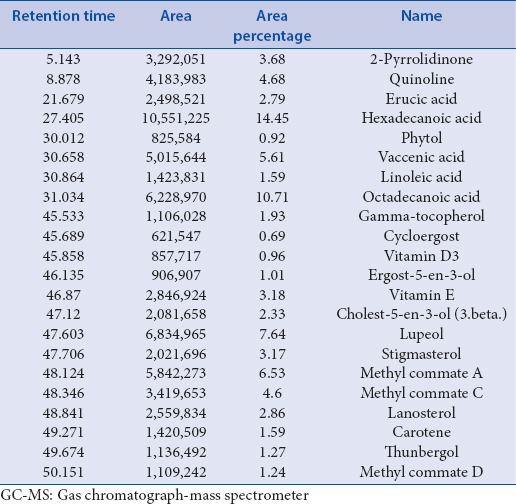
Table 1.
GC-MS analysis of ethanolic extract of Cissus quadrangularis Linn.
Cissus quadrangularis extract significantly decreases the viability of KB cells
Cell viability was assessed by MTT assay, which is a colorimetric assay for measuring the activity of cellular enzymes that reduce the tetrazolium dye, MTT, to its insoluble formazan, giving a purple color. For the evaluation of inhibition of cell viability of KB cancer cells, different doses of CQ extract (25–300 µg/ml) were administered for 24 h. It was found that the number of cells decreased as the dose increases [Figure 2] and at approximately 200 µg/ml dose of extract, 50% of the cell viability was lost as compared to untreated cells (control). The percentage of cells viability was determined by calculating the O.D. of treated against the Blank.
Figure 2.
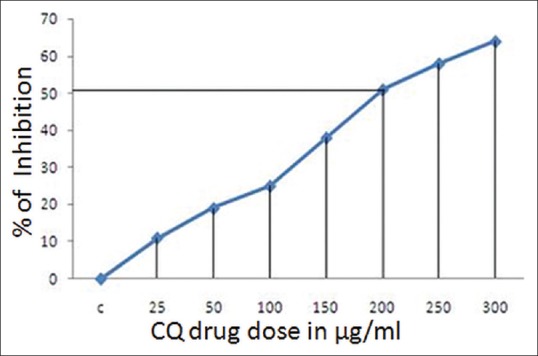
Percentage inhibition of cells and dose-dependent cytotoxic effect of Cissus quadrangularis Linn. extract over a concentration range of 25–300 μg/ml against KB cells by 3-(4, 5-dimethyl thizol-2-yl)-2, 5-diphenyl tetrazolium bromide assay at 24 h. IC50 of Cissus quadrangularis extract against KB cells is 200 μg/ml
KB cells undergo membrane morphological changes after treatment with CQ extract
Morphological changes, including plasma membrane blebbing, changes to the cell membrane, such as loss of membrane asymmetry and attachment and cell shrinkage, are the early stages of apoptosis. The treated cancer cells show features of apoptosis whereas the nontreated (control) cells did not exhibit any changes characteristic of apoptosis [Figure 3]. H/E staining clearly shows that as the dose increases the number of cells decrease with altered morphology [Figure 3b i.e. 100 µg/ml and 3c, i.e. 200 µg/ml dose of CQ extract] as compared to control/non treated KB cells [Figure 3a].
Figure 3.
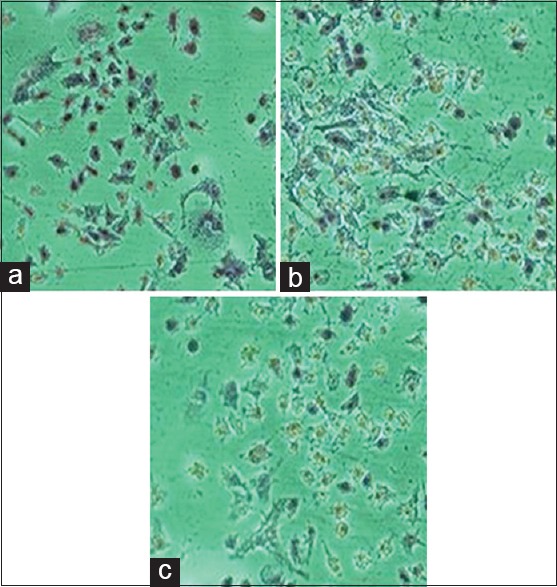
Hematoxylin/eosin staining for morphological analysis: The images show change in morphology of KB cells after Cissus quadrangularis extract treatment (a: Control, b: 100 μg/ml and c: 200 μg/ml dose of Cissus quadrangularis extract)
Cissus quadrangularis extract changes nuclear morphology of KB cells
CQ treated KB cells showed significant nuclear changes [Figure 4] as observed by PI staining. Treatment with CQ resulted in nuclear condensation and formation of apoptotic bodies, demonstrated by PI staining. The results showed a marked difference between the control and CQ extract treated KB cells. The PI positive cells were observed in the treated ones with morphological changes similar to apoptotic cells whereas the untreated cells appeared round without any changes.
Figure 4.
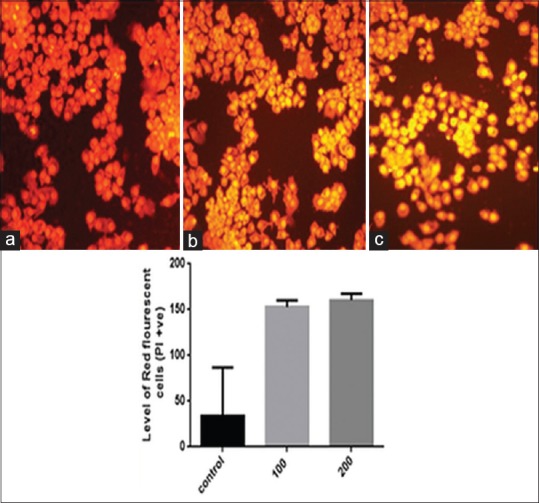
Cissus quadrangularis extract induces cytotoxicity to KB cells. Propidium iodide staining reveals that the number of propidium iodide positive cells indicating dead cells increases as the Cissus quadrangularis dose increases (a: Control, b: 100 μg/ml, c: 200 μg/ml) as shown in respective histogram
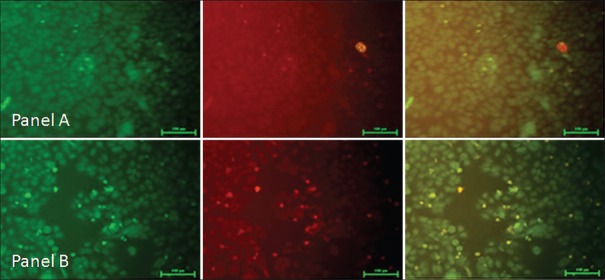
Cissus quadrangularis extract liberates reactive oxygen species in KB cells
It has recently been described that ROS overproduction can trigger intrinsically mediated apoptosis and induce cancer cell death selectively. It has been shown that some natural phytochemicals cause increased ROS production and mitochondrial outer membrane permeabilization specifically in cancer cells.[23] DCFDA, a nonfluorescent cell-membrane permeable probe penetrates the cells, reacts with cellular esterase and ROS, and metabolizes into fluorescent 2’,7’-dichlorofluorescein which can be detected by fluorescence microscopy. In this study, we show that CQ extract treatment increased ROS production and loss of mitochondria membrane potential (MMP) in KB oral carcinoma cells. We have found significant increase in number of fluorescent KB cells after treatment with CQ 100 and 200 µg/ml extract [Figures 5b and 5c], as compared to the control cells [Figure 5a].
Figure 5.
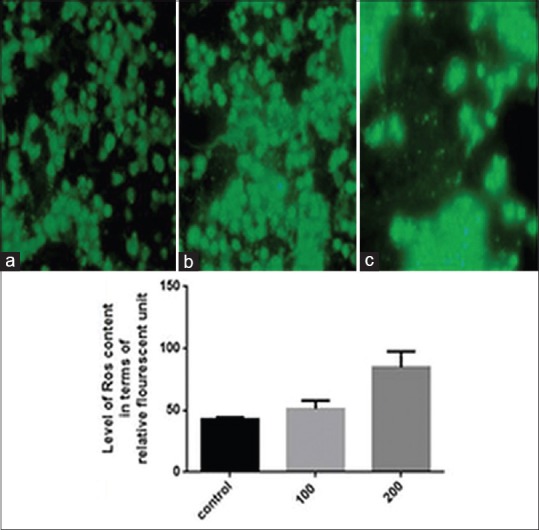
Cissus quadrangularis extract liberates reactive oxygen species. 2’,7’-dischloro fluorescein diacetate staining shows increased green fluorescence after treatment with 100 μg/ml (b) and 200 μg/ml (c) of Cissus quadrangularis extract as compared to control (a) in KB cells indicating generation of reactive oxygen species
Cissus quadrangularis induces apoptosis detected by 4’, 6-diamidino-2-phenylindole
Apoptotic cells were detected with DAPI staining. The apoptotic cells were determined by condensed chromatin and fragmented nuclei as shown in Figure 6b (200 µg/ml CQ treated KB cells), as compared to the control cells [Figure 6a]. under inverted fluorescent microscope (Nikon).
Figure 6.
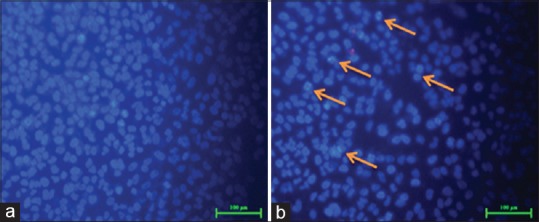
4’, 6-diamidino-2-phenylindole staining shows the presence of blue fluorescent cells in Cissus quadrangularis extract treated ones shown by arrows in the Figure b whereas low fluorescence in control section (a) control. However the number of cells also decreased after the Cissus quadrangularis extract treatment (b)
Cissus quadrangularis extract changes mitochondrial membrane potential
Loss of MMP (Δψm) is as an early event in apoptosis. We evaluated the changes in the MMP in the presence of CQ extract. When the cells are stained with 5,5’,6,6’ tetrachloro-1,1’,3,3’-tetraethyl benzimidazolyl carbocyanine iodide (JC-1), the loss of (Δψm) is indicated by the decrease of red fluorescence and the increase of green fluorescence. After 24 h treatment of CQ extract with 200 µg/ml dose on KB cancer cells, followed by the JC-1 staining, there was significant green fluorescence in majority of cells indicating changes in MMP which may be a reason for its potent apoptotic activity. The results indicated that CQ extract possesses promising apoptotic potential as shown in Figure 7.
Figure 7.
JC1 stained fluorescence image of KB cells after 24 h treatment of (Cissus quadrangularis 200 μg/ml). Panel (a) Represents control and panel (b) Represents the IC50 dose of Cissus quadrangularis ethanolic extract. The green fluorescence indicates a decrease in mitochondrial membrane potential, an early event in apoptosis as observed in b treated with 200 μg/ml of Cissus quadrangularis extract as compared to panel (a) Control KB cancer cell
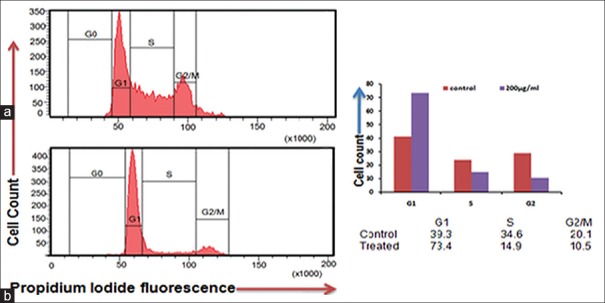
Cissus quadrangularis extract disturbs cell division cycle and induces G1 arrest
CQ extract inhibit the cell cycle, as we resolved its effect on cell cycle distribution analyzed by flow cytometer. Flow histograms represent cell cycle distribution in KB cells after 24 h and exposure of CQ extract as shown in Figure 8. Extract exposed KB cells resulted in the 73.4% cells at G1 fraction as compared to control 39.3% cells, which was accompanied by a decrease in S phase and G2 M phase cells as shown in Figure 8b as compare to unexposed cells in Figure 8a. Thus, CQ extracts mediated growth inhibition and led to G1 phase arrest of KB cells.
Figure 8.
Cissus quadrangularis extract induced apoptosis in KB cells assayed by flow cytometry by propidium iodide staining. Cells were treated with 200 μg/ml of Cissus quadrangularis for 24 h, stained by propidium iodide and followed by flow cytometry to determine the hypodiploid DNA (fragmented DNA) proportions. The percentage of hypodiploid cells (sub G1 peak) was calculated on the basis of the respective histograms. (a) Untreated cells, (b) 200 μg/ml Cissus quadrangularis treated KB cells. Respective histogram shows G1 phase arrest in treated ones as compares to control
p53 activation and Bcl-2 down-regulation is involved in Cissus quadrangularis extract-induced apoptosis
p53 selectively regulates target genes and instigates various stress responses, including cell cycle arrest, apoptosis and/or senescence, to exert its key tumor suppression function. Protein immunoblotting results indicated that the expression of p53 was up-regulated after the treatment of CQ (200 µg/ml for 24 h) in KB oral carcinoma cells after comparing with control. The level of p53 and Bcl-2 is expressed as the ratio of densitometric analysis of the sample to the corresponding beta-actin as an internal control as shown in Figure 9.
Figure 9.
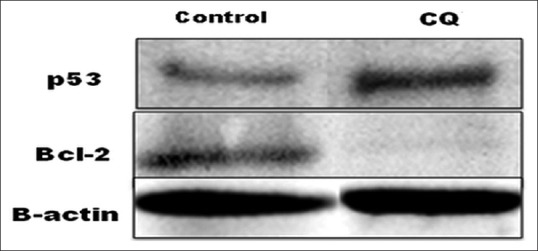
Western blot of p53 and Bcl-2 protein showed the up-regulation of p53 and down regulation of Bcl-2 in KB cell after treatment with Cissus quadrangularis extract (200 μg/ml)
Therefore, it specifies the involvement of p53 in inducing apoptosis and cell cycle arrest in CQ treated KB cells. Overexpression of the Bcl-2 anti-apoptotic proteins has been demonstrated to inhibit cell death induced by many stimuli, including growth factor deprivation, hypoxia, and oxidative stress. However, all traditional anti-cancer drugs appear to depend in large measure on Bcl-2/Bax-dependent mechanisms for killing cancer cells, where anti-apoptotic proteins such as Bcl-2 prevent apoptosis by acting as guardians of the mitochondria outer membrane and preserve its integrity by opposing Bax and Bak (pro-apoptotic proteins).[24,25] Bcl-2 was down-regulated after the treatment of KB cells with CQ extract along with the loss of MMP.
In silico molecular interaction study
To analyze the Bcl-2 function loss, in the presence of all chief chemical compounds (data given as supplementary data), of the plant extract, initially Bcl-2 (4IEH) was selected and downloaded from RCSB protein databases. String database used for the identification of key function partner protein of Bcl-2 and as per the generated network TP53 protein selected as a preferential functional partner among others. Bcl-2 was docked with all chief chemical compounds, of the plant extract and their interactions were analyzed as shown in Table 2. Gamma-Tocopherol showed highest binding competence with Bcl-2–6.30 Kcal/Mol and then stigmast-4-en-3-one-5.55 as shown in Figure 10. Few more molecular docking studies were conducted, in order to identify the preferential binding affinity of all chief chemical compounds, of the plant extract over Bcl-2 and TP53, so this result depicted that all chief chemical compounds, of the plant extract having greater binding affinity toward the Bcl-2 than TP53, it means if both biomolecules were present together in cell vicinity then all chief chemical compounds, of the plant extract preferential bind with Bcl-2 as shown in Table 2.
Table 2.
Binding energy and interaction analysis of Bcl-2 and TP53 with chief chemical compounds
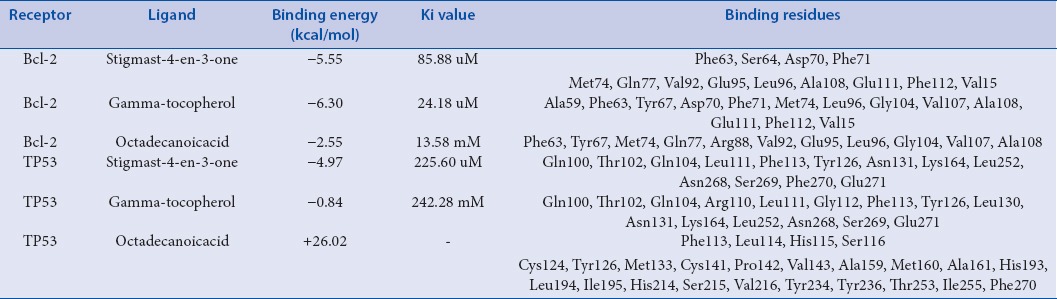
Figure 10.
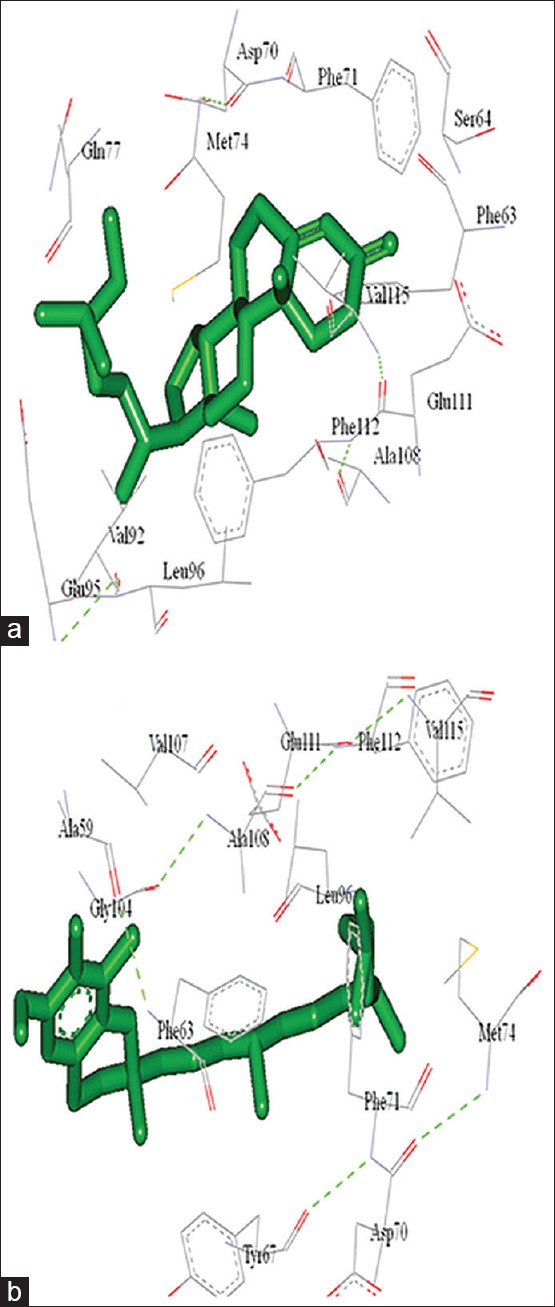
Molecular interaction of Bcl-2 with (a) Stigmast-4-en-3-one and (b) Gamma–tocopherol
After that to elucidate the mechanism of anti-cancerous property induced by all chief chemical compounds, of the plant extract, we implemented protein-protein docking technique. In order to evaluate the probable loss of function of an Bcl-2 after binding with all chief chemical compounds, of the plant extract, Hex scores among protein-protein (Bcl-2 with their preferential functional partner TP53 as per the evidence of string database) and protein-complex (Bcl-2 + all chief chemical compounds, of the plant extract with their functional partner TP53) were compared. Among all chief chemical compounds 7 chief chemical compounds, of the plant extract binding caused reduction in docking score of Bcl-2 with their respective functional partner TP53 as shown in Table 3, and the maximum reduction in Hex score was from −753.41 (Bcl-2 with TP53) to −702.16 (Bcl-2-stigmast-4-en-3-one bound with TP53).
Table 3.
Bcl-2-TP53 binding energy, interaction and highest function loss analysis of Bcl-2 by chief chemical compounds; stigmast-4-en-3-one and octadecanoicacid
DISCUSSION
In the search for novel, effective and safe drugs from natural origin targeting the cancerous cells, we have attempted to identify the molecular mechanisms involved in apoptosis induction in KB oral epidermoid carcinoma cells by CQ stem ethanolic extract. In this study, it was found that the CQ ethanolic extract exerts anti-proliferative and pro-apoptotic effects on KB cells by decreasing cell viability in a dose-dependent manner as observed by MTT assay. The CQ treated KB cells were evaluated for altered morphology, genotoxic effect, and appearance of a sub-G1 population as observed by H/E staining, DAPI staining and fluorescence-activated cell sorting analysis, respectively. The two major apoptosis signaling pathways have been recognized as the intrinsic or mitochondrial pathway and the extrinsic or death receptor related pathway. Further, the intrinsic pathway involves mitochondria-dependent processes, the consequential release of cytochrome c and activation of caspase-9. So, we have concentrated on molecular mechanisms that were triggered early in the intrinsic apoptotic pathway. We observed that CQ causes a rapid decrease in MMP. Identification of supposed CQ mitochondrial target could lead to the synthesis of derivatives that are more efficacious growth inhibitory and apoptotic agents in chemoprevention and chemotherapy as suggested in GC-MS profiling [Table 1]. p53 cDNA sequence analysis was found by Hoe-Jin and Young-Joo that both mouth epidermoid carcinoma (KB) cells contained wild-type p53 gene. In addition to p53 gene status, they have also detected p53 protein expression by Western blot after treatment of Zelkova serrata twig extract.[26] Accordingly, we have also performed the Western blot and observed up-regulation of wild type p53 after the treatment of 200 µg/ml of CQ extract on KB cells. Recently, it was shown that purified fraction of acetone extract of CQ stem altered Bax–Bcl-2 ratio, the release of cytochrome c from mitochondria and PARP cleavage.[27] Taken together, our results point to a general mechanism of ROS-mediated apoptosis induced by CQ extract in KB cells that involves a progressive loss of MMP and activation of the intrinsic mitochondrial specific pathway that confirm previous findings. Mitochondria plays an essential role in intrinsic apoptotic signaling by endowing amplification factors such as cytochrome-c, Smac/DIABLO and apoptotic inducer factors, and this execution pathway is regulated by the Bcl-2 family.[28] This is normally a function of a balance between the pro-apoptotic members such as Bax and Bak, and their anti-apoptotic counterparts Bcl-2 and Bcl-xL. Bax translocates to the mitochondria and induces mitochondrial outer membrane potential, which facilitates the way of pro-apoptotic factors for the downstream apoptosome assembly during apoptotic signaling.[29] On the contrary, over-expression of Bcl-2 prevents the oligomerization of Bax at the mitochondria, and thereby inhibits death signaling.[30] However, we show here that the apoptosis triggered by CQ extract in KB cells was associated with the down-regulation of Bcl-2 gene expression and critical involvement of MMP and ROS in inducing apoptosis. The cellular redox homeostasis is maintained by a tight balance between the rates of ROS production and the ability of the various anti-oxidant defence systems to modulate intracellular redox status. It is now well established that the cellular redox status has an important role in a variety of cellular processes such as gene expression, protein function, proliferation, cell survival, and cell death.[31] In addition to the membrane nicotinamide adenine dinucleotide phosphate oxidase system, mitochondria are an important source of ROS due to the high flux of electrons through the electron transport chain (ETC) that predisposes to leakage of electrons onto oxygen to generate O2−.[32] Interestingly, exposure of KB cells to CQ extract resulted in a significant increase in ROS production, which could be due to its ability to inhibit complex II of the ETC. Therefore, the findings suggest that CQ extract could be mediating apoptosis via directly targeting the mitochondria, dissipation of MMP, an increase in intracellular ROS production as well as a significant arrest of cells in G1 phase.
On other side in silico studies also support the wet lab results, molecular docking studies clearly indicated that stigmast-4-en-3-one and octadecanoic acid both of the chief constituents of the extract are key players that cause the highest loss of normal functioning of Bcl-2 and TP53 binding. The extent of the loss was observed from −753.41 (Bcl-2 with TP53) to −702.16 and −714.03 respectively. As per earlier studies, it has been reported that Bcl-2 inhibits apoptosis by safeguarding the mitochondrial integrity, thereby preventing cytochrome c release and activation of caspase 9. Bcl-2 also constitutively suppresses p53 functions such as tumor suppression in many tumor types, growth arrest, and p53 dependent apoptosis,[33] so as per the verification of in silico studies stigmast-4-en-3-one and Octadecanoic acid both reduce the normal binding affinity of Bcl-2 and TP53, so that Bcl-2 is unable to suppress TP53. Our results also indicate that the chief constituents of our extract preferentially sequester Bcl-2 with a higher affinity as compared to p53, as a result p53 seems to be freely available in the cytoplasm for induction of growth arrest and apoptosis.
CONCLUSION
It can be concluded by the results that the CQ stem ethanolic extract contains several bioactive compounds that showed remarkable anti-cancer property towards KB oral epidermoid carcinoma cells. The CQ extract downregulates the Bcl-2 and up-regulates the p53 protein expression. Apart from this in silico studies suggest the two chief compounds, that is, stigmast-4-en-3-one and octadecanoic acid may act as key mediators of anti-cancer activity of CQ extract. Also, induction of cancer cell apoptosis by CQ extract suggests that this material could be developed as a promising p53-dependent cancer therapeutic agent in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Dr. Snober S. Mir
Dr. Snober S. Mir is working as an Associate Professor in the Department of Bioengineering, Integral University, Lucknow. Her area of specialization is Molecular Cell Biology and Translational Cancer Biology. She obtained her Master's degree in Biotechnology from IBU, Aligarh Muslim University and her Ph.D in Biotechnology from Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. Dr. Mir worked as a Postdoctoral Fellow at the Medical College of Georgia, USA. Her work has been published in several peer reviewed international journals including, Molecular Cell Biology, Molecular Cancer Research, Molecular Microbiology, Molecular Cytogenetics, PLOS One, BBRC, CNSNDDT, Journal of Neurodegenerative Diseases etc.

Mrs. Saba Sheikh
Mrs. Saba Sheikh is working as a Senior Research Fellow of Maulana Aazad National Fellow in Department of Biosciences, Integral University Lucknow. She obtained her Master's in Biotechnology from Integral University and purusing her PhD in Cytogenetics and Plant Extracts.

Dr. Anupam Dhasmana
Mr. Anupam Dhasmana is working as a Senior Research Fellow of Indian Council of Medical Research in Department of Bioengineering, Integral University Lucknow. He obtained his Master's of Technology degree in Biotechnology from Integral University and pursuing his PhD in Environmental Carcinogens, Computational Biology and In vitro Toxicology.

Mrs. Safia
Mrs. Safia is working as a Senior Research Fellow of Maulana Aazad National Fellow in Department of molecular Biosciences, Integral University Lucknow. She obtained her Master's in Biotechnology from Integral University and pursuing her PhD in Molecular Cancer Biology.

Mr. Ejazul Haque
Mr. Ejazul Haque is working as a Senior Research Fellow of Maulana Aazad National Fellow in Department in Department of Biosciences, Integral University Lucknow. He obtained his Master's degree in Biochemistry from Integral University and pursuing his PhD in Molecular Cancer Biology and p53 rescue drugs.

Mr. Mohammad Kamil
Mr. Mohammad Kamil is working as a Junior Research Fellow of Department of Biotechnology, Government of India in, Integral University Lucknow. He obtained his Master's degree in Biotechnology from Integral University and purusing his PhD in Molecular Cancer Biology and Chaperone Biology.

Dr. Mohtashim Lohani
Dr. Mohtashim Lohani has done his graduation (B.Sc.) and Post graduation (M.Sc.) from Lucknow University. He is Professor at the dept. of Biosciences, Integral University, Lucknow. He did his Ph.D. from Jamia Hamdard University, New Delhi, while he worked for his thesis at Industrial Toxicology Research Centre (CSIR), Lucknow. During Ph.D. he was invited twice to Rostock University, Germany, to receive training on Multicolor FISH technique. He worked at the Case Western Reserve University, Cleveland Ohio, USA as Post Doc. He also supervised a short project at KSA. He has published more than 40 research papers in high impact factor International Journals of high repute. 4 of his students have been awarded Ph.D., 4 theses are under review, and five students are under way of thesis submission. He has completed a funded project from UP-CST, and 4 of his proposals are under review.

Dr. Md Arshad
Dr. Md Arshad is Assistant Professor in Department of Zoology, University of Lucknow, Lucknow has completed Ph.D. from Division of Endocrinology, C.D.R.I., Lucknow. Dr. Arshad did his postdoctoral research study from Center of Osteoarticular & Dental Tissue Engineering, INSERM-9903, Nantes Cedex, France in 2003-2004. He has about 10 years teaching and 12 years research experience in the field of reproductive Endocrinology, osteoporosis and cancer cell line. He has published his research articles in journals of international and national repute and associated with various scientific and academic societies.

Mr. Sahabjada Siddiqui
Mr. Sahabjada Siddiqui has completed his B.Sc from CSJM Kanpur, M.Sc. from Aligarh Muslim University and also submitted his PhD thesis to Department of Zoology of Lucknow University on the topic of, “Osteogenic Prospective of Indian Medicinal Plant: Celluar & Molecular Study.”
Acknowledgment
The authors sincerely acknowledge infrastructural support accorded by the Departments of Bioengineering and Biosciences, Integral University, Lucknow. For the financial assistance, Saba Sheikh thanks University Grants Commission, Government of India for providing the Maulana Azad National Fellowship (Senior Research Fellowship) for research.
REFERENCES
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Chew BH, Taher SW. Primary care for cancers: Epidemiology, causes, prevention and classification – A narrative review. J Fam Med Community Health. 2014;1:1002. [Google Scholar]
- 3.Pavelic J. Editorial: Combined cancer therapy. Curr Pharm Des. 2014;20:6511–2. doi: 10.2174/1381612820666140826154834. [DOI] [PubMed] [Google Scholar]
- 4.Sankaran M, Kandhan K, Veluchamy V. Anti-proliferative potential of Pergularia daemia (forsk.) on human oral epidermoid carcinoma (kb) cells by inducing apoptosis and modifying oxidant anti-oxidant status. Asian J Pharm Clin Res. 2014;7:89–95. [Google Scholar]
- 5.Shilpa PN, Sivaramakrishnan V, Niranjali S. Oral cancer prevention and control, the approach of the World Health Organization. Asian Pac J Cancer Prev. 2012;13:2753–8. doi: 10.7314/apjcp.2012.13.6.2753. [DOI] [PubMed] [Google Scholar]
- 6.Eswaran R, Anandan A, Doss A, Sangeetha G, Anand SP. Analysis of chemical composition of Cissus quadrangularis linn by GC-MS. Asian J Pharm Clin Res. 2012;2:139–40. [Google Scholar]
- 7.Potu BK, Bhat KM, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR, et al. Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics (Sao Paulo) 2009;64:993–8. doi: 10.1590/S1807-59322009001000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parag A, Pednekar BR. Assessment of Semecarpus anacardium (linn.f) leaf methanolic extract for their anti-bacterial, anti-fungal and anti-oxidant activity. Int J Pharm Pharm Sci. 2013;5:1. [Google Scholar]
- 9.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 10.Turk M, Rzayev ZM, Kurucu G. Interaction of boron-containing and PEO branched derivatives of poly (MA-altlMEVE) with HeLa cells. Health. 2010;2:51–61. [Google Scholar]
- 11.Zamai L, Falcieri E, Marhefka G, Vitale M. Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry. 1996;23:303–11. doi: 10.1002/(SICI)1097-0320(19960401)23:4<303::AID-CYTO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Ansil PN, Wills PJ, Varun R, Latha MS. Cytotoxic and apoptotic activities of Amorphophallus campanulatus tuber extracts against human hepatoma cell line. Res Pharm Sci. 2014;9:269–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Saha SK, Sikdar S, Mukherjee A, Bhadra K, Boujedaini N, Khuda-Bukhsh AR. Ethanolic extract of the Goldenseal, Hydrastis canadensis, has demonstrable chemopreventive effects on HeLa cells in vitro : Drug-DNA interaction with calf thymus DNA as target. Environ Toxicol Pharmacol. 2013;36:202–14. doi: 10.1016/j.etap.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Li CJ, Tsang SF, Tsai CH, Tsai HY, Chyuan JH, Hsu HY. Momordica charantia extract induces apoptosis in human cancer cells through caspase- and mitochondria-dependent pathways. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/261971. 261971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhasmana A, Sajid Jamal QM, Mir SS, Bhatt ML, Rahman Q, Gupta R, et al. Titanium dioxide nanoparticles as guardian against environmental carcinogen benzo[alpha] pyrene. PLoS One. 2014;9:e107068. doi: 10.1371/journal.pone.0107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alshatwi AA, Hasan TN, Shafi G, Syed NA, Al-Assaf AH, Alamri MS, et al. Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC-3 human prostate cancer cells by assessment of apoptosis-related genes. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/103026. 103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera – A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–74. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006;25:247–60. doi: 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Morris GM, Goodsell DS, Huey R, Olson AJ. Distributed automated docking of flexible ligands to proteins: Parallel applications of AutoDock 2.4. J Comput Aided Mol Des. 1996;10:293–304. doi: 10.1007/BF00124499. [DOI] [PubMed] [Google Scholar]
- 22.Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW. HexServer: An FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 2010;38:W445–9. doi: 10.1093/nar/gkq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin C, Karnik A, McNulty J, Pandey S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol Cancer Ther. 2011;10:57–68. doi: 10.1158/1535-7163.MCT-10-0735. [DOI] [PubMed] [Google Scholar]
- 24.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 25.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang HJ, Jang YJ. Selective apoptotic effect of Zelkova serrata twig extract on mouth epidermoid carcinoma through p53 activation. Int J Oral Sci. 2012;4:78–84. doi: 10.1038/ijos.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhujade A, Gupta G, Talmale S, Das SK, Patil MB. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013;4:338–46. doi: 10.1039/c2fo30167a. [DOI] [PubMed] [Google Scholar]
- 28.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 29.Ghibelli L, Diederich M. Multistep and multitask Bax activation. Mitochondrion. 2010;10:604–13. doi: 10.1016/j.mito.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–83. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 31.Burhans WC, Heintz NH. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radic Biol Med. 2009;47:1282–93. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Maillet A, Yadav S, Loo YL, Sachaphibulkij K, Pervaiz S. A novel Osmium-based compound targets the mitochondria and triggers ROS-dependent apoptosis in colon carcinoma. Cell Death Dis. 2013;4:e653. doi: 10.1038/cddis.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M, Milner J. Bcl-2 constitutively suppresses p53-dependent apoptosis in colorectal cancer cells. Genes Dev. 2003;17:832–7. doi: 10.1101/gad.252603. [DOI] [PMC free article] [PubMed] [Google Scholar]