Abstract
Context:
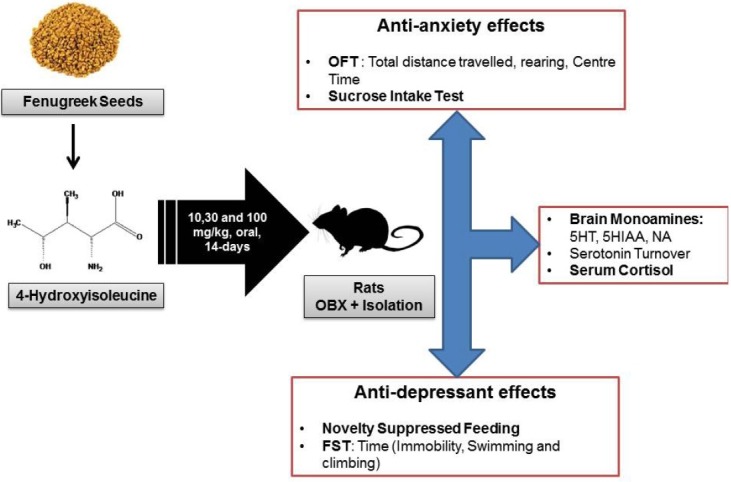
Antidepressant-like effects of (2S, 3R, 4S)-4-hydroxyisoleucine (4-HI), a major amino acid from fenugreek seeds, has been reported in the animal model of acute depression.
Aims:
To evaluate effects of subacute administration of 4-HI in animal model of stress-induced depression namely socially isolated olfactory bulbectomized rats.
Materials and Methods:
Bilateral olfactory bulbectomy (OBX) were induced in 30 Sprague-Dawley rats. After recovery period of 14 days, rats were randomized into five groups of 6 rats each and stressed with social isolation (individual housing). The rats were orally treated with either vehicle (OBX-Iso), positive control, fluoxetine (30 mg/kg) or 4-HI (10, 30, 100 mg/kg) once a day from day 14 onward. Separate group of rats with social isolation but without OBX (Sham-Iso) was also maintained. The behavioral depression and anxiety related parameters using open field test (OFT), sucrose intake test, novelty suppressed feeding (NSF) and forced swim test (FST), and neurochemical estimation (brain monoamines viz., serotonin and nor-adrenaline, serotonin turnover, and serum cortisol) were performed.
Statistical Analysis Used:
Data was analyzed by either two-way ANOVA (OFT and FST) or one-way ANOVA (sucrose intake test, NSF, and neurochemical estimation) followed by Dunnett's multiple comparisons test. Differences were considered significant at P < 0.05.
Results:
The significant and dose-dependent protection from behavioral and neurochemical changes were observed in 4-HI co-administrated OBX-Iso rats.
Conclusion:
4-HI demonstrated the antidepressant and antianxiety effects in socially isolated stress-induced OBX rats with possible involvement of multiple stress relieving mechanisms.
HIGHLIGHTS OF PAPER
In this study, the subacute pretreatment of 4-HI showed strong and dose-dependent prevention of isolation stress related behavioral and neurochemical responses in olfactory bulbectomized rats. The prevention of hyperactive HPA axis in OBX-Iso stress-induced rats can be envisaged as probable mechanism of antidepressant and antianxiety effects of 4-HI.
SUMMARY
Effect of 4-hydroxyisoleucine (4-HI) in olfactory bulbectomized and socially isolated (Iso) rats was evaluated
4-HI showed significant and dose-dependent antidepressant effects during novelty suppressed feeding (NSF) and forced swim test (FST)
4-HI showed significant and dose-dependent antianxiety effects during OFT (open field test) and sucrose intake test
4-HI showed protection from OBX-Iso stress-induced brain monoamines, serotonin turnover, and serum cortisol level elevation.
Abbreviations used: SSRI: Selective Serotonin Reuptake Inhibitor; 4-HI: (2S, 3R, 4S)-4-hydroxyisoleucine; OBX: Olfactory bulbectomy; CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals; OFT: Open Field Test; NSF: Novelty Suppressed Feeding; FST: Forced Swimming Test; 5HT: 5-Hydroxytryptamine; 5-HIAA: 5-Hydroxyindoleacetic Acid; NA: Nor-adrenaline; and HPA: Hypothalamic-Pituitary Adrenal.
Keywords: Anxiety, depression, fenugreek seeds, 4-hydroxyisoleucine, olfactory bulbectomy, social isolation
INTRODUCTION
Depression is a chronic recurrent syndrome, often refractive to drug treatment affecting the quality of life and the overall productivity. In recent years, clinical depression has been recognized as a major public health problem. According to the World Health Organization in its 1998 report, depression (including complications) affected about 12% of the world population and was supposed to be the second greatest cause of premature death and disability world-wide by the year 2020.[1] The high prevalence of suicide in depressed patients (up to 15%), along with the complications arising from mental stress and its effects on the cardiovascular system, have confirmed depression as a contributory factor to fatal coronary disease.[2] Depression is a syndrome that affects a person's mood, physical health, and alters behavior. Patients with major depressive disorder have symptoms that reflect changes in brain monoamine neurotransmitters, specifically serotonin, nor epinephrine and dopamine.[3]
Despite the advent of new molecules in the pharmacotherapy of depression, many cases of depression are remained undiagnosed and untreated. Although, several classes of antidepressants are currently being used, due to clinical limitations and adverse effects there is critical interest in development of efficient and safe drugs for treatment of depression. The most widely used antidepressants namely selective serotonin reuptake inhibitors (SSRIs) increase extracellular availability of serotonin. However, SSRIs are known for their severe side effects like sexual dysfunction,[4] extra pyramidal side effects[5] increased risk of suicidal thoughts and behavior in young population,[6] and absence or delayed sexual desire (libido), arousal, ejaculation and orgasm.[4,7,8]
Deficiency of some dietary amino acids reported to cause reduction in brain serotonin leading to genesis of depression.[9,10,11] The changes in amino acid concentrations in the blood modulates the rate of production of important neurotransmitters[12] and potentially regulates a variety of behavioral functions such as depression and anxiety.[13] Two endogenous amino acids, Leucine, and isoleucine are known to modulate brain serotonin concentration in rats.[14,15] Therefore, leucine and isoleucine related amino acid constituents from dietary source need to be explored as potential antidepressant agents.
The branched chain amino acid, (2S, 3R, and 4S)-4-hydroxyisoleucine (4-HI) is constituent of fenugreek seeds (Trigonella foenum-graecum L. Family: Fabaceae), a commonly used medicinal spice. 4-HI constitutes about 80% of total free amino acids in the fenugreek seeds and proven antidiabetic agents through pancreatic and extra pancreatic mechanisms.[16] Recently, isolation, characterization of 4-HI from fenugreek seeds and excellent antidepressant activity in animal models of depression on acute administration through serotonergic mechanisms is reported.[17] However, management of clinical depression takes several days or weeks for evident therapeutic effect in patients.[18,19] Furthermore, strong relations between depression, anxiety (common co-existing condition), and stress related central nervous system functions (e.g., serotonergic system) exist.[20] Therefore, antidepressant agents need to be investigated for long-term effects (subacute) in stress-induced animal model of depression.
Social isolation (Iso) and olfactory bulbectomy (OBX) are widely employed methods of induction of stress-induced depression in rodents[21] and mimics the depressed patients in clinic.[22] Furthermore, social isolation is reported to facilitate the characterization of depressive-like states in olfactory bulbectomized OBX) rats.[23] Therefore, this study was undertaken with an objective to evaluate effects of 4-HI on behavioral, neuroendocranial parameters related to depression, anxiety, and stress in socially isolated Bulbectomized rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (250–270 g) were purchased from National Toxicology Centre, Pune India. The animals were housed at a temperature of 25°C ± 1°C and relative humidity of 45–55% under 12:12 light: Dark cycle. The rats had a free access to feed pellets (Chakan Oil Mills Ltd., Sangli, India) and tap water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee and as per norm of Committee for the Purpose of Control and Supervision of Experiments on Animals, India. All observations were recorded between 8:00 and 13:00 h and each animal was used only once. To avoid subjective bias, the observer was not aware about the treatment given (blind study). Each experimental group consisted of six animals unless otherwise stated. Rats were transported from the animal house to the testing area in their housing cages and were allowed to adapt to the new environment before actual testing.
The test compound
The sample of test compound, 4-HI, was provided by Indus Biotech Private Limited, Pune, India. The 4-HI was prepared from fenugreek (Trigonella foenum-graecum L.) seeds that are collected from state of Madhya Pradesh in India during month of November) and authenticated at Agharkar Research Institute, Pune, India with voucher specimen deposition. The characterization using NMR[24] and high-performance liquid chromatography fingerprinting of 4-HI sample was reported earlier.[17] The purity of received sample was 98.89%. The stock solution of 4-HI was freshly prepared (100 mg/ml) for each experiment by dissolving in saline (0.9% NaCl solution) with shaking on a vortex-mixing device (Cryo-centrifuge machine, no. 5810, Eppendroff, Hamburg, Germany) for about 30 s and suitably diluted for oral administration to the rats in volume of 1 ml/kg.
Bilateral OBX surgery
OBX was performed on anesthetized rats as per reported procedure.[25] Briefly, the male Sprague-Dawley rats were anaesthetized with ketamine (80 mg/kg i.p.). Each rat was placed in stereotaxic frame (Inco, Ambala city, India), head was shaven, midline scalp sagittal incision (1 cm) was made, bilateral burr holes (2 mm diameter) was drilled 8 mm anterior to bregma, and 2 mm lateral from midline. Both main and accessory olfactory bulbs were aspirated through both burr holes using a blunt hypodermic needle attached to water pump without damaging the frontal cortex. The burr holes were then plugged with a hemostatics sponge to control further bleeding. Sham operations were done in the same way, but with the bulbs left intact. Povidone iodine solution was applied to the wounds and the rat was allowed to recover for the period of 14 days after surgery.
Treatment schedule
The social isolation procedure was performed for 14 days, starting immediately after OBX/sham surgery during recovery period as per reported procedure.[23] The rats were single-housed in their home cages. The rats were randomly divided into experimental groups of 6 rats each during 14-day of recovery period and treated as follows for next 14 days: Group 1 - Sham-Iso (treated with vehicle), Group 2 - OBX-Iso control (treated with vehicle), Group 3 – Fluo + OBX-Iso: Treated with fluoxetine (30 mg/kg p.o.), Group - 4, 5 and 6 were 4-HI- + OBX-Iso and treated with 4-HI at dose of 10, 30, 100 mg/kg p.o., respectively.
Behavioral assessment
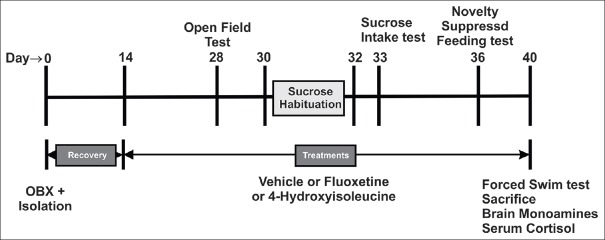
After completion of 14-day of recovery period and end of treatment period, each of the group was subjected to the battery of behavioral test as per reported procedure.[23] These tests were performed during the light phase and rats were transported to the experimental room at least 30 min before the start of each experiment to acclimatize with environment. The tests were conducted in a way so as to be least to most stressful, and leaving an interval between them to minimize any potential order effects. The following sequence of tests [Figure 1] was followed as per reported procedure.[23] Open field test (OFT) → sucrose intake test → novelty suppressed feeding (NSF) → forced swimming test (FST).
Figure 1.
The schedule of experiment with treatment details and tests (behavioral and neurochemical) carried out on socially isolated olfactory bulbectomized rats
Open field test
The effects of various treatments were assessed using OFT as per reported procedure.[23] The open field apparatus was a brightly lit white wooden box (50 cm × 50 cm × 30 cm) with a white floor and luminescent walls. Rats were released at the center of the apparatus and responses were videotaped for 5 min. The behavioral parameters (total distance travelled, time spent in the center, and number of rearings) were measured from recorded videos by observer who was blind to the treatments.
Sucrose intake test
To evaluate the effects of treatments in sucrose intake test, the reported procedure was followed.[23] Sucrose (1%; LobaChemie Private Limited, Mumbai, India) was dissolved in drinking water and animals were trained to drink sucrose solutions during 2 days with free access to water and sucrose in their home cage, and the total fluid consumption per day was measured. Then they were deprived of any drinking solution for 24 h and subsequently each animal was given free access to sucrose solutions for 1 h in its home cage. The milliliters (ml) of sucrose consumed by each rat were recorded and the mean sucrose intake was compared.
Novelty suppressed feeding
The NSF test was performed after 24 h of food deprivation (water available ad libitum) as per reported procedure.[23] The test was done in a dimly lit (30–50 lx) open arena (50 cm × 50 cm × 30 cm) containing clean wood chip bedding and with a home cage food pellet (2 g) placed on the center. Each mouse was removed from the home cage, placed in one corner of the arena. The latency to begin a feeding episode was recorded (maximum time was 600 s) were recorded by observer who was blind to the treatments.
Forced swim test
To evaluate the effects of treatments in FST, the reported procedure was followed.[23] The rats were individually placed in clear plastic cylinders (height 24 cm, internal diameter 12 cm) filled with 22 cm of tap water at 25–27°C. Each rat was videotaped from above for a total of 5 min. The immobility time (floating in the water without struggling, and making only those movements necessary to keep the head above water) and swimming time (movement usually horizontal throughout the swim chamber) were manually scored by observer who was blind to the treatments.
Brain monoamine estimation
The concentrations of monoamines namely 5-hydroxytryptamine (5-HT), its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were estimated using spectrofluorometer by reported procedure.[26] The whole brain was homogenized in cold acidified n-butanol. 2–5 ml of the supernatant solution was pipetted into a 25 ml glass stoppered tube and mechanically shaken for the period of 5 min with n-heptane and hydrochloric acid containing L-cysteine. The phases were then separated by centrifugation and organic phase was retained for the 5-HIAA determination.
To determine concentration of 5-HT, 0.1 ml samples of the aqueous phase were pipetted into test tubes and 0.004% of O-phthalaldehyde (OPT) was added in 1.0 N HCI. After mixing and heating in a boiling water bath, the tubes were cooled in water and fluorescence was measured. Activation and fluorescent wavelengths were 360 nm and 470 nm, respectively. Blanks were prepared by reacting the OPT solution with 0.1 ml HCl + cysteine solution only. To determine concentration of 5-HIAA, the organic phase was pipetted into a glass tube containing phosphate buffer (pH 7.0) and shaken mechanically for the period of 10 min. 0.2 ml of the aqueous phase was pipetted into two test tubes, A and B. 1% cysteine solution was added to test tube A and to 0 02% sodium periodate solution was added to test tube B. Then concentrated HCl was added to both A and B. After this OPT solution and periodate solution was added to tube A. After 30 min, the cysteine and OPT solutions were added to tube B. The tubes were then placed in a boiling water bath for 10 min, cooled in water and read at activation: 360 nm fluorescence: 470 nm. The brain serotonin turnover for each rat (the ratio of 5-HIAA/5-HT) was calculated from 5-HT and 5-HIAA concentrations each rat.
To concentration of nor-adrenaline (NA) in brain homogenate was measured using miniaturization of the trihydroxyindole method. To 0.02 ml of the HCl phase, 0.005 ml 0.4 M HCl and 0.01 ml EDTA/sodium acetate buffer (pH 6.9) were added, followed by 0.01 ml iodine solution (0.1 M in ethanol) for oxidation. The reaction was stopped after 2 min by addition of 0.01 ml Na2SO3, in 5M NaOH. Acetic acid (0.01 ml, 10 M) was added 1.5 min later. The solution was then heated to 100°C for 6 min. When the sample again reached room temperature, excitation and emission spectra were read in the micro cuvette using same parameters as that of 5-HT estimation.
Measurement of serum cortisol levels
Serum cortisol levels were measured before OFT paradigm (day 28). Blood was withdrawn from each rat by retro orbital puncture method under the anesthesia. Blood samples were collected in appropriate tube, centrifuged at 2500 rpm, and serum was separated. The serum was used for cortisol estimation using 96-well ELISA kit (No: EIA-1887, DRG Diagnostics, Germany) as per manufacturer's instructions.
Statistical analysis
All data were expressed as a mean value ± standard error of mean (SEM) and were analyzed with the help of software Prism (v 6.0, GraphPad Software Inc., San Diego, CA, USA). Data of parameters obtained from OFT and FST were analyzed by two-way ANOVA and Dunnett's multiple comparisons test. Data obtained from sucrose intake test, NSF and brain monoamines (5-HT, 5-HIAA, 5-HIAA/5-HT ratio and NA), and serum cortisol levels were analyzed by separate one-way ANOVA and Dunnett's multiple comparisons test. Differences were considered significant at P < 0.05.
RESULTS
Effect of treatments on OBX and social isolated stress-induced rats during open field test
The data of behavioral observations during OFT is presented as Table 1. Two-way ANOVA of OFT data showed significant values for interactions (F [10, 90] =24.00, P < 0.001). The measurements of total distance travelled and number of rearings (but not time spent in center) in OBX-Iso control group showed significant (P < 0.001) increase as compared with Sham-Iso group (Dunnett's multiple comparison test).
Table 1.
Effect of treatments on OBX and social isolation stress-induced rats during OFT
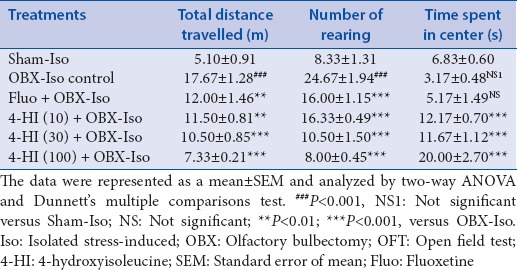
Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.01) decrease in total distance travelled as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant and dose-dependent decrease in total distance travelled as compared with OBX-Iso control group.
Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) decrease in number of rearings as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant and dose-dependent decrease in number of rearings as compared with OBX-Iso control group.
The measurements of time spent in center in OBX-Iso control group did not show significant decrease as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed increase (not significant) in time spent in center as compared to OBX-Iso control group. However, subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant and dose-dependent increase in time spent in center as compared with OBX-Iso control group.
Effect of treatments on OBX and social isolation stress-induced rats during sucrose intake test
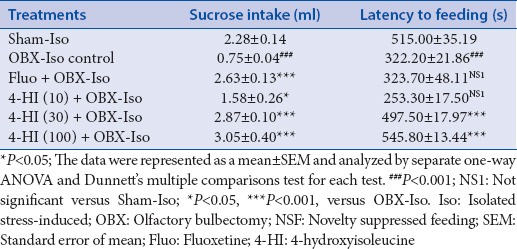
The data obtained from sucrose intake test is presented as Table 2. One-way ANOVA of the data obtained from sucrose intake test showed significant (P < 0.001) effects of treatments (F [5, 30] =16.93) on sucrose intake. Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) decrease in sucrose intake by OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) increase (in sucrose intake as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant and dose-dependent increase in sucrose intake as compared with OBX-Iso control group.
Table 2.
Effect of treatments on OBX and social isolation stress-induced rats during sucrose intake test and NSF
Effect of treatments on OBX and social isolation stress-induced rats during novelty suppressed feeding
The data obtained from NSF is presented as Table 2. One-way ANOVA of the data obtained from NSF showed significant (P < 0.001) effects of treatments (F [5, 30] = 19.05) on latency to feeding in NSF. Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) decrease (in latency to feeding by OBX-Iso control group) as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed increase (not significant) in sucrose intake as compared to OBX-Iso control group. The subacute treatment of 4-HI (30 and 100 mg/kg but not at 10 mg/kg dose) showed significant increase in latency to feeding as compared with OBX-Iso control group.
Effect of treatments on depression related parameters of OBX and social isolation stress-induced rats during forced swim test
The data obtained from FST is presented as Table 3. Two-way ANOVA of the data obtained from FST showed significant interaction (F [10, 90] = 198.4, P < 0.001). Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) increase in immobility and swimming time and significant (P < 0.001) decrease in climbing time in OBX-Iso control group as compared with Sham-Iso group.
Table 3.
Effect of treatments on depression related parameters of OBX and social isolation stress-induced rats during FST
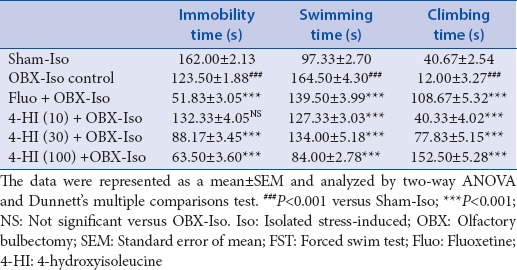
Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) decrease in immobility time as compared to OBX-Iso control group. The subacute treatment of 4-HI (10 mg/kg) showed increase (not significant) in immobility time. The subacute treatment of 4-HI (30 and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent decrease in immobility time as compared with OBX-Iso control group.
Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) decrease in swimming time as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30, and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent decrease in swimming time as compared with OBX-Iso control group.
Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) increase in climbing time as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30, and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent increase in climbing time as compared with OBX-Iso control group.
Effect of treatments on brain monoamines and serum cortisol levels of OBX and social isolation stress-induced rats
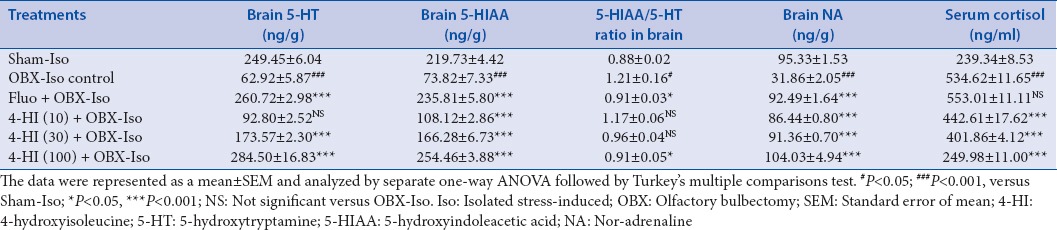
The data obtained from brain monoamine and serum cortisol estimation is presented as Table 4. Separate one-way ANOVA of the data obtained from each of brain monoamines estimation and serum cortisol showed significant effects of treatments with following F statistics: Brain 5-HT (F [7, 40] =130.0, P < 0.001), brain 5-HIAA (F [7, 40] = 220.9, P < 0.001), 5-HIAA/5-HT ratio in brain (F [7, 40] = 220.9, P < 0.001), and brain NA (F [7, 40] = 3.499, P < 0.001).
Table 4.
Effect of treatments on brain monoamines and serum cortisol levels of OBX and social isolation stress-induced rats
Effects on brain 5-hydroxytryptamine levels
Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) decrease in brain 5-HT concentration in OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) increase in brain 5-HT concentration as compared to OBX-Iso control group. The subacute treatment of 4-HI (30 and 100 mg/kg, oral but not 10 mg/kg dose) showed significant (P < 0.001) and dose-dependent increase in brain 5-HT concentration as compared with OBX-Iso control group.
Effects on brain 5-hydroxyindoleacetic acid levels
Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) decrease in brain 5-HIAA concentration in OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) increase in brain 5-HIAA concentration as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent increase in brain 5-HIAA concentration as compared with OBX-Iso control group.
Effects on brain 5-hydroxyindoleacetic acid to 5-hydroxytryptamine ratio (serotonin turnover)
Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.05) increase in brain 5-HIAA/5-HT ratio in OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.05) decrease in brain 5-HIAA/5-HT as compared to OBX-Iso control group. The subacute oral treatment of 4-HI (100 mg/kg, but not 10 and 30 mg/kg dose) showed significant (P < 0.05) decrease in brain 5-HIAA/5-HT as compared with OBX-Iso control group.
Effects on brain nor-adrenaline levels
Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) decrease in brain NA concentration in OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed significant (P < 0.001) increase in brain NA concentration as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent increase in brain NA concentration as compared with OBX-Iso control group.
Effects on serum cortisol levels
Post-hoc group-wise comparisons using Dunnett's multiple comparisons test showed significant (P < 0.001) increase in serum cortisol concentration in OBX-Iso control group as compared with Sham-Iso group. Subacute treatment of fluoxetine (30 mg/kg, oral) showed small increase (not-significant) in serum cortisol concentration as compared to OBX-Iso control group. The subacute treatment of 4-HI (10, 30 and 100 mg/kg, oral) showed significant (P < 0.001) and dose-dependent decrease in serum cortisol concentration as compared with OBX-Iso control group.
DISCUSSION
In the last few years, several plant derived natural substances have been introduced with potential to fight various human pathologies, particularly neuropsychiatric disorders including stress, depression, and anxiety. This is reflected in the large number of naturally occurring substances assessed in preclinical models depression, including medicinal plants, such as St John's wort.[27]
In this study, we are reporting the effects of one of promising natural branched chain amino acid, 4-HI, derived from fenugreek seeds on social isolated OBX rats. Social isolation is common cause of anxiety disorders in clinic.[28,29] Social isolation in rodents is often viewed as an animal model of stress and causes a variety of behavioral changes, including anxiety,[30] and mood related behavior.[31] Furthermore, nonphysical nature of social isolation minimizes discomfort yet, reveals stress responses in animals, which stimulate the hypothalamic-pituitary-adrenal (HPA) axis activity.[23,32] Thus, results from social isolation induced stress method has good predictive validity for SSRI induced suicidal behavior related traits in clinic.[33] Therefore, we used social isolation as a stress inducing model system in the present study.
The hyperactivity and exploratory behavior of the OBX syndrome is known to enhance when animals are socially isolated.[30] Our results showed increased exploratory activity (in OFT), decreased immobility and climbing time (in FST), increased swimming time (in FST), and decreased sucrose intake and feeding latency time (in NSF) in OBX-Iso control rats as compared with Sham-Iso rats are indicative of increase in behavioral hyperactivity and in line with earlier reports.[23]
Anxiety-related responses in OFT (total distance and rearing) were significantly increased, whereas the central time (time spent in center) reduced (but not significantly) in OBX-Iso rats as compared with Sham counterparts. These observations in anxiety related parameters in OFT are in line with previous observations of increased anxiety-like manifestations induced by OBX[30,34] alone or social isolation alone[32] or their combination.[23] These anxiety related changes were prevented in subacute treatment of fluoxetine and 4-HI indicating their anxiolytic potential.
Anhedonia, the inability to experience pleasure from activities usually found enjoyable, e.g., exercise is a cardinal symptom of depression.[35] Recently, anhedonia has emerged as one of the most promising endophenotypes of clinical depression.[36] Chronic mild stress-induced anhedonia is considered to be a realistic animal model of depression.[37] Therefore, we studied effects of treatments on OBX-isolation stress (a chronic stress) anhedonia using the sucrose intake test, a validated measure of anhedonia in laboratory animals.[38]
Our results from sucrose intake test confirm the anhedonia response of OBX-Iso control rats as evidenced by a decreased sucrose intake as compared to Sham-Iso rats[39] indicating depressive-like state. The subacute administration of fluoxetine and 4-HI prevented OBX-Iso induced anhedonia. The reversal effects of fluoxetine against anhedonia in our study are in agreement with earlier reports of reversal of chronic mild stress-induced anhedonia.[40,41] The subacute administration of 4-HI showed dose-dependent prevention of OBX-Iso (a chronic stress) during our study. Recently, down-regulation of hippocampal serotonin transporter (5-HTT) protein expression is reported as signature change associated with anhedonia.[42] Taken together, our results from sucrose intake test provided strong support to our results from other parameters (FST and serotoninergic system measurements) and suggested 4-HI as a potential antidepressant agent.
In this study, the latency to feeding of OBX-Iso rats was found to decrease as compared with Sham counterpart during NSF. This finding is contrary to expected behavior of increased feeding in a depressive/anxious-like situation. This reduced latency can be attributed to an increased exploratory behavior or an enhanced impulsivity induced by combined OBX and Iso stress, or locomotor hyperactivity as reported earlier.[23] This notion is supported by the reports of increased exploratory behavior in different paradigms[43] and impulsivity in the hole-board test in OBX animals.[44] The heightened anxious behavior observed in the OFT in this study also strengthens such possibilities.
In our study, 4-HI could prevent OBX-Iso induced anxiety-like behavior during OFT and NSF. However, fluoxetine failed to prevent the center time (in OFT) and feeding latency decrease (in NSF). Our results of failure of fluoxetine in anxiety related models can be attributed to adaptation ability to novel stimuli especially under stressful stimuli such as OBX[45] without affecting anxiety behavior[46,47] On the other hand, 4-HI treatment to OBX-Iso rats showed significant prevention of anxiety-like behavior in OFT and NSF tests indicating anxiolytic potential. In the past, antidepressant-like effect demonstrated by of 4-HI in mice is suggested to have serotonergic mediation.[17] The differential effects of fluoxetine and 4-HI in anxiety related parameters in this study supports the possible role of other mechanism such as stress reliving pathways (e.g., stress hormones) in the anxiolytic action of 4-HI.
In this study, OBX-Iso group was shown to decrease immobility and increase in swimming time as compared to Sham-Iso control. Furthermore, the subacute treatment of fluoxetine and 4-HI was shown to prevent OBX-Iso induced increase in swimming time indicating antidepressant like effects. These results are in line with earlier reports[23] and indicate no significant influence of social isolation on FST parameters. The FST is one of the most commonly used animal models for assessing the physiological changes in response to stress-related conditions.[48,49] including depression.[50] Our results from behavioral effects in FST are in line with many earlier reports[51,52] and can be explained based on the hyperactivity shown by OBX induced animals under highly stressful situations.[34]
Our results of significant decreased climbing behavior in FST (an active struggling response to escape from a threatening event) in OBX-Iso rats as compared with Sham counterpart is in agreement with earlier report.[23] Incapability to actively face stressful situations is postulated as cause of these reduced escape attempts.[23] The significant prevention of such decrease in 4-HI treated group's shows role of adrenergic neurotransmission in the mechanism of 4-HI.[53] It is suggested that immobility involves the serotonergic system and climbing involves the adrenergic neurotransmission. The SSRIs decrease immobility behavior while NA reuptake inhibitors increase climbing behavior.[54] Interestingly, differential levels in brain levels of NA, and 5-HT in OBX animals have been reported.[55] Our results showed reduced levels of 5-HT and NA concentration in brain samples of rats from OBX-Iso group as compared with their Sham counterpart. Further, subacute administration of 4-HI showed significant prevention of OBX-Iso induced decline of monoamine levels provided additional support to the reported antidepressant-like effects of 4-HI in FST model in non-OBX rats.[17]
During our study, we observed simultaneous prevention of OBX-induced decrease in brain concentrations of 5-HT and its metabolite 5-HIAA in 4-HI (100 mg/kg) treated rats. Therefore, beneficial antistress effects of 4-HI against OBX-Iso induced behavior can be attributed to increase in serotonergic anabolism. The brain serotonergic turnover is characterized by the 5-HIAA/5-HT ratio. The 5-HIAA/5HT could also serve to detect impairments in the function of enzymes or transporters. In this study, the elevated 5-HIAA and 5-HT with decreased serotonin turnover in 4-HI treated OBX-iso rats indicated increased serotonergic anabolism as a mechanism of antidepressant action of 4-HI.
The increase in brain serotonin after social isolation stress[56] plays an important role in the activation of the HPA axis and copes well as stress reliever through various neuronal pathways.[57] Social isolation stress is reported to cause dysregulation of the HPA axis.[58] Furthermore, dysfunction of HPA axis has been linked with stress-related psychiatric conditions such as depression, or trauma in general.[59]
HPA axis dysregulation, characterized by elevated cortisol concentrations are observed in many patients suffering from affective disorders.[60] Activation of the HPA axis causes an increase in cortisol, which can induce physiological changes in response to the stressor.[61] Cortisol levels are reported as reliable indicator of stress reactivity.[62] The cortisol level rise is reported after social stress[61] and is among most promising stress related biomarker in animals.[63] The correlation between cortisol levels and clinical depression is also confirmed during many studies.[64] Increased stress leading to exaggerated secretion of stress hormones (e.g., cortisol) in part, due to negative feedback to the HPA axis.[65] The elevated levels of serum cortisol in the OBX-Iso stress control rats in this study are in line with these observations.
In this study, we investigated effects of fluoxetine and 4-HI on serum cortisol levels on socially isolated OBX rats during our study. The subacute pretreatment fluoxetine failed to prevent OBX-Iso stress-induced elevation in cortisol levels and in agreement with reports of elevated cortisol levels after subchronic treatment of fluoxetine to OBX mice.[66] In this study, the subacute pretreatment of 4-HI showed strong and dose-dependent prevention of OBX-Iso stress-induced rise in serum cortisol levels. Our results from anxiety related behavioral (OFT and NSF) and neurochemical (serotonin and cortisol) parameters suggest prevention of hyperactive HPA axis in OBX-Iso stress-induced rats as probable mechanism of action.
CONCLUSION
4-HI demonstrated promising antidepressant and antianxiety effects in OBX-Iso stress-induced rats probably through stress relieving action on hyperactive HPA axis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Padmaja Kalshetti
Padmaja Kalshetti, is Assistant Professor, Department of Pharmacology, MAEER'S Maharashtra Institute of Pharmacy, Pune and is a doctoral student at Jawaharlal Nehru Technological University Hyderabad, India. Graduated and Post graduated in Bharati Vidyapeeth Deemed University, India. Her doctoral research is focused on the pharmacological evaluation of some phytoconstituents in behavioral disorders. She has teaching experience in the areas of Pharmacology.

Dr. Ramesh Alluri
Dr. Ramesh Alluri, is a Professor and Principal of Vishnu Institute of Pharmaceutical Education and Research. He has around 15 years of teaching and 6 years research experience. He is Ph.D. from Jawaharlal Nehru Technological University Hyderabad, India. Presently he is Professor, Shri Vishnu College of Pharmacy, Andhra Pradesh, India and the Secretary of Association of Pharmaceutical teachers of India (APTI), Andhra Pradesh State. He has guided many M. Pharm and Ph.D. students at various Universities, has 49 (including many awarded) research publications, and received grants from foundation of MSME and DST (cognitive science) worth of 41 lakhs.

Vishwaraman Mohan
Vishwaraman Mohan, is Senior Vice President at Indus Biotech Private Limited, Pune, India. He has 42 years of professional experience in reputed pharmaceutical industries like Cipla, Ranbaxy etc., with 42 publication in peer-reviewed international journals and 33 papers in conference proceedings. His research interest is isolation and characterization of phytochemicals and computer aided drug design and discovery of bioactive phytoconstituents.

Dr. Prasad Thakurdesai
Dr. Prasad Thakurdesai, is GM-Scientific affairs, Indus Biotech Private Limited, Pune, India. He is PhD from Nagpur University, Nagpur, India and has 24 years of professional experience with 77 research publications in international peer reviewed journals, 49 papers in international conferences, 1 book and 1 book chapter. He has guided 2 PhD and more than 30 M Pharm students. His research interest area includes medicinal plants research, nutraceuticals, and patient reported outcome (PRO).
Acknowledgment
The authors would like acknowledge Dr. B. S. Kuchekar, Principal, Maharashtra Institute of Pharmacy, Pune University, Pune, India for providing necessary facilities to carry out the study.
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global burden of disease study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Cheung BM, Au T, Chan S, Lam C, Lau Sh, Lee R, et al. The relationship between hypertension and anxiety or depression in Hong Kong Chinese. Exp Clin Cardiol. 2005;10:21–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi MH, Hollander E, Nutt D, Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69:246–58. doi: 10.4088/jcp.v69n0211. [DOI] [PubMed] [Google Scholar]
- 4.Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: A critical review. J Clin Psychopharmacol. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Lane RM. SSRI-induced extrapyramidal side-effects and akathisia: Implications for treatment. J Psychopharmacol. 1998;12:192–214. doi: 10.1177/026988119801200212. [DOI] [PubMed] [Google Scholar]
- 6.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–9. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 7.de Jong TR, Veening JG, Olivier B, Waldinger MD. Oxytocin involvement in SSRI-induced delayed ejaculation: A review of animal studies. J Sex Med. 2007;4:14–28. doi: 10.1111/j.1743-6109.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: A review of the evidence for drug-induced sexual dysfunction. J Affect Disord. 2002;69:119–40. doi: 10.1016/s0165-0327(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 9.Haenisch B, Bönisch H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2011;129:352–68. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Venero JL, Herrera AJ, Machado A, Cano J. Changes in neurotransmitter levels associated with the deficiency of some essential amino acids in the diet. Br J Nutr. 1992;68:409–20. doi: 10.1079/bjn19920098. [DOI] [PubMed] [Google Scholar]
- 11.Yokogoshi H, Nomura M. Effect of amino acid supplementation to a low-protein diet on brain neurotransmitters and memory-learning ability of rats. Physiol Behav. 1991;50:1227–32. doi: 10.1016/0031-9384(91)90587-e. [DOI] [PubMed] [Google Scholar]
- 12.Fernstrom JD. Aromatic amino acids and monoamine synthesis in the central nervous system: Influence of the diet. J Nutr Biochem. 1990;1:508–17. doi: 10.1016/0955-2863(90)90033-h. [DOI] [PubMed] [Google Scholar]
- 13.Coppola A, Wenner BR, Ilkayeva O, Stevens RD, Maggioni M, Slotkin TA, et al. Branched-chain amino acids alter neurobehavioral function in rats. Am J Physiol Endocrinol Metab. 2013;304:E405–13. doi: 10.1152/ajpendo.00373.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M, Lin AH, Verkerk R, Delmeire L, Van Gastel A, Van der Planken M, et al. Serotonergic and noradrenergic markers of post-traumatic stress disorder with and without major depression. Neuropsychopharmacology. 1999;20:188–97. doi: 10.1016/S0893-133X(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 15.Takada A, Grdisa M, Diksic M. Blood-brain barrier transfer of L-Trp and alpha-MTrp in Li-treated rats. Neurochem Int. 1992;21:513–9. doi: 10.1016/0197-0186(92)90082-3. [DOI] [PubMed] [Google Scholar]
- 16.Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab. 2004;287:E463–71. doi: 10.1152/ajpendo.00163.2003. [DOI] [PubMed] [Google Scholar]
- 17.Gaur V, Bodhankar SL, Mohan V, Thakurdesai P. Antidepressant-like effect of 4-hydroxyisoleucine from Trigonella foenum graecum L. seeds in mice. Biomed Aging Pathol. 2012;2:121–5. [Google Scholar]
- 18.Vetulani J, Sulser F. Action of various antidepressant treatments reduces reactivity of noradrenergic cyclic AMP-generating system in limbic forebrain. Nature. 1975;257:495–6. doi: 10.1038/257495a0. [DOI] [PubMed] [Google Scholar]
- 19.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence 1965. J Neuropsychiatry Clin Neurosci. 1995;7:524–33. doi: 10.1176/jnp.7.4.524. [DOI] [PubMed] [Google Scholar]
- 20.File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- 21.Dandekar MP, Singru PS, Kokare DM, Subhedar NK. Cocaine- and amphetamine-regulated transcript peptide plays a role in the manifestation of depression: Social isolation and olfactory bulbectomy models reveal unifying principles. Neuropsychopharmacology. 2009;34:1288–300. doi: 10.1038/npp.2008.201. [DOI] [PubMed] [Google Scholar]
- 22.Song C, Leonard B. The effect of olfactory bulbectomy in the rat, alone or in combination with antidepressants and endogenous factors, on immune function. Hum Psychopharmacol Clin Exp. 1995;10:7–18. [Google Scholar]
- 23.Linge R, Pazos Á, Díaz Á. Social isolation differentially affects anxiety and depressive-like responses of bulbectomized mice. Behav Brain Res. 2013;245:1–6. doi: 10.1016/j.bbr.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan H, Mohan V. Isolation of (2S,3R,4S)-4-hydroxyisoleucine from Trigonella foenum graecum seeds. Asian J Chem. 2014;26:3082–4. [Google Scholar]
- 25.Redmond AM, Kelly JP, Leonard BE. Behavioural and neurochemical effects of dizocilpine in the olfactory bulbectomized rat model of depression. Pharmacol Biochem Behav. 1997;58:355–9. doi: 10.1016/s0091-3057(97)00259-1. [DOI] [PubMed] [Google Scholar]
- 26.Schlumpf M, Lichtensteiger W, Langemann H, Waser PG, Hefti F. A fluorometric micromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue. Biochem Pharmacol. 1974;23:2437–46. doi: 10.1016/0006-2952(74)90235-4. [DOI] [PubMed] [Google Scholar]
- 27.do Rego JC, Benkiki N, Chosson E, Kabouche Z, Seguin E, Costentin J. Antidepressant-like effect of hyperfoliatin, a polyisoprenylated phloroglucinol derivative from Hypericum perfoliatum (Clusiaceae) is associated with an inhibition of neuronal monoamines uptake. Eur J Pharmacol. 2007;569:197–203. doi: 10.1016/j.ejphar.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–32. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 29.Teo AR, Lerrigo R, Rogers MA. The role of social isolation in social anxiety disorder: A systematic review and meta-analysis. J Anxiety Disord. 2013;27:353–64. doi: 10.1016/j.janxdis.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, et al. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res. 2007;178:262–73. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Võikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: Assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–52. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 32.Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–21. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Malkesman O, Pine DS, Tragon T, Austin DR, Henter ID, Chen G, et al. Animal models of suicide-trait-related behaviors. Trends Pharmacol Sci. 2009;30:165–73. doi: 10.1016/j.tips.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucignat-Caretta C, Bondí M, Caretta A. Time course of alterations after olfactory bulbectomy in mice. Physiol Behav. 2006;89:637–43. doi: 10.1016/j.physbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 38.Willner P, Muscat R, Papp M. An animal model of anhedonia. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):550A–1A. doi: 10.1097/00002826-199201001-00286. [DOI] [PubMed] [Google Scholar]
- 39.Romeas T, Morissette MC, Mnie-Filali O, Piñeyro G, Boye SM. Simultaneous anhedonia and exaggerated locomotor activation in an animal model of depression. Psychopharmacology (Berl) 2009;205:293–303. doi: 10.1007/s00213-009-1539-y. [DOI] [PubMed] [Google Scholar]
- 40.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–16. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 1992;109:433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- 42.Tang M, Lei J, Sun X, Liu G, Zhao S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013;1513:127–34. doi: 10.1016/j.brainres.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Zueger M, Urani A, Chourbaji S, Zacher C, Roche M, Harkin A, et al. Olfactory bulbectomy in mice induces alterations in exploratory behavior. Neurosci Lett. 2005;374:142–6. doi: 10.1016/j.neulet.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 44.Saitoh A, Hirose N, Yamada M, Yamada M, Nozaki C, Oka T, et al. Changes in emotional behavior of mice in the hole-board test after olfactory bulbectomy. J Pharmacol Sci. 2006;102:377–86. doi: 10.1254/jphs.fp0060837. [DOI] [PubMed] [Google Scholar]
- 45.Mar A, Spreekmeester E, Rochford J. Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology (Berl) 2000;150:52–60. doi: 10.1007/s002130000400. [DOI] [PubMed] [Google Scholar]
- 46.Wang YQ, Tu ZC, Xu XY, Li R, Qu WM, Urade Y, et al. Acute administration of fluoxetine normalizes rapid eye movement sleep abnormality, but not depressive behaviors in olfactory bulbectomized rats. J Neurochem. 2012;120:314–24. doi: 10.1111/j.1471-4159.2011.07558.x. [DOI] [PubMed] [Google Scholar]
- 47.Mar A, Spreekmeester E, Rochford J. Fluoxetine-induced increases in open-field habituation in the olfactory bulbectomized rat depend on test aversiveness but not on anxiety. Pharmacol Biochem Behav. 2002;73:703–12. doi: 10.1016/s0091-3057(02)00881-x. [DOI] [PubMed] [Google Scholar]
- 48.Sakakibara H, Ishida K, Izawa Y, Minami Y, Saito S, Kawai Y, et al. Effects of forced swimming stress on rat brain function. J Med Invest. 2005;52(Suppl):300–1. doi: 10.2152/jmi.52.300. [DOI] [PubMed] [Google Scholar]
- 49.Nagaraja H, Jeganathan P. Forced swimming stress-induced changes in the physiological and biochemical parameters in albino rats. Indian J Physiol Pharmacol. 1999;43:53–9. [PubMed] [Google Scholar]
- 50.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–14. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 51.Han F, Nakano T, Yamamoto Y, Shioda N, Lu YM, Fukunaga K. Improvement of depressive behaviors by nefiracetam is associated with activation of CaM kinases in olfactory bulbectomized mice. Brain Res. 2009;1265:205–14. doi: 10.1016/j.brainres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Smaga I, Pomierny B, Krzyzanowska W, Pomierny-Chamiolo L, Miszkiel J, Niedzielska E, et al. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: Behavioral and biochemical analyses in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:280–7. doi: 10.1016/j.pnpbp.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 54.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–44. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, et al. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–6. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002;926:10–7. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- 57.Sorenson AN, Sullivan EC, Mendoza SP, Capitanio JP, Higley JD. Serotonin transporter genotype modulates HPA axis output during stress: Effect of stress, dexamethasone test and ACTH challenge. Transl Dev Psychiatry. 2013;1:21130. doi: 10.3402/tdp.v1i0.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler TR, Ariwodola OJ, Weiner JL. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci. 2014;7:102. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young R. Trauma, attempted suicide, and morning cortisol in a community sample of adolescents. J Trauma Stress. 2010;23:288–91. doi: 10.1002/jts.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–8. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 61.Stafford M, Gardner M, Kumari M, Kuh D, Ben-Shlomo Y. Social isolation and diurnal cortisol patterns in an ageing cohort. Psychoneuroendocrinology. 2013;38:2737–45. doi: 10.1016/j.psyneuen.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–51. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preti A. Animal model and neurobiology of suicide. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:818–30. doi: 10.1016/j.pnpbp.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 64.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Rittenhouse PA, López-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–18. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 66.Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Grando J, et al. Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol Biochem Behav. 2012;103:220–9. doi: 10.1016/j.pbb.2012.08.024. [DOI] [PubMed] [Google Scholar]