Abstract
Background:
Emblica officinalis (Euphorbiaceae), popularly known as Indian gooseberry or “Amla” in India, is used in Ayurveda as “rejuvenating herb” since ancient times.
Objective:
This study was carried out to estimate toxicity, anti-inflammatory, and antioxidative activities of the methanolic extract of Emblica officinalis fruit (MEO) in an animal model.
Materials and Methods:
Antioxidative property of MEO was assessed by in vitro assays such as phosphomolybdenum assay (total antioxidant capacity), free radical scavenging assays 1,1-diphenyl-2-picrylhydrazyl and 2,2’-azino-bis and 3-ethylbenzthiazoline-6-sulphonic acid (DPPH and ABTS method) and lipid peroxidation assay (LPO). The anti-inflammatory property was evaluated by carrageenan-induced acute inflammation in rats by measuring rat paw volume at different time intervals and toxicological analysis using mice.
Results and Discussion:
High performance liquid chromatography studies revealed the presence of gallic acid (2.10%), mucic acid (4.90%), ellagic acid (2.10%), quercetin (28.00%), rutin (3.89%), and β-glucogallin (1.46%). MEO showed highest antioxidant activities by using DPPH (17.33–89.00%), ABTS (23.03–94.16%), nitric oxide scavenging activity (12.94–70.16%), LPO (56.54%), and phosphomolybdenum assay (142 ± 6.09 μg/ml). The LD50 was found to be approximately 1125 mg/kg (p.o). High dose of MEO showed significant reduction (72.71%) in the inflammation after 4 h of treatment, which was comparable to diclofenac (10 mg/kg) (61.57%) treated group. Significant reduction (P < 0.05) in the inflammatory cytokine (interleukin-1β and tumor necrosis factor-α) markers were also observed (57.25% and 35.41%, respectively) in serum of MEO treated animals as compared to control.
Conclusion:
Taken together, phenolic compounds of MEO may serve as a potential herbal drug for amelioration of acute inflammation due to their modulatory action on free radicals.
SUMMARY
The methanolic extract of Emblica officinalis fruit (MEO) has potent antioxidant activity as assessed by DPPH, ABTS and LPO assays
MEO has potent anti-inflammatory activity in carrageenan induced paw edema model
The phenolic compounds of MEO might be a potential herbal drug for amelioration of acute inflammation.
Abbreviations used: ROS, reactive oxygen species; RNS, reactive nitrogen species, LPO, lipid peroxidation, NO, nitric oxide, IL, interleukin; TNF α tumor necrosis factor alpha; NSAIDs, nonsteroidal anti inflammatory drugs; AA, ascorbic acid; MEO, methanolic extract of Emblica officinalis fruit; ABTS+; 2,2’ azino bis 3 ethylbenzthiazoline 6 sulphonic acid; DPPH, 1,1 diphenyl 2 picrylhydrazyl; HPLC, high performance liquid chromatography; MDA, malondialdehyde; DMSO, dimethyl sulphoxide; ELISA, enzyme linked immunosorbent assay.
Keywords: 1-diphenyl-2-picrylhydrazyl, acute inflammation, amla, antioxidant, Emblica officinalis, high performance liquid chromatography
INTRODUCTION
There is an escalating demand for herbal drug therapy, many plant species are being explored and are used in various human cultures around the world for their medicinal properties.[1] These natural drugs help in maintaining the homeostasis. When cells are under stress, they produce free radicals like reactive oxygen species (ROS) and reactive nitrogen species (RNS) that damage the cells in many ways such as lipid peroxidation (LPO), protein denaturation, and DNA damage.[2] Nitric oxide (NO) is an important mediator of many physiological functions and its role in the inflammatory process is gaining recognition.[3] Natural antioxidants have an important role in protecting the cells against damage by ROS/RNS, which can lead to several afflictions such as inflammation[4,5] and diabetes.[6]
Inflammation is one of the first responses to cell and tissue damage inflicted by diverse factors such as infections and chemicals and physical injuries. Inflammation is caused by the release of various inflammatory mediators such as cytokines ([interleukin [IL]-1β, IL-6), tumor necrosis factor alpha (TNF-α), prostaglandins, bradykinin, histamine, growth factors, neurogenic factors, and followed by central sensitization and hypersensitivity.[7] During such conditions, nonsteroidal anti-inflammatory drugs (NSAIDs) are used to alleviate pain and reduce inflammation.[8] However, long-term use of these NSAIDs may lead to certain types of ulcers and other disorders,[9] and hence there is a prompt need to search for an alternative to these synthetic drugs where herbal medicines can be suitable candidates.
Emblica officinalis Gaertn., syn. Phyllanthus emblica L., popularly known as Indian gooseberry, is a small to medium-sized deciduous tree, native to India and the Middle East, belonging to the family Euphorbiaceae. It is commonly known as “Amla” in India and is used in Ayurveda as “rejuvenating herb” since ancient times.[10] E. officinalis extract contains several antioxidants such as emblicanin A and B, gallic acid, ellagic acid, ascorbic acid (AA) (Vitamin C), which are used to treat several medical conditions.[11] It also possesses several attributes like antipyretic, analgesic, antimicrobial, antifungal, antitussive, chemopreventive, immunostimulatory, hepatoprotective, cardioprotective, radioprotective, and potential to increase hemoglobin.[12] Despite its wide-ranging medicinal uses, there are very few scientific evidence showing correlation between in vivo anti-inflammatory effects and antioxidant properties for this wonder berry. Hence, the present report emphasizes on in vivo anti-inflammatory effectiveness of methanolic extract of Emblica officinalis fruit (MEO) using carrageenan-induced rat paw oedema model and a wide spectrum of in vitro antioxidant studies which includes models like 2,2’-azino-bis 3-ethylbenzthiazoline-6-sulphonic acid (ABTS+), 1,1-diphenyl-2-picrylhydrazyl (DPPH), molybdenum reduction method, NO determination, and LPO, along with quantitative analysis of potential antioxidant compounds of MEO by high performance liquid chromatography (HPLC).
MATERIALS AND METHODS
Chemicals and reagents
Acetic anhydride, ammonium hydroxide, AA, butylated hydroxytoluene, 2-deoxy-2-ribose, DPPH, ferric chloride, gallic acid, magnesium ribbon, quercetin, sodium nitrite, and trichloroacetic acid were obtained from HiMedia Laboratories, Pvt., Ltd., Mumbai, India. Thiobarbituric acid was obtained from Loba Chemical, Mumbai, India. Ammonium persulphate, benzene, chloroform, di-potassium hydrogen phosphate, ethanol, ethylenediaminetetraacetic acid, glacial acetic acid, hydrogen peroxide, methanol, 5% o-phosphoric acid, 0.01% naphthylethylene diamine, potassium di-hydrogen phosphate, potassium ferricyanide, potassium hydroxide, sodium hydroxide, and sulfanilamide were obtained from Rankem, Mumbai, India. ABTS and gallic acid (3,4,5-trihydroxy benzoic acid) were obtained from Sigma, USA. Conc. HCl, conc. H2 SO4, Fehling's solution, Mayer's reagents, and sodium carbonate were procured from Merck, Mumbai, India. Folin-Ciocalteu reagent was from Sisco Research Laboratory, Mumbai, India. Aluminium chloride was obtained from SD Fine Chemicals Limited, Mumbai, India. All chemicals and solvents were of analytical grade.
Preparation of plant material
Methanolic extract was prepared using 500 g of coarse dried fruit powder, which was extracted in Soxhlet apparatus with 2 l of methanol for 24 h, in a three cycle process. Then, the mixture was filtered, concentrated, and lyophilized (LyoQuest, Telstar, Spain).[13] The MEO was dissolved in double-distilled water in desired concentrations before analysis.
In vitro assays
3-ethylbenzthiazoline-6-sulphonic acid radical scavenging assay
ABTS radical cations were produced by mixing ABTS and MEO and incubating the mixture at room temperature for 16 h in dark condition. This was followed by addition of 20 µl of 10 mM phosphate buffer saline (pH 7.4), test solutions of various concentrations, and 230 µl of ABTS radical solution (0.238 mM).[14] The absorbance was measured immediately at 734 nm spectrophotometer (Elico, SL 210). A control reaction was carried out without the test sample. Linear graph of concentration against percentage inhibition was constructed, and IC50 value was calculated.
1,1-diphenyl-2-picrylhydrazyl radical scavenging assay
Different concentrations of test solution and 50:l of DPPH (0.659 mM) solution was incubated at 25°C for 20 min, after which the absorbance was read at 510 nm using spectrophotometer (Elico, SL 210).[15] A control reaction was carried out without the test sample. Linear graph of concentration against percentage inhibition was constructed, and IC50 value was calculated. The percentage inhibition was calculated according to the following equation:
% inhibition = (A0 − At)/A0 × 100
where A0 was the absorbance of the control (blank, without extract), and At was the absorbance in the presence of the extract.
Determination of total antioxidant capacity by phosphomolybdenum assay
The total antioxidant capacity of MEO was assessed by phosphomolybdenum method.[16] To 0.3 mL of extract solution (1 mg/mL), 3 mL reagent solution (28 mM sodium phosphate, 6 M sulfuric acid and 4 mM ammonium molybdate) was added and incubated at 95°C for 90 min. Then, the absorbance of the solution was measured at 695 nm against blank spectrophotometer (Elico, SL 210). The antioxidant capacity of the extract was evaluated as AA equivalents.
Lipid peroxidation assay
Malondialdehyde (MDA), a marker of LPO was estimated by the method of Okhawa et al.[17] and as modified by Middha et al.[18] using 1,1,3,3-tetramethoxy propane as the standard, measured at 532 nm and expressed in nM/mg protein.
Quantitative analysis of antioxidant compounds
High-performance liquid chromatography analysis
HPLC (Waters Corporation, Singapore) protocol was followed as per Middha et al.[19] Active constituent in the MEO extract was dissolved in a mixture of methanol and water (6:4 v/v), and identified by comparison of the retention time in chromatogram with standard Vitamin C, gallic acid, rutin, quercetin, and ellagic acid (Sigma Chemical Co., St Louis, USA). Data were analyzed using Empower software (Waters Corporation, Singapore).
In vivo assay
Animals
Male Wistar albino rats (140–160 g) and Swiss albino mice (25–30 g) were ordered from Indian Institute of Sciences (Bangalore, India), housed in groups of 4 and given 7 days to acclimate to the housing facility in polypropylene, polycarbonate, and stainless steel cages. Rats were randomly selected and sheltered under standard laboratory conditions of light and dark cycles of 7:00 am to 7:00 pm, temperature of 25°C ± 2°C, lighting of 350 lux, and 68% ± 1% relative humidity and given access to rat maintenance food (Lipton India Ltd., Bangalore, India) and tap water ad libitum. Cages (size 421 mm × 290 mm × 190 mm with a gap of 7 mm between wires) and corn cob bedding were changed every alternate day to ensure that animals are clean and dry as per the National Institutes of Health guidelines.[20] During housing, animal's health status was monitored twice daily, and no adverse events were detected. The study protocol was approved by Maharani Lakshmi Ammanni College Ethical Committee, clearance from Ethical Committee (1368/ac/10/CPCSEA), Bangalore, India.
Acute toxicity test
Swiss albino mice (25–30 g) of both sexes were divided into six groups of 10 each. Animals were housed 5 per cage and were starved overnight but were given water preceding the experiment. MEO was administered to each group at different dose levels of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 g/kg BW/ml. The mice were kept under observation for 24 h, mortality was recorded, and LD50 (median lethal dose) was determined as per Middha et al.[18]
Anti-inflammatory activity
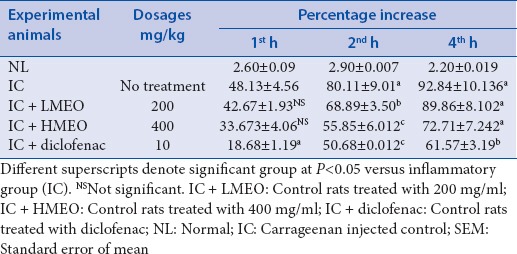
The carrageenan-induced rat paw edema test[21] was used as an experimental model for testing the anti-inflammatory activity of MEO. The rats were divided into five groups of six rats each as shown in Table 1. The investigated MEO, dissolved in dimethyl sulphoxide (DMSO) were administered orally in doses of 200 mg/kg and 400 mg/kg; these doses were selected based on an initial toxicity screening for up to 24 h. Diclofenac (10 mg/kg) (Sigma Chemical Co., St. Louis, USA) dissolved in DMSO, was used as a reference drug. The control animals were given the vehicle (1 ml/kg p.o. DMSO). 1 h after the oral administration of the extracts or diclofenac, carrageenan-saline solution (0.5% w/v) and saline were injected in a volume of 0.1 ml into the plantar surface of the right and left hind paw, respectively. Left paw served as the control (noninflamed) paw. The animals were observed for 0th h, 1st h, 2nd h, and 4th h; the paw volume was measured using plethysmometer (PanLab, Barcelona, Spain).[21] The anti-inflammatory effect was calculated using the equation below:
Table 1.
Paw volume variation due to anti-inflammatory effects of methanolic extract of Emblica officinalis fruit
Anti-inflammatory effect (%) = k − e/k × 100
where k is the difference in the paw weight in the control group and e is the difference in the paw weight in the treatment group.
Finally, after drug treatments, rats were euthanized using 0.3 ml/100 g i.p ketamine (300 mg/kg) +0.15 ml/100 g xylazine (30 mg/kg) as recommended by Committee for the Purpose of Control and Supervision of Experiments on Animals. After euthanization, the carcasses were placed in a non-PVC containing, sealable, and transparent plastic bag and were sent to incinerator. Blood samples were collected by heart puncture method, and serum was separated for biochemical estimations.
Measurement of interleukin-1β and tumor necrosis factor-α levels
The serum concentration of IL-1β was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit method (Biosource, San Diego, California)[22] and TNF-α was measured with another ELISA kit (Endogen, Woburn, MA, USA).[23]
Statistical analysis
All the measurements were repeated six times and expressed as a mean ± standard error of means and Statistically analyzed by two way analysis of variance followed by Tukey's multiple ranges using GraphPad prism (GraphPad, San Diego, CA, USA)
RESULTS
Yield of the extract
The total yield of the extract was found to be 9.8%.
In vitro activity
3-ethylbenzthiazoline-6-sulphonic acid radical scavenging assay
A dose-dependent inhibition of scavenging activity was displayed by MEO to ABTS radical [Figure 1]. The percentage of ABTS scavenging by MEO was found to be at 23.03% and 94.16% at 10 µg/ml and 100 µg/ml, respectively.
Figure 1.
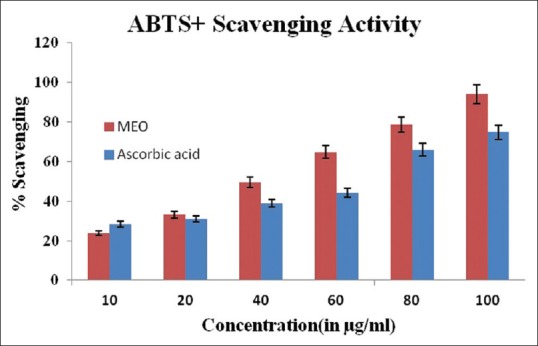
ABTS+ radical scavenging activity of methanolic extract of Emblica officinalis fruit compared to standard ascorbic acid
1-diphenyl-2-picrylhydrazyl radical scavenging assay
MEO showed radical scavenging activity as measured by DPPH, across concentrations ranging from 0.1 to 1.0 µg/ml [Figure 2]. The values for maximum % inhibition at 0.1–1 mg/ml ranged from 7.33% to 83.33% in MEO, whereas that of standard (AA) was found to be 90%. The high DPPH scavenging activities of the methanolic extract have already been reported by other researchers.[24] IC50 of MEO was found to be 0.628 µg/ml.
Figure 2.
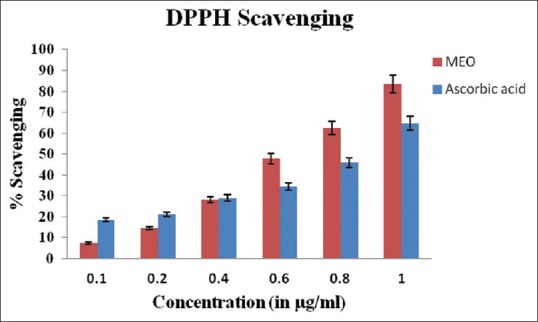
DPPH scavenging activity of methanolic extract of Emblica officinalis fruit compared to standard ascorbic acid
Determination of total antioxidant capacity by phosphomolybdenum assay
The principle includes the reduction of Mo (VI) to Mo (V) by the extract that contains antioxidant compounds. In this study, MEO was more effective in reduction of Mo (VI) to Mo (V) (142.0 ± 6.09 μg/ml). The reduction of Mo (VI) to Mo (V) by administration of reference chemical, AA (IC50 81.3 ± 4.12 μg/ml), suggested the presence of effective antioxidants in MEO.
Lipid peroxidation assay
Decrease in the MDA levels is a good indication of an effective antioxidant. There was a significant dose-dependent reduction in MDA levels by MEO. Maximum percentage reduction in MDA levels of 56.54% was observed at 300 μg/ml concentration of MEO.
High performance liquid chromatography analysis
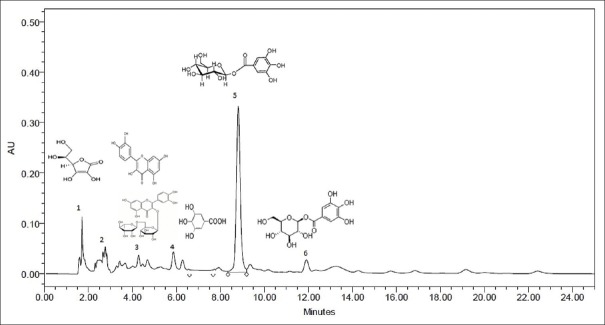
HPLC method has been reported to be simple, easy to use, and effective enough for the identification and quantification of major phenolic compounds found in aromatic plants,[19,25] despite the fact that the separation of procyanidins is not satisfactory with these phases.[25] To deduce a better separation of phenolic acids and flavonoids in aromatic plants, we have used Spherisorb C18 stationary phase, which provided a quite fine outcome. After extraction and acid hydrolysis, the content of phenolic substances was determined. Quantification was done via calibration with standards. The amount of phenolic acids such as quercetin (28.00 mg/100 g), rutin (3.89 mg/100 g), β-glucogallin (0.146 mg/ml), gallic acid (2.10 mg/100 g), and mucic acid (4.90 mg/100 g) were detected in the analyzed samples [Figure 3]. Quercetin has been reported to be present in a large number of herbals.[19,26] Rutin in some cases can be hydrolyzed to quercetin (aglycone). This could have happened in our sample of interest. Thus, hydrolysis was needed for the identification and quantitative determination of phenolic acids. Time of harvest and storing conditions might have an effect on the variations in the phenolic compounds.
Figure 3.
High performance liquid chromatography chromatogram of ascorbic acid (1), quercetin (2), rutin (3), gallic acid (4), mucic acid (5), and beta-glucogallin (6) in methanolic extract of Emblica officinalis fruit
Assessment of pharmacological activity (in vivo)
Acute toxicity
In vivo median lethal dose or LD50 of the extract was 1125 mg/kg BW. Other parameters such as body weight and survival rate showed no side effects to MEO (data not shown).
Anti-inflammatory activity
Carrageenan-induced paw edema: MEO significantly (P < 0. 01) exhibited anti-inflammatory activity in a dose-dependent manner at 4 h. The reduction in carrageenan-induced paw edema by high dosage of MEO (HMEO) after 4 h was 72.71% while edema reduction by the standard drug, diclofenac (10 mg/kg) was 61.57% [Table 1]. MEO produced a dose-dependent inhibition of paw edema, which was comparable to the effect of known anti-inflammatory drug, diclofenac.
Effect of methanolic extract of Emblica officinalis on different cytokine levels
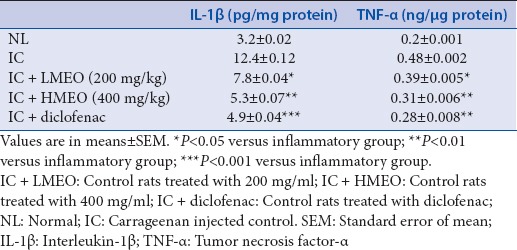
Cytokines are small glycoproteins counteracted in response to an antigen and initially defined as a mediator for regulating the innate and adaptive immune reactions. At the time of inflammation, IL-1β and TNF-α are the two major cytokines, which play a major role in leading mechanisms.[7] The levels of these cytokines should be suppressed to reduce the severity of the inflammatory reaction. Table 2 indicates that inflammatory reaction induced by carrageenan significantly increased the concentration of IL-1β and TNF-α (P < 0.05). MEO treatment decreased the level of these cytokines significantly as compared to the inflammation-induced group.
Table 2.
Cytokines levels IL-1β and TNF-α of experimental animals (n=6)
DISCUSSION
Quite a lot of present day diseases are due to the shift in the balance of pro-oxidant and antioxidant homeostasis in the body.[13,27] Inflammation is the response to injury of cells and body tissues through different factors such as infections and chemicals and mechanical injuries.[22,28] The pathogenesis of inflammation is brought about by free radicals.[22,29,30] Plants have many compounds which show different bioactivities, including antioxidant and anti-inflammatory, which are experimented on because of their numerous mechanisms of actions with non-toxic nature.[19] Antioxidants interfere with the oxidative processes by scavenging free radicals and chelating free catalytic metals.[31] There are numerous NSAIDs existing in the market, but these drugs have been shown to have certain side effects that may cause cardiovascular and gastrointestinal disorders; therefore, it is important to endorse the drugs from natural sources.[32]
Emblica has been used for the treatment of arthritis and other inflammatory diseases.[32] A wide variety of in vitro methods such as DPPH and ABTS are used to assess radical scavenging ability of certain agents from natural and synthetic sources. DPPH is a stable free radical, and its activity is based on its ability to decolorize from deep purple color at 517 nm to light yellow in the presence of antioxidants forming a stable DPPH molecule.[33] MEO showed an effective radical scavenging ability by this assay. ABTS is a blue colored chromophore and upon addition of MEO, it is reduced and this reduction process is concentration dependent. E. officinalis is a rich source of Vitamin C, alkaloids, phenolics, flavonoids, and tannins.[12] In agreement to this, our study revealed that the MEO has a high level of radical scavenging capacity as evidenced in both DPPH and ABTS assays. This might be because of more phenolic compounds in MEO, which help in scavenging of radicals in the two studied assays and hence attribute to their higher antioxidant activity.[13,33] The phosphomolybdate results further confirmed the potent antioxidant activity of MEO, which increased in a concentration-dependent manner.
In vivo toxicological estimation was performed to evaluate the extent to which a drug can cause deleterious effects to the cells; these tests are essential and widely accepted to know the optimum drug concentration to be administered. LD50 of MEO was measured to check lethal toxicity and was found to be 1125 mg/kg BW. Treatment with MEO at a dose of 200mg/kg and 400mg/kg to their respective group of mice for 1 month did not show any toxic side effect. The selection of the 2 doses 200 mg/kg and 400 mg/kg was based on an initial toxicity study where until 1 month after administration of these doses of the extract, the animals were healthy without any visual signs or symptoms of illness (data not shown). Previously, Golechha et al., 2014[34] showed that a 300–500 mg/kg dose of Emblica has not shown any adverse effect.
Carrageenan induced edema of rat paw is used widely as a working model of acute inflammation in search for new anti inflammatory drugs.[35] During the first 2 h (Phase I), liberation of histamine and serotonin begins in rat paw after carrageenan injection, whereas in Phase II release of kinin, prostaglandin, bradykinin, leukotrienes, and polymorphonuclear cells in the damaged tissue in surroundings cells also increases depending upon the complement system for their activation.[34] Results of standard drug diclofenac sodium, normal saline, and two doses of test extracts (LMEO and HMEO) were compared. Both the tested doses of MEO have shown a significant reduction in paw volume as compared to control group; the efficiency of MEO is very much comparable to that of the standard drug (P < 0.05).
Our in vivo results were strongly in accordance to previous studies.[36] In case of infection, inflammation, and immune activation, IL-1β and TNF-α play a major role. Sometimes, TNF-α can also stimulate the release of IL-1β.[4] The levels of IL-1β and TNF-α of experimental animals showed a significant decrease after administration of MEO as compared to the saline-treated animal group. Rats treated with high dosage (400 mg/ml) showed more potent effect than lower dosage (200 mg/ml) which is very much comparable to the standard drug.
The role of phenolic compounds in inflammatory conditions is well established.[37,38] They also inhibit polymorphonuclear lipoxygenase, an enzyme involved in inflammatory conditions.[37] In this context, E. officinalis has caught the attention of the researchers as it is a potential source of phenolic compounds and flavonoids.[36] These effects may be due to high levels of quercetin, rutin, and other phenolic compounds found in the MEO.[23,28]
CONCLUSIONS
In summary, the results of this study suggest that MEO not only is a storehouse of natural antioxidant but also possesses strong anti-inflammatory activity which might be helpful in preventing or slowing the progress of various oxidative stress-related diseases. The anti-inflammatory activity of MEO may probably be due to the presence of several bioactive anti-inflammatory principals as elucidated by HPLC analysis. However, it needs isolation, structural elucidation, and screening of any of the various active principle (s) to point out the real activity of the drug. Even, an extensive clinical study needs to be hypothesized to ascertain the efficacy and safety of this agent to illustrate an improvement over currently available treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Sushil Kumar Middha
Sushil Kumar Middha: Currently working as a Lecturer/Assistant Professor at the department of Biotechnology, Maharani Lakshmi Ammanni College For Women, Bangalore, India and having expertise in bioinformatics tools, in silico drug discovery methods, pre clinical studies and antioxidants.

Arvind Kumar Goyal
Arvind Kumar Goyal: Currently working as a guest instructor at the Bamboo technology, department of Biotechnology and having expertise in Bamboo tissue culture, biomarkers (RAPD, AFLP) and Phytochemistry.

Dinesh Babu
Dinesh Babu: Currently working as a research scholar at the department of Gastro-intestinal Neuropharmacology, Heymans Institute of Pharmacology, Faculty of Medicine and Health Sciences; Ghent University, Belgium and having expertise in vitro studies (cell lines method) and antioxidant assays.

Prakash Lokesh
Prakash Lokesh: Currently working as a Lecturer/Assistant Professor at the Jain University and proficient in handling HPLC and having expertise in Phytochemistry and Proteomics.

Varsha Yardi
Varsha Yardi: Student in Biochemistry at the Maharani Lakshmi Ammanni College For Women, Bangalore, India.

Lavanya Mojamdar
Lavanya Mojamdar: Student in Biotechnology at the Maharani Lakshmi Ammanni College For Women, Bangalore, India.

Deepthi Sudhir Keni
Deepthi Sudhir Keni: Student in Biotechnology at the Sapthagiri College of Engineering, Bengaluru, Karnataka, India.

Talambedu Usha
Talambedu Usha: Currently working as a Lecturer/Assistant Professor at the department of Biochemistry, Maharani Lakshmi Ammanni College For Women, Bangalore, India and having expertise in biochemical assays, bioinformatics tools and antioxidants.
Acknowledgments
This work was partially supported by the grants from BT-Finishing School, Govt. of Karnataka and Maharani Lakshmi Ammanni College for Women, Bangalore.
REFERENCES
- 1.Middha SK, Mittal Y, Ushal T, Kumar D, Srinivasan R, Vashisth L, et al. Phyto-mellitus: A phyto-chemical database for diabetes. Bioinformation. 2009;4:78–9. doi: 10.6026/97320630004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal AK, Basistha BC, Sen A, Middha SK. Antioxidant profiling of Hippophae salicifolia growing in sacred forests of Sikkim, India. Funct Plant Biol. 2011;38:697–702. doi: 10.1071/FP11016. [DOI] [PubMed] [Google Scholar]
- 3.Damodara Reddy V, Padmavathi P, Gopi S, Paramahamsa M, Varadacharyulu NC. Protective effect of Emblica officinalis against alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian J Clin Biochem. 2010;25:419–24. doi: 10.1007/s12291-010-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik D, Kumar A, Kaushik P, Rana AC. Analgesic and anti-inflammatory activity of Pinus roxburghii Sarg. Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/245431. 245431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad MP, Shekhar S, Amit B. Phytochemical analysis and antioxidant potential of Piper species and its molecular characterization by RAPD markers. Int J Fundam Appl Sci. 2012;1:71–3. [Google Scholar]
- 6.Usha T, Middha SK. A new vista an in vitro new vista to identify hypoglycemic activity. Int J Fundam Appl Sci. 2012;1:27–9. [Google Scholar]
- 7.Gresa-Arribas N, Viéitez C, Dentesano G, Serratosa J, Saura J, Solà C. Modelling neuroinflammation in vitro: A tool to test the potential neuroprotective effect of anti-inflammatory agents. PLoS One. 2012;7:e45227. doi: 10.1371/journal.pone.0045227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009;112:587–94. [Google Scholar]
- 9.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–22. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 10.Khopde SM, Indira Priyadarsini K, Mohan H, Gawandi VB, Satav JG, et al. Characterizing the antioxidant activity of amla (Phyllanthus emblica) extract. Curr Sci. 2001;81:185–90. [Google Scholar]
- 11.Khan KH. Roles of Emblica officinalis in medicine – A review. Bot Res Int. 2009;2:218–28. [Google Scholar]
- 12.Khosla S, Sharma S. A short description on pharmacogenetic properties of Emblica officinalis. Spatula DD. 2012;2:187–93. [Google Scholar]
- 13.Singh R, Singh MK, Chandra LR, Arora MS, Naiwal TK, Pande V. In vitro Antioxidant and free radical scavenging activity of Dolichos biflorus (Gahat dal) from Kumauni region. Int J Fundam Appl Sci. 2012;1:9–11. [Google Scholar]
- 14.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 15.Goyal AK, Middha SK, Sen A. Evaluation of the DPPH radical scavenging activity, total phenols and antioxidant activities in Indian wild Bambusa vulgaris “Vittata” methanolic leaf extract. J Nat Pharm. 2010;1:34–9. [Google Scholar]
- 16.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Middha SK, Bhattacharjee B, Saini D, Baliga MS, Nagaveni MB, Usha T. Protective role of Trigonella foenum graceum extract against oxidative stress in hyperglycemic rats. Eur Rev Med Pharmacol Sci. 2011;15:427–35. [PubMed] [Google Scholar]
- 19.Middha SK, Usha T, Pande V. HPLC evaluation of phenolic profile, nutritive content, and antioxidant capacity of extracts obtained from Punica granatum fruit peel. Adv Pharmacol Sci 2013. 2013 doi: 10.1155/2013/296236. 296236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.8th ed. Washington, DC: The National Academies Press; 2010. National Institutes of Health. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 21.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 22.Usha T, Middha SK, Bhattacharya M, Lokesh P, Goyal AK. Rosmarinic acid, a new polyphenol from Baccaurea ramiflora Lour leaf: A probable compound for its anti-inflammatory activity. Antioxidants. 2014;3:830–42. doi: 10.3390/antiox3040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–92. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 24.Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH* method. J Sep Sci. 2007;30:1250–4. doi: 10.1002/jssc.200600532. [DOI] [PubMed] [Google Scholar]
- 25.Proestos C, Komaitis M. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after silylation. Foods. 2013;2:90–9. doi: 10.3390/foods2010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal 2013. 2013 doi: 10.1155/2013/162750. 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiran B, Lalitha V, Raveesha KA. Psoralea Corylifolia L a potent medicinal plant with broad spectrum of medicinal properties. Int J Fundam Appl Sci. 2013;2:20–2. [Google Scholar]
- 28.Alam B, Akter F, Parvin N, Sharmin Pia R, Akter S, Chowdhury J, et al. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna J Phytomed. 2013;3:112–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Abotsi W, Ainooson G, Woode E. Anti-inflammatory and antioxidant effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt (Phytolaccaceae) Afr J Tradit Complement Altern Med. 2012;9:138–52. doi: 10.4314/ajtcam.v9i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambeoku GJ, Kabatende J. Antinociceptive and anti inflammatory activities of leaf methanol extract of Cotyledon orbiculata L (Crassulaceae) Adv Pharmacol Sci 2013; Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/862625. 862625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal AK, Middha SK, Sen A. In vitro antioxidative profiling of different fractions of Dendrocalamus strictus (Roxb) Nees leaf extracts. Free Radic Antioxid. 2011;1:42–8. [Google Scholar]
- 32.Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goyal AK, Mishra T, Bhattacharya M, Kar P, Sen A. Evaluation of phytochemical constituents and antioxidant activity of selected actinorhizal fruits growing in the forests of Northeast India. J Biosci. 2013;38:797–803. doi: 10.1007/s12038-013-9363-2. [DOI] [PubMed] [Google Scholar]
- 34.Golechha M, Sarangal V, Ojha S, Bhatia J, Arya DS. Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: Involvement of possible mechanisms. Int J Inflam 2014. 2014 doi: 10.1155/2014/178408. 178408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valencia E, Feria M, Díaz JG, González A, Bermejo J. Antinociceptive, anti-inflammatory and antipyretic effects of lapidin, a bicyclic sesquiterpene. Planta Med. 1994;60:395–9. doi: 10.1055/s-2006-959517. [DOI] [PubMed] [Google Scholar]
- 36.Muthuraman A, Sood S, Singla SK. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology. 2011;19:327–34. doi: 10.1007/s10787-010-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang F, Dusting GJ. Natural phenolic compounds as cardiovascular therapeutics: Potential role of their antiinflammatory effects. Curr Vasc Pharmacol. 2003;1:135–56. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- 38.Mogana R, Teng-Jin K, Wiart C. Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq.(Burseraceae Kunth) Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/734824. 734824. [DOI] [PMC free article] [PubMed] [Google Scholar]