Abstract
Background:
Cordyceps sinensis (CS) is a traditional Chinese medicine contains potent active metabolites such as nucleosides and polysaccharides. The submerged cultivation technique is studied for the large scale production of CS for biomass and metabolites production.
Objective:
To optimize culture conditions for large-scale production of CS1197 biomass and metabolites production.
Materials and Methods:
The CS1197 strain of CS was isolated from dead larvae of natural CS and the authenticity was assured by the presence of two major markers adenosine and cordycepin by high performance liquid chromatography and mass spectrometry. A three-level Box-Behnken design was employed to optimize process parameters culturing temperature, pH, and inoculum volume for the biomass yield, adenosine and cordycepin. The experimental results were regressed to a second-order polynomial equation by a multiple regression analysis for the prediction of biomass yield, adenosine and cordycepin production. Multiple responses were optimized based on desirability function method.
Results:
The desirability function suggested the process conditions temperature 28°C, pH 7 and inoculum volume 10% for optimal production of nutraceuticals in the biomass. The water extracts from dried CS1197 mycelia showed good inhibition for 2 diphenyl-1-picrylhydrazyl and 2,2-azinobis-(3-ethyl-benzo-thiazoline-6-sulfonic acid-free radicals.
Conclusion:
The result suggests that response surface methodology-desirability function coupled approach can successfully optimize the culture conditions for CS1197.
SUMMARY
Authentication of CS1197 strain by the presence of adenosine and cordycepin and culturing period was determined to be for 14 days
Content of nucleosides in natural CS was found higher than in cultured CS1197 mycelium
Box-Behnken design to optimize critical cultural conditions: temperature, pH and inoculum volume
Water extract showed better antioxidant activity proving credible source of natural antioxidants.
Keywords: Chinese medicinal mushroom, Cordyceps sinensis, desirability function, large scale, submerged cultivation
INTRODUCTION
Cordyceps sinensis (CS) is one of the valuable entamophagous fungus described in traditional Chinese medicines as rare and exotic medicinal fungi. CS has been treasured throughout Asia as one of the most effective natural tonics to strengthen the vitality and promote longevity. In view of the many positive health effects attributed to CS, it is hailed as wonder herb of traditional Chinese medicines since many centuries.[1] CS parasitizes caterpillar stage of ghost moths (Hepialus armoricanus) and produces a fruiting body assessed as an herbal remedy. It is mainly distributed in China, Tibetan Plateau, Bhutan, Nepal, and the northern part of India at an altitude of 3500–5000 m above sea level. Harvesting and trade activities of CS usually begin in June to July every year. In China, it is called “Dong Chong Xia Cao” which means “winter worm summer grass”[2] and often known as a Himalayan Viagra. In Chinese Pharmacopeia, CS has been regarded as a celebrated drug since 1963 and cited to have similar medical effects as ginseng and deer velvet.[3] CS possess many health benefits such as anti-oxidative effect,[4,5] anti-tumor,[6,7,8] potentiating immune response,[9] anti-inflammatory,[9,10] anti-stress and anti-fatigue,[11] and anti-myocarditis.[12] CS contains a major class of active ingredients such as nucleosides, polysaccharides, sterols,[13] and products formulated with CS have gained great popularity in Eastern medicines.
During the last decade, trade activities have established huge market demand for CS particularly in China, Tibet, Nepal, and Himalayan region. In rural Tibet, CS collection is an important source of income and contributed 40% to local households, 8.5% of the Gross Domestic Product in 2004. The annual production of CS in Tibetan Plateau was estimated in 2009 at 80–175 tonnes and 1 kg of caterpillars traded for US$ 3000 (lowest quality) to over US$ 18000 (best quality, largest larvae) in 2008[14] and highly valued in Nepal and India.[15,16] Overharvesting of CS pose a great threat to the environment and cause serious ecological imbalance. It is feared that the increased pressure of harvesting may lead to the complete disappearance of this species in future. Convention on International Trade in Endangered Species Management Authority of China officially classified CS fungus as an endangered species.[17] Hence regulatory policies, practices, and alternative strategies for large scale production are needed to save CS species. The effective management strategies are required to secure the long-term existence of CS. The review on this important fungus analyzing various aspects such as extraction methods of bio-actives, characterization techniques, and bio-active linked biological functions has appeared recently.[13]
In view of the recent demand for the CS, there are many attempts at cultivating the CS artificially. The initial attempts to develop an efficient technology for cultivation of fruiting bodies became futile. Artificial production of CS in the bioreactor is essential to meet human needs and to mitigate the pressure on natural resources of the species. A successful large-scale production of CS by fermentation is necessary so that fungal strains can be easily isolated from natural CS and manufactured in large quantities by fermentation technology.[18,19,20,21,22] Solid state and submerged fermentations are widely used for the production of CS biomass and its components. Cultivation on the solid medium is adopted by many manufacturers in Japan and United States of America. Although this methodology harvests the mycelium with a maximum recovery of bio-actives, and this can be well-known low-priced technique, but the main disadvantage is that mycelia contain a high content of grain matter than actual CS substance. Liquid or submerged fermentation is a preferred system for efficient production of desired bioactive compounds by mycelia because of the ease with which the conditions can be manipulated and optimized with high mycelia production as demonstrated for various fungi. Dong and Yao successfully optimized the nutritional requirements for mycelia growth of CS in semi-synthetic liquid media by orthogonal matrix method.[23] Other than nutritional requirements, the culture conditions such as temperature, pH, inoculum volume, and dissolved O2 content are crucial for CS.[24] Many studies aimed to isolate CS strain from wild specimens and successfully scaled up the biomass and metabolites production are in literature.[25,26]
Designing experiments building models evaluating the effects of factors and searching optimum condition of factors for desirable responses are the major tasks in any process scale up. The conventional optimization technique, e.g., one factor at a time method, is not only tedious and time consuming, but also misleading in interpretation of results, especially for the interactions among different factors which they are unable to detect. The orthogonal array method is a cost-effective optimization strategy,[27] but it cannot fit the results into a regression equation to locate the optimum level through the entire space of the tested independent variables. On the other hand, the response surface methodology (RSM) is proven to be an efficient statistical technique for the optimization of multiple variables with a minimum number of experiments in order to predict the best conditions.[28,29] RSM involves studying the response of the statistically designed combinations, estimating the coefficients of the mathematical model that best fits the experimental data, predicting the response of the fitted model and validating the adequacy of the model. The most popular experimental designs, that is, central composite design and Box-Behnken design (BBD) of the principal RSM have been widely used in literature.[28,29,30] Box-Behnken, a spherical and revolving design, has been applied to optimization of chemical and physical processes[31] because of its reasoning design and excellent outcomes.
Desirability function analysis is widely used in multi-response optimization of the process to determine desirable solutions when more than one response is involved. Such an approach was originally introduced by Harrington.[32] Derringer and Suich developed another version of desirability function[33] which is now being used widely by researchers.
The objectives of the present work are as follows: (a) To find culturing period for the CS1197 strain. (b) Quantification of major biomarkers adenosine and cordycepin in water extracts of CS1197 dried mycelium and natural CS by high performance liquid chromatography (HPLC) (c) optimization of culturing conditions temperature, pH, and inoculum volume by RSM based on BBD and desirability function analysis to maximize biomass yield, adenosine and cordycepin content.
MATERIALS AND METHODS
Reagents
Potato dextrose broths (PDB), potato dextrose agar (PDA) from Hi-Media Laboratories Pvt. Ltd., India and adenosine (≥99%), cordycepin (≥99%) standards, 2,2-azinobis-(3-ethyl-benzo-thiazoline-6-sulfonic acid (ABTS), Trolox, and 2 diphenyl-1-picrylhydrazyl (DPPH•) from Sigma-Aldrich were purchased. Water and methanol were HPLC grade, and formic acid was analytical grade.
Fungal material
Natural CS (Berk.) Sacc. Specimens were collected from Uttarakhand region, India. The CS1197 strain used in this study was isolated from dead larvae of natural CS, which was collected from the same region by National Type Culture Collection, Forest Pathology Division, Forest Research Institute, Dehradun, India.
Fermentation
The CS fungal strain CS1197 was maintained on PDA slants and sub-cultured every month. Slants were incubated at 24°C for 10 days and stored at 4°C. For seed culture preparation, initially CS1197 was grown on PDA for 7 days, loop of inoculum was transferred to PDB, incubated at 24°C for 7 days and seed culture was used for large-scale production of CS1197 biomass and metabolites.
Growth curve of Cordyceps sinensis in liquid culture
The culturing period for CS1197 was determined to study further the effect of culturing conditions on yields subsequently. Growth curve study was conducted for 8 weeks where 50 ml PDB is having pH 5 ± 1 in 250-ml Erlenmeyer flask was inoculated with CS1197, incubated at 24°C under dark and static condition. Samples were collected at various intervals from the flasks for analyzing biomass dry weight by filtering through Whatman filter paper No. 1 (Whatman TM, GE healthcare, UK), washed thrice with triple distilled water and dried at 50°C overnight.
Water extraction, high-performance liquid chromatography, and mass spectrometry
Adenosine and cordycepin content in natural CS was measured and compared with artificially produced CS1197 biomass. Wild specimens were washed with distilled water to remove dirt and cut to separate dead larvae and fruiting portion. Both dead larvae and fruiting body were dried at 50°C for 3 h. Both dried CS1197 mycelium and natural CS were ground to fine powder and analyzed through a set of sieves (1, 0.5, 0.25, and 0.125 mm) to get powder with different mean particle size. The dried powder with an average particle size of 0.375 mm was used for water extraction. Natural CS (larvae and fruiting body) and CS1197 dried mycelium were extracted with triple distilled water at 80°C for 3 h in shaking hot water bath set at 100 oscillations per min. The samples were centrifuged at 10,000 rpm for 30 min, supernatant collected and water extract yield (wt/wt %) were calculated.
Samples were filtered through 0.22 µm syringe filter (Nupore Filtration Systems Pvt. Ltd., Ghaziabad, India) prior to HPLC. Stock solutions of adenosine and cordycepin standards at 1 mg/ml were prepared by dissolving accurate amounts in a mobile phase and injected at different concentrations for calibration. Water extracts at proper concentration were analyzed using HPLC-8A system (Shimadzu Corporation, Kyoto, Japan), pump with Rheyodyne injector, SPD-M10A VP photo-diode array detector, system controller SCL-10A VP, and software for HPLC peaks integration was Class VP. A prepacked column Waters, Symmetry® C18 (5 µm, 4.6 mm × 250 mm) was used for elution of nucleosides. Isocratic elution method was adopted with a flow rate of 1.0 ml/min for separation of standards and samples using mobile phase consisting of water:methanol:formic acid (95:4:1, V/V). The detection wavelength of photodiode array was 260 nm, and the column temperature was kept 25°C.
Mass spectrometric (MS) experiments were performed on MS, Quadrupole-time-of-flight, Ultima Global, Waters, UK with an electrospray ionization (ESI) interface. MS of each compound was obtained by positively scanning the ratio of mass to electric charge from 50 to 350. MS detection conditions for both ESI + mode was as follows: Capillary voltage - 3.50 kV, cone voltage - 100V, source temperature - 120°C, desolvation temperature - 300°C, cone gas - 50 L/h, and desolvation gas - 500 L/h. Software used was Mass lynx 4.0 (Waters Inc., India).
Effect of culture conditions
The preliminary studies were conducted to select culture conditions such as temperature, pH, inoculum volume, and medium volume that have an influence on biomass, adenosine, and cordycepin yield in CS, CS1197. Liquid cultures were inoculated with CS1197 and incubated statically for 14 days at temperatures 4, 12, 18, 24, and 28°C to determine the effect of temperature on mycelia growth. The effect of media pH on mycelia growth was estimated by setting different pH values. The pH of the medium was adjusted to 4.0, 5.0, 6.0, 7.0, 8.0, and 9.0 with 1N HCl or 1N NaOH solutions and incubated at 24°C for 14 days. The pH was measured using the Cyberscan pH meter (Eutech Instruments, Singapore). Further, the different volumes of seed culture at 2%, 5%, 10%, 15%, or 20% (volume ratio) were inoculated into a 250-ml flask containing 50 ml medium to study the effect of inoculum volume on mycelia growth, and the growth response of CS1197 to levels of medium volume was investigated by filling 50, 75, 100, 125, 150 or 200 ml medium into a 250-ml Erlenmeyer flask incubated at 24°C for 14 days.
Experimental design and statistical analysis
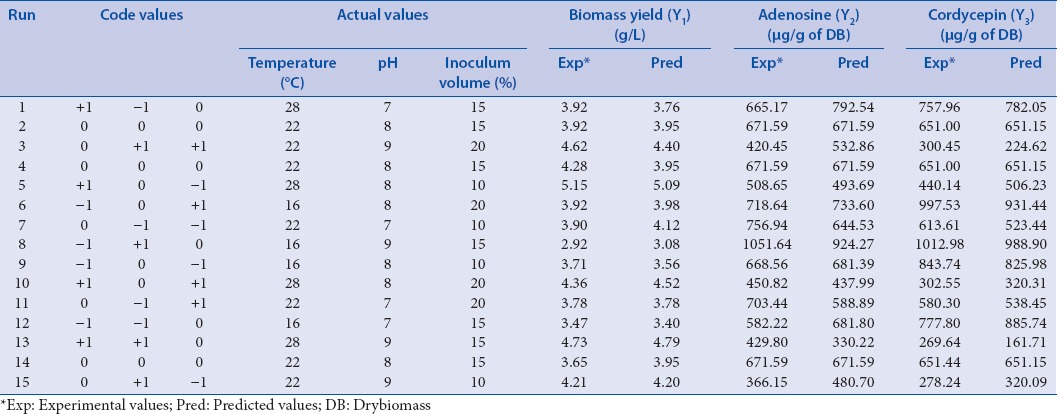
A three-level BBD was employed in the present study, and the optimal conditions were determined through a minimal set of experiments compared with other designs.[34] BBD has been successfully applied to optimize culture conditions for submerged cultivation of Cordyceps spp.[35,36] Fifteen experiments as per the BBD were conducted to explore the effect of culture conditions on three responses biomass yield (Y1), adenosine (Y2), and cordycepin (Y3) contents in the submerged cultivation of CS1197. As shown in Table 1, the three factors chosen for this study were temperature (X1), pH (X2), and inoculum volume (X3) with each factor at three levels high (coded as +1), middle (coded as 0), and low (coded as −1). The design included three replicates at the center point to provide a measure of process stability and inherent variability. All experiments were performed in duplicates. A second-order polynomial model was fitted to correlate the relationship between independent variables and responses. The general form of second order polynomial equation is:
Table 1.
Box-Behnken design with experimental and predicted values of biomass yield, adenosine and cordycepin content
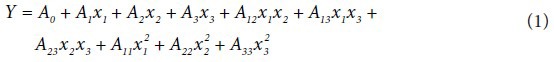
Where Y is the predicted response; A0 is the model constant; A1, A2, and A3 are linear coefficients; A12, A13, and A23 are cross product coefficients; and A11, A22, and A33 are the quadratic coefficients. x1, x2, and x3 are coded values of independent variables x1, x2, and x3, respectively. The quality of fit of the polynomial model to experimental data was expressed by the coefficient of determination R2. ANOVA analysis and regression of the model were carried out using JMP 5.1 statistical software and optimization (JMP 5.1.1, SAS Institute Inc., Cary, NC, USA) of the model maximizing the responses was carried out using “Model fit” option under “Analyze” in JMP 5.1. Response surfaces with contour lines explaining the effect of variables on the responses were plotted using KyPlot software (beta version 2.0, KyensLab Inc., Tokyo) keeping two factors varying while one factor kept constant at the middle level.
Desirability function
Desirability function is a transformation of each response (Yi) to an individual desirability function (di) varying over the range 0–1 scale. If di = 0, the response is completely undesirable and di = 1 then, the response is the most desirable. Several responses are simultaneously optimized, by combining each of these di by means of the geometric mean to create the overall desirability (D).
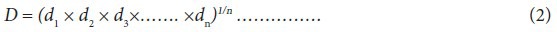
Derringer and Suich proposed different desirability functions di depending on whether a particular response Yi is to be minimized, maximized, or assigned a target value. Li, Ui, and Ti are the lower, upper, and target values, respectively, that are desired for the response Yi with the condition Li ≤Ti ≤Ui. The present study involves determining a single set of process conditions maximizing the 3 responses viz., biomass, adenosine and cordycepin simultaneously.
In our case, the individual desirability function is:
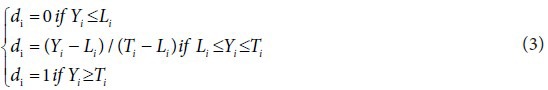
Anti-oxidant activity
Two methods were employed to determine the antioxidant effect of water extracts from CS1197, including the scavenging effect on DPPH• radicals and Trolox equivalent antioxidant capacity (TEAC) assay.
Scavenging effect of CS1197 water extract on DPPH radicals was determined as per the procedure reported in the earlier article.[37]
The TEAC assay was carried out as per method reported with slight modification.[38,39] Briefly, 7 mmol/L ABTS and 2.45 mmol/L potassium per sulfate were prepared and mixed in a ratio of 1:1 by volume, reaction mixture was allowed to stand in a dark for 16 h at room temperature to produce dark green ABTS•+ radicals. The stock ABTS•+ radical solution was diluted with phosphate buffer solution (pH = 7.4) to an absorbance of 0.7 at 734 nm and used further in a study. A volume of 200 µl of the diluted water extract was mixed with 3.8 mL ABTS•+ solution, and the reaction mixture was incubated at room temperature for 2 h, and then the absorbance was read at 734 nm. Trolox solution was used as a reference standard, and the results were expressed as µg of Trolox/mg of extract. Decrease in the green color of reaction mixture indicates increasing scavenging effect on ABTS•+ radicals.
RESULTS AND DISCUSSION
Culturing period for CS1197
Growth response in liquid media over a typical time course was observed in Erlenmeyer flasks at 24°C under dark and static conditions. Figure 1 shows the growth of CS1197 and also the amount of adenosine and cordycepin present in dried mycelium harvested over 8 weeks. The biomass yield, adenosine and cordycepin were found to be 2.13 g/L (dry weight), 714 ± 31.74 and 673 ± 44.58 (µg/g of mycelium), respectively, at the end of 14 days of growth period. The mycelia growth was found stabilized in the 5th week and reached declining phase thereafter. It was noticed that both the nucleosides were produced in significant amount in first 2 weeks, but adenosine quantity reduced suddenly in the 3rd week probably due to structural transformation from adenosine to cordycepin since both possesses similar structural features. Though the biomass kept increasing till 5th week, but the production of nucleosides became very negligible. Hence, the growth period for CS1197 was restricted to 14 days in subsequent optimization studies.
Figure 1.
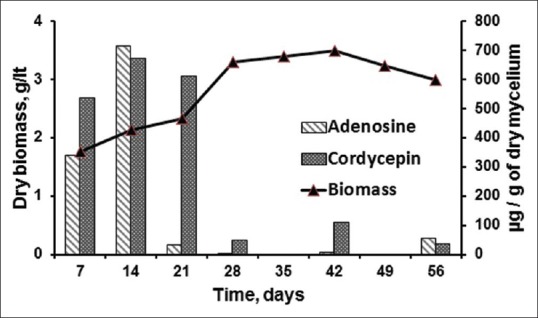
Growth of Cordyceps sinensis CS1197 in liquid media and adenosine and cordycepin content in dried mycelia
Identification of adenosine and cordycepin by high-performance liquid chromatography and mass spectrometry
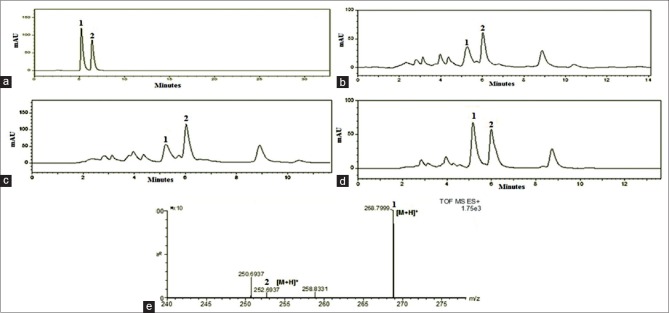
It is well-known that adenosine and cordycepin are the major biomarkers in Cordyceps sp.[40,41,42] and HPLC method has been widely used in the determination of adenosine and cordycepin from CS.[43,44] In the present study, the presence of both nucleosides was confirmed in natural CS and fermented CS1197 mycelium assuring authenticity. Under an ideal chromatographic conditions including flow rate, mobile phase, detection wavelength and column temperature, the elution time is short, and it has a good separation of two nucleosides. The chromatogram for standards mixture of adenosine and cordycepin [Figure 2a] was compared with chromatograms of water extract obtained from dead larvae [Figure 2b] and fruiting body of natural CS [Figure 2c] and CS1197 mycelium [Figure 2d]. It was observed that the amount of adenosine and cordycepin in fruiting body (862 ± 9.12 and 1469 ± 6.16 µg/g, respectively) was found to higher than in dead larvae of natural CS (561 ± 14.27 and 757 ± 12.51 µg/g, respectively). The amount of adenosine and cordycepin in in vitro grown CS1197 mycelium were quiet lesser than that present in CS collected from natural habitat [Figure 1]. The molecular masses of adenosine and cordycepin in CS1197 mycelium were confirmed by analyzing water extracts in MS [Figure 2e].
Figure 2.
High performance liquid chromatography chromatograms for (a) Adenosine and cordycepin standard mixtures; (b) natural Cordyceps sinensis (dead larvae); (c) natural Cordyceps sinensis (fruiting body); (d) artificial Cordyceps sinensis CS1197 mycelium; (e) mass spectra of nucleosides present in CS1197 mycelium
Culture conditions
The results of preliminary studies showed that temperature, pH, and initial inoculum volume were found to be critical conditions for submerged cultivation. Based on the preliminary work, the range of process conditions for optimization study was selected: Temperature 18–28°C, pH 7–9, and inoculum volume 10–20% (v/v). It was also observed that CS1197 could grow in medium volume 50–200 ml; but mycelia dry weight increased with decreasing medium volume from 200 to 50 ml in a 250-ml flask [Table 2]. The maximum mycelia dry weight was obtained with the medium volume of 50 ml in a 250-ml flask. Hence, 20% of culturing flask capacity was selected for medium volume in further studies.
Table 2.
Effect of medium volume on mycelial growth of Cordyceps sinensis CS1197

Response surface methodology
The experimental data showing the considerable variation in the biomass yield, adenosine and cordycepin content with respect to changes in all the three factors was in Table 1. The canonical analysis was carried out to determine the shape of the fitted response and the stationary point for biomass, adenosine, and cordycepin. Accordingly, the predicted response at the stationary point was found lying outside the range of variables. Further to know the type of stationary point, eigenvalues were calculated for three responses and found that stationary point for this model was a saddle point and, therefore, the estimated surface did not have a unique optimum.
Biomass
RSM has been successfully applied to predict the effect of the process conditions on biomass yield. The validity of the model was verified by F-test and coefficient of determination (R2) of 0.90 indicates more than 90% of the variability in the biomass yield could be predicted by the model. In order to gain a better understanding of the results, the predicted models are presented in Figure 3a–c as three dimensional surface plots. Figure 3a shows the effect of the temperature and pH on the biomass yield. The increase in pH and temperature caused an increase in biomass yield with better yields observed at higher values of both factors. Figure 3b demonstrates the effect of the temperature and inoculum volume on biomass yield: Above 24°C and at inoculum volume of ~14%, high biomass yield is predicted. Figure 3c shows the effect of pH and inoculum volume on biomass yield: Higher biomass yield is indicated at pH ~8 and both low and high levels of inoculum volume.
Figure 3.
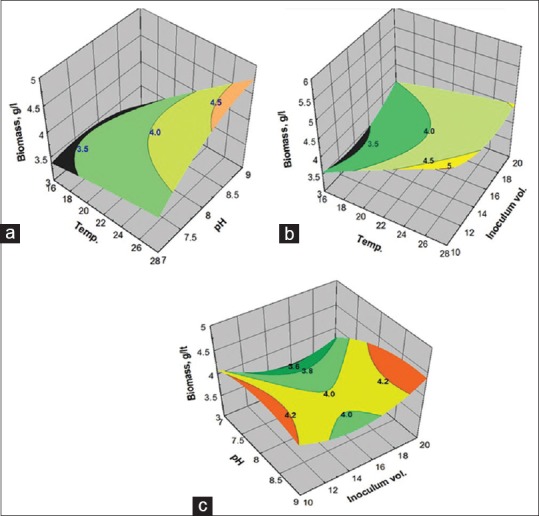
Response surface plots showing the (a) effect of temperature and pH; (b) effect of temperature and inoculum volume; (c) pH and inoculum volume on biomass yield
The regression analysis of second-order polynomial model was performed, and polynomial equation for predicting the biomass yield is below:
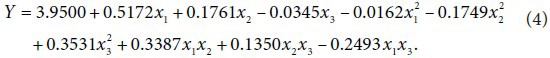
Adenosine
Adenosine is one of the major biomarkers in CS that is reported to show many pharmacological actions. RSM was applied to predict the adenosine content in the submerged fermentation of CS1197 where the model fit was confirmed with high R2 (0.75) [Table 3]. The predicted model is presented in Figure 4a–c. It was observed that lower temperature favors higher yield of adenosine [Figure 4a and b] and lower pH level supports increased the yield of adenosine [Figure 4a and c]. Furthermore, the higher levels of inoculum volume decreased the yield of adenosine.
Table 3.
Analysis of variances in the regression model for optimization of following responses in cultured mycelium of Cordyceps sinensis CS1197
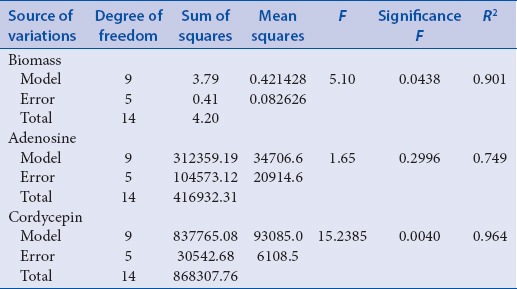
Figure 4.
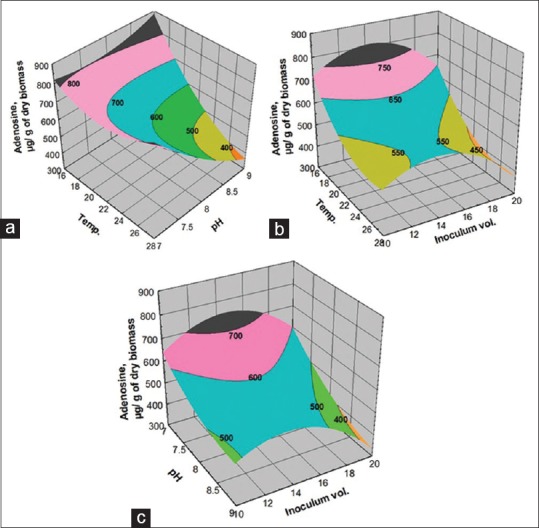
Response surface plots showing the (a) effect of temperature and pH; (b) effect of temperature and inoculum volume; (c) pH and inoculum volume on adenosine yield
The second order polynomial equation for predicting adenosine yield is as below:
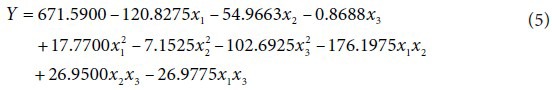
Cordycepin
Cordycepin is a bioactive constituent of CS shown to regulate homeostatic function.[45] As an adenosine analog, it is possible that cordycepin goes through a similar metabolic pathway. It is one of the effective ingredients, reported to show anti-cancer effect.[46,47] The fitness of the model to experimental cordycepin yield was confirmed by higher F = 15.23 and R2 = 0.967 [Table 3] indicating more than 96% of the variability in the cordycepin yield could be predicted by the model. The predicted model is presented in Figure 5a–c. The effect of temperature, pH, and inoculum volume on cordycepin yield was observed to behave in a similar way as that predicted for adenosine.
Figure 5.
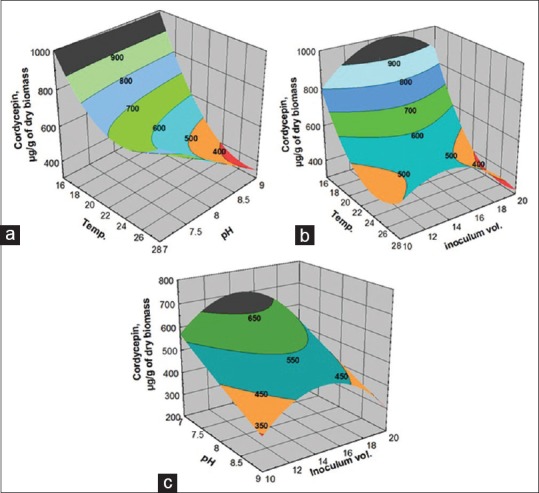
Response surface plots showing the (a) Effect of temperature and pH; (b) effect of temperature and inoculum volume; (c) pH and inoculum volume on cordycepin yield
The second order polynomial equation for predicting cordycepin yield:
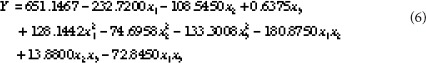
Optimization of culture conditions by desirability functions
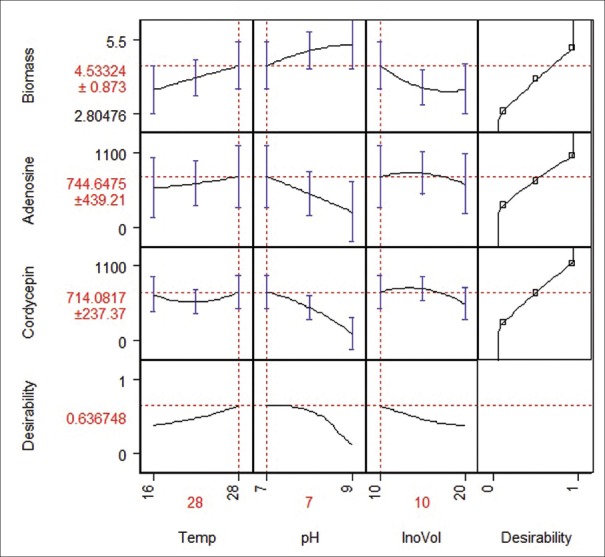
Individual maximum desirability for each of three responses and overall desirability for three responses were calculated using statistical tool JMP 5.1 under “Prediction Profile” option. Table 4 gives various process conditions for obtaining the maximum yield of biomass, adenosine and cordycepin at their individual maximum desirability. Table 4 also gives different scenarios to yields when the process is run at different conditions, whereas maximum biomass is obtained at a high temperature (28°C) with lower yields for adenosine and cordycepin, low temperature (16°C) favors higher yields for adenosines and cordycepin. If the process is run for maximum yield of biomass (5.29 g/L), desirability function analysis suggest individual desirability of 0.99 and poor overall desirability that will result in 75.6% reduction in adenosine and 89.9% reduction in cordycepin from the respective maximum yields [option I in Table 4]. Similarly, if the process was aimed at the maximum yield of adenosine (0.931 mg/g), suggesting 0.81 individual desirability and 0.32 overall desirability, that will result in 39.9% reduction in biomass yield and 1.73% reduction in cordycepin yield [option II in Table 4]. Aiming a maximum yield of cordycepin (1.041 mg/g) at the individual desirability of 0.87 will result in 6.45% reduction in adenosine and 35.8% reduction in biomass yield [option III in Table 4]. If the process is carried out as suggested by overall desirability function analysis at maximum desirability [Figure 6] of 0.64 at process conditions 28°C, pH of 7, and 10% inoculum volume [option IV in Table 4], one obtains yield of biomass 4.53 g/L which is 14.4% reduction from maximum yield. Similarly, adenosine and cordycepin yields will be reduced by 20% and 31.5%, respectively, from their maximum yields.
Table 4.
Process conditions for predicted yield of biomass, adenosine and cordycepin yield under different scenarios
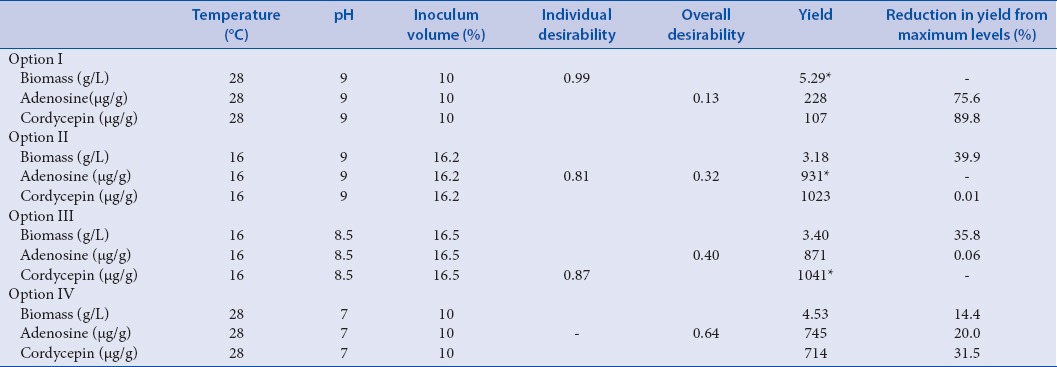
Figure 6.
Overall desirability prediction for optimization
From the above discussion, one can consider the option IV as the best choice of process conditions compared to other options since option IV results in the best yields in both adenosine and cordycepin while giving a reasonable biomass yield. However, it must be mentioned that economical process conditions are determined based on the cost of energy for processing, which will vary from country to country. It is generally accepted that the process is run nearer to ambient conditions.
Antioxidant activity
Free radicals are associated with cellular necrosis and a variety of pathological conditions such as cancer, degenerative disease in neurons, hepatopathies, atherosclerosis, and even aging. Supplementation with antioxidants could represent an important therapeutic potential to minimize the damage. Gradually growing attention has been paid to the discovery and development of efficient and safe antioxidants from natural resources. As a rich reservoir of bioactive resources, some medicinal fungi have been demonstrated to be excellent producers of antioxidant metabolites.[48]
Among them, CS is an excellent natural remedy for chronic diseases. Zheng et al. observed free radicals scavenging ability of an aqueous extract of CS mycelium and reported that scavenging rate was increased from 10.7% to 80.1% when extract concentration increased from 0.5 to 4.5 mg/mL.[49] In the present study, water extracts of dried CS1197 mycelium grown at a different temperature, pH, and inoculum volume were tested for their free radicals scavenging ability. The extracts showed inhibition ranging from 5.3% to 18% at 1 mg/ml concentration, which is comparable to literature values.[49]
Yu et al. studied the tendency for CSE to scavenge ABTS•+ free radicals and samples in the range of 0–4.0 mg/mL displayed scavenging effect ranging from 0 to ~60 µg of Trolox.[5] Water extracts from CS1197 showed good inhibition ranging from 27 to 39 µg of Trolox at 1 mg/ml concentration, which is comparable to literature values.[5]
CONCLUSION
Submerged cultivation of CS has been studied for optimum production. The presence of important biomarkers adenosine and cordycepin has been confirmed to be present in the biomass. Optimization of the process parameters temperature, pH, and inoculum volume for the responses biomass, adenosine and cordycepin yield using the RSM was studied. Based on the desirability function analysis method, the maximum overall desirability for the biomass, adenosine and cordycepin can be achieved at process conditions temperature 28°C, pH 7, and inoculum volume 10%.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Shashidhar M. Ghatnur
Shashidhar M. Ghatnur is currently Senior Research Fellow at Food Engineering Centre, CSIR-Central Food Technological Research Institute, Mysore, India. He obtained his Master degree in Food Technology (Food Process Engg.) from Allahabad Agricultural Institute-Deemed University, Allahabad, India in August 2011. He has been awarded Senior Research Fellowship from CSIR, India in April 2012 to pursue PhD programme in Engineering Sciences under AcSIR, CSIR-CFTRI, Mysore, India. He is author of 4 peer reviewed publications and has presented poster communications at national and international conferences. His research interest includes: Supercritical CO2 extraction of bio-actives from medicinal herbs, characterization and encapsulation of bioactive ingredients by supercritical mediated process.

Giridhar Parvatam
Dr. Giridhar Parvatam, currently scientist at Plant Cell Biotechnology Department of CSIR-CFTRI, Mysore, has about 16 years R&D experience in the area of plant molecular biology, in vitro production and regulation of secondary metabolites from food value plants. He is currently an author of 130 publications, 12 reviews, 3 chapters in books, 10 Indian, one US and one EU patents, and presented several invited lectures at national and international conferences. He is a fellow of Indian Botanical Society, Society for Applied Biotechnology and Academy of Plant Sciences and Elected member of NASI, Allahabad, and Plant Tissue Culture Association of India.

Manohar Balaraman
Dr. Manohar Balaraman, currently scientist at Department of Food Engineering, CSIR-Central Food Technological Research Institute, Mysore, India, has about 30 years of experience in the area of food process engineering. He has 65 research publications in peer-reviewed international journals and 40 presentations in national and international conferences. His major research interests include supercritical fluid extraction, molecular distillation and biotechnological approaches to extract bio-actives from natural materials.
Acknowledgments
The author (SMG) acknowledges NTCC, Forest Pathology Division, FRI, Dehradun for providing CS fungal strain and the award of a senior research fellowship from CSIR, New Delhi, India. The authors also acknowledge the Director, CSIR-CFTRI, Mysore, India for the encouragement given.
REFERENCES
- 1.Mizuno T. Medicinal effects and utilization of Cordyceps (Fr.) Link (Ascomycetes) and Isaria Fr. (Mitosporic Fungi) Chinese caterpillar fungi. Int J Med Mushrooms. 1991;1:251–61. [Google Scholar]
- 2.Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–84. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 3.Beijing, China: The People's Medical Publishing House; 1964. CPCMH (Committee of Pharmacopeia Chinese Ministry of Health). Chinese pharmacopeia (1963 edition); Part one. [Google Scholar]
- 4.Choi JW, Ra KS, Kim SY, Yoon TJ, Yu KW, Shin KS, et al. Enhancement of anti-complementary and radical scavenging activities in the submerged culture of Cordyceps sinensis by addition of citrus peel. Bioresour Technol. 2010;101:6028–34. doi: 10.1016/j.biortech.2010.02.083. [DOI] [PubMed] [Google Scholar]
- 5.Yu HM, Wang BS, Huang SC, Duh PD. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J Agric Food Chem. 2006;54:3132–8. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 6.Pao HY, Pan BS, Leu SF, Huang BM. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. J Agric Food Chem. 2012;60:4905–13. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 7.Chen YJ, Shiao MS, Lee SS, Wang SY. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997;60:2349–59. doi: 10.1016/s0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agric Food Chem. 2010;58:11645–52. doi: 10.1021/jf1028976. [DOI] [PubMed] [Google Scholar]
- 9.Yoon TJ, Yu KW, Shin KS, Suh HJ. Innate immune stimulation of exo-polymers prepared from Cordyceps sinensis by submerged culture. Appl Microbiol Biotechnol. 2008;80:1087–93. doi: 10.1007/s00253-008-1607-y. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Li P, Zhao D, Tang H, Guo J. Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation. 2011;34:639–44. doi: 10.1007/s10753-010-9273-5. [DOI] [PubMed] [Google Scholar]
- 11.Koh JH, Kim KM, Kim JM, Song JC, Suh HJ. Antifatigue and antistress effect of the hot-water fraction from mycelia of Cordyceps sinensis. Biol Pharm Bull. 2003;26:691–4. doi: 10.1248/bpb.26.691. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Gao XY, Rao BF, Liu L, Dong B, Cui LQ. Effects of Cordyceps sinensis alcohol extractive on serum interferon-gamma level and splenic T lymphocyte subset in mice with viral myocarditis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22:321–3. [PubMed] [Google Scholar]
- 13.Shashidhar M, Giridhar P, Udaya Sankar K, Manohar B. Bioactive principles from Cordyceps sinensis : A potent food supplement: A review. J Funct Foods. 2013;5:1013–30. doi: 10.1016/j.jff.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler D. Caterpillar fungus (Ophiocordyceps sinensis) production and sustainability on the Tibetan Plateau and in the Himalayas. Asian Med. 2009;5:291–316. [Google Scholar]
- 15.Sharma S. Trade of Cordyceps sinensis from high altitudes of the Indian Himalaya: Conservation and biotechnological priorities. Curr Sci. 2004;86:1614–9. [Google Scholar]
- 16.Jeffrey C. BBC News; 2012. [Last retrieved on 2012 Jul 09]. The Viagra Transforming Local Economies in India. Available from: http://www.bbc.com/news/magazine-18735544 . [Google Scholar]
- 17.CITES Management Authority of China. Announcement of Adjustment for Catalogue of Exporting and Importing Wild Animals and Plants (in Chinese); In Proceedings of the Meeting Held on 24-26 May, 2012, Thimphu, Bhutan (CoP16 Inf. 49) 2012 [Google Scholar]
- 18.Wang G. Beijing: Science and Technology Reference Press; 1995. Cordyceps species: Ecology, cultivation and application; p. 307. [Google Scholar]
- 19.Yin D, Tang X. Advances in the study on artificial cultivation of Cordyceps sinensis. Zhongguo Zhong Yao Za Zhi. 1995;20:707–9. [PubMed] [Google Scholar]
- 20.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis part II. J Altern Complement Med. 1998;4:429–57. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 21.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 22.Yue K, Ye M, Lin X, Zhou Z. The artificial cultivation of medicinal caterpillar fungus, Ophiocordyceps sinensis (Ascomycetes): A review. Int J Med Mushrooms. 2013;15:425–34. doi: 10.1615/intjmedmushr.v15.i5.10. [DOI] [PubMed] [Google Scholar]
- 23.Dong CH, Yao YJ. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. J Appl Microbiol. 2005;99:483–92. doi: 10.1111/j.1365-2672.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- 24.Dong CH, Yao YJ. On the reliability of fungal materials used in studies on ophiocordyceps sinensis. J Ind Microbiol Biotechnol. 2011;38:1027–35. doi: 10.1007/s10295-010-0877-4. [DOI] [PubMed] [Google Scholar]
- 25.Cha SH, Lim JS, Yoon CS, Koh JH, Chang HI, Kim SW. Production of mycelia and exo-biopolymer from molasses by Cordyceps sinensis 16 in submerged culture. Bioresour Technol. 2007;98:165–8. doi: 10.1016/j.biortech.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Leung PH, Zhang QX, Wu JY. Mycelium cultivation, chemical composition and antitumour activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J Appl Microbiol. 2006;101:275–83. doi: 10.1111/j.1365-2672.2006.02930.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang QD, Yang J. Food experiment design and statistical analysis. China: China Agricultural University Press; 2003. pp. 1–521. [Google Scholar]
- 28.Box GE, Hunter WG, Hunter JS. New York: Wiley; 1978. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building; pp. 510–39. [Google Scholar]
- 29.Akitha Devi MK, Giridhar P. Isoflavone augmentation in soybean cell cultures is optimized using response surface methodology. J Agric Food Chem. 2014;62:3143–9. doi: 10.1021/jf500207x. [DOI] [PubMed] [Google Scholar]
- 30.Dean AM, Voss D. Design and Analysis of Experiments. New York: Springer-Verlag, Inc; 1999. pp. 547–58. [Google Scholar]
- 31.Muthukumar M, Mohan D, Rajendran M. Optimization of mix proportions of mineral aggregates using Box Behnken design of experiments. Cement Concrete Compos. 2003;25:751–8. [Google Scholar]
- 32.Harrington EC. The desirability function. Ind Qual Control. 1965;21:494–8. [Google Scholar]
- 33.Derringer GC, Suich R. Simultaneous optimization of several response variables. Qual Technol. 1980;12:214–9. [Google Scholar]
- 34.Dong CH, Xie XQ, Wang XL, Zhan Y, Yao YJ. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioproducts Process. 2009;87:139–44. [Google Scholar]
- 35.Shih IL, Tsaib KL, Hsieh C. Effects of culture conditions on the mycelia growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Eng J. 2007;33:93–201. [Google Scholar]
- 36.Jian DC, Li QY. Optimization of culture conditions on mycelial grown in submerged culture of Cordyceps militaris. Int J Food Eng. 2011;7:1556–3758. [Google Scholar]
- 37.Ghatnur SM, Sonale RS, Balaraman M, Kadimi US. Engineering liposomes of leaf extract of seabuckthorn (SBT) by supercritical carbon dioxide (SCCO2)-mediated process. J Liposome Res. 2012;22:215–23. doi: 10.3109/08982104.2012.658576. [DOI] [PubMed] [Google Scholar]
- 38.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 39.Song FL, Gan RY, Zhang Y, Xiao Q, Kuang L, Li HB. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int J Mol Sci. 2010;11:2362–72. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan H, Li S, Xiang J, Lai C, Yang F, Gao J, et al. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC–ESI–MS/MS) Anal Chim Acta. 2006;567:218–28. [Google Scholar]
- 41.Guo C, Zhu J, Zhang C, Zhang L. Determination of adenosine and 3’-deoxyadenosine in Cordyceps militaris (L.) Link. by HPLC. Zhongguo Zhong Yao Za Zhi. 1998;23:236–7. 256. [PubMed] [Google Scholar]
- 42.Hsu TH, Shiao LH, Hsieh C, Chang DM. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom Dong Chong Xia Cao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002;78:463–9. [Google Scholar]
- 43.Guo FQ, Li A, Huang LF, Liang YZ, Chen BM. Identification and determination of nucleosides in Cordyceps sinensis and its substitutes by high performance liquid chromatography with mass spectrometric detection. J Pharm Biomed Anal. 2006;40:623–30. doi: 10.1016/j.jpba.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda R, Nishimura M, Sun Y, Wada M, Nakashima K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomed Chromatogr. 2008;22:630–6. doi: 10.1002/bmc.980. [DOI] [PubMed] [Google Scholar]
- 45.Tsai YJ, Lin LC, Tsai TH. Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of Cordyceps sinensis in rat. J Agric Food Chem. 2010;58:4638–43. doi: 10.1021/jf100269g. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3’-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 2006;26:43–7. [PubMed] [Google Scholar]
- 47.Yoshikawa N, Nishiuchi A, Kubo E, Yamaguchi Y, Kunitomo M, Kagota S, et al. Cordyceps sinensis acts as an adenosine A3 receptor agonist on mouse melanoma and lung carcinoma cells, and human fibrosarcoma and colon carcinoma cells. Pharmacol Pharm. 2011;2:266–70. [Google Scholar]
- 48.Sun C, Shan CY, Gao XD, Tan RX. Protection of PC12 cells from hydrogen peroxide-induced injury by EPS2, an exopolysaccharide from a marine filamentous fungus Keissleriella sp. YS4108. J Biotechnol. 2005;115:137–44. doi: 10.1016/j.jbiotec.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Zheng LP, Gao LW, Zhou JQ, Sima YH, Wang JW. Antioxidant activity of aqueous extract of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. Afr J Biotechnol. 2008;7:3004–10. [Google Scholar]