Abstract
Context:
Anisochilus carnosus (L.f.) wall belonging to the family Lamiaceae is a plant that is widely used in folk medicine for treating eczema, cold, cough, and fever.
Objective:
In the present study, we explored the anticancer potential of A. carnosus leaves against Ehrlich ascites carcinoma (EAC) and estimated the quantity of luteolin present in various extracts and fractions of A. carnosus by high-performance thin layer chromatography (HPTLC) fingerprinting.
Materials and Methods:
Various factors such as tumor volume, tumor cell viability, tumor weight, prolongation of lifespan, and hematological parameters were assessed.
Result:
We observed a significant lowering in tumor volume, tumor weight, and cell viability in EAC-induced mice following intervention with A. carnosus extracts. Also, there was a considerable prolongation of host lifespan and restoration of hematological parameters to almost normal levels with A. carnosus treatment. HPTLC fingerprinting of various extracts and fractions of A. carnosus along with luteolin as the reference standard revealed the occurrence of luteolin in all tested extracts and fractions of A. carnosus with the highest concentration being reported in the ethanol fraction.
Conclusion:
A. carnosus exhibits potent anti-tumor potential which can most likely be attributed to the occurrence of different phytochemicals such as phytosterols, terpenoids, and flavonoids in the plant. Further studies to isolate compounds from A. carnosus and understand the mechanism of anti-tumor activity would be worthwhile.
SUMMARY
EAC induced mice that received A. carnosus treatment exhibited significant reduction in tumor volume, tumor weight and tumor cell viability. Their life span was considerably prolonged. We detected luteolin in A. carnosus aqueous and ethanol extract using HPTLC. Hence, anticancer activity of A. carnosus can be partly attributed to the presence of luteolin.
Keywords: Cytotoxicity, Ehrlich ascites carcinoma, high-performance thin layer chromatography, luteolin
INTRODUCTION
Cancer refers to a group of diseases characterized by anomalous cells that can swiftly proliferate, pervade, and metastasize to different vital organs of the body. Death due to this dreaded disease has been on the rise with a probability of almost 5, 89, 430 cancer deaths anticipated to occur in 2015.[1] Although on-going researchers from numerous scientific disciplines are doing their best to find a cure for this malady, the ultimate, perfect one is yet to be discovered.[2] Moreover, patients who undergo the current cancer therapies in the clinical setting go through a number of distressing after-effects such as nausea, hair loss, depression, and constipation[3] making it absolutely essential to find a more effective alternative treatment. Incidentally, plant-based immunomodulators have long been used as a supportive therapy to reduce these distressing side effects and improve the overall health.[4]
Anisochilus carnosus (L.f.) wall (Lamiaceae) commonly known as “Thick leaved lavender” is an annual herb indigenous to the Western Ghats of India. The folkloric benefits of this plant are many. For instance, the tribes in Bhilla district of Maharashtra in India use the roots of this plant for a respite from cold, cough, and fever.[5] The Paliyar tribal community of Tamil Nadu in India smear the leaf paste over the affected regions for treating various skin diseases such as eczema and psoriasis.[6,7] Phytochemically, A. carnosus comprises plentiful secondary metabolites such as flavonoids, triterpenoids, phytosterols, tannins, and essential oils[8,9,10] all of which have tremendous chemopreventive, antioxidant, and anticancer properties.[11,12,13] In the current study, the anticancer activity of aqueous and ethanol extract of A. carnosus has been examined in in vivo utilizing the Ehrlich ascites carcinoma (EAC) model. Since luteolin has been acclaimed to have potent anticancer activity, we have endeavored to quantify its concentration in various extracts and fractions of A. carnosus by high-performance thin layer chromatography (HPTLC) analysis.
MATERIALS AND METHODS
Plant collection
A. carnosus leaves were collected from Udupi in the month of September 2010 and authenticated by Dr. Richard Lobo, Associate Professor, Manipal College of Pharmaceutical Sciences, Manipal, Karnataka, India. It was given voucher number PP 573 and placed in the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal, Karnataka, India.
Extraction
The leaves of A. carnosus (300 g) were dried in the shade, coarsely ground, and extracted using soxhlet extractor with ethanol (3 L). The crude A. carnosus ethanolic extract (AE) was concentrated under reduced pressure to dryness in a rotary evaporator and stored in a desiccator. For preparation of A. carnosus aqueous extract (AA), coarsely powdered leaves (150 g) were macerated in water: Chloroform (99:1). After a week, the extract was strained using a muslin cloth and concentrated on a steam bath and kept in a desiccator. The percentage yield of AE and AA were 12.3% w/w and 16.43% w/w, respectively. Both the extracts were suspended in 0.05% acacia gum (vehicle) for the animal study.
Reagents and chemicals
The reagents sodium chloride, acacia gum, methylene blue, trypan blue, sodium sulfate, methyl violet, and propylene glycol were procured from Merck Specialties Pvt. Ltd., Mumbai, India. Ethanol was bought from Ranbaxy Fine Chemicals Ltd., Sikanderpur, Punjab, India. The standards cisplatin and luteolin were procured from Sigma-Aldrich, US. solvents and chemicals purchased were of analytical grade.
Animals
Eight weeks old Swiss albino mice (20–25 g) were adapted to an investigational room maintained at controlled temperature (22 ± 2°C), humidity (50 ± 5%), and an alternate light and dark phase of 12 h each. They were kept in polypropylene cages with a maximum of two mice per cage and were given water and standard food pellets ad libitum. The experiment was performed after attaining ethical clearance from the Institutional Animal Ethics Committee of KMC, Manipal, No. IAEC/KMC/76/2011-2012.
Acute toxicity study and dose calculation
In order to estimate the safe dose for administration, acute toxicity studies were performed by the up and down method in accordance with the OECD Test Guidelines 425.[14] Briefly, the mice were fasted overnight prior to oral administration of A. carnosus extracts at various doses (5–2000 mg/kg body weight [b. wt.]). After dosing, they were observed for physical indications of toxicity such as tremors, salivation, convulsion, diarrhea, and coma for the next 1 h, after 4 h, after 24 h, and regularly thereafter for 14 days. Based on these observations, it was noted that AE and AA extracts were safe up to doses of 5 and 1 g/kg b. wt., respectively. Hence, we decided to treat the mice at doses corresponding to 50 and 30 mg/kg b. wt. of AE extract and 50 and 100 mg/kg b. wt. of AA extract.
Tumor model and transplantation
EAC cell lines procured from Dr. Ramadasan Kuttan (Director, Amala Cancer Research Centre, Thrissur, Kerala) were preserved and proliferated in an aseptic environment by serial intraperitoneal (i.p.) transplantation. Using an 18 ml sterile syringe, the ascitic fluid was drawn and checked for ascitic tumor cell viability by trypan blue exclusion assay. In Neubauer's hemocytometer, all cells stained with trypan blue were counted. Once the cells were found to be more than 99% viable, the suspension was diluted with normal saline to obtain a final concentration of 1 × 107 cells/ml. From this stock solution, 0.25 ml was injected i.p. for the formation of a liquid tumor. This was presumed to be day 0 of the experiment.
Experimental design
Swiss albino mice were randomized and split into seven groups (n = 12). Animals in every group except Group 1 were EAC induced. Treatment was given consecutively for 13 days in mice belonging to Group 3, 4, 5, and 6. Treatment regimen (via i.p. route) was as follows:
Group 1: Normal control (0.05% acacia gum)
Group 2: EAC control
Group 3: EAC + AE I (30 mg/kg b. wt.)
Group 4: EAC + AE II (50 mg/kg b. wt.)
Group 5: EAC + AA I (50 mg/kg b. wt.)
Group 6: EAC + AA II (100 mg/kg b. wt.)
Group 7: EAC + cisplatin (3.5 mg/kg b. wt.) on the first day.
Evaluation of anticancer activity
A day following final dosage and 18 h of fasting, six mice in each group was sacrificed by CO2 exposure following which cervical dislocation was carried out to determine the tumor cell viability, tumor weight, tumor volume, and hematological parameters. Remaining six animals in each group were given water and food ad libitum to estimate the prolongation of lifespan in the host.
Tumor weight
Body weight of mice was noted before and after the ascitic fluid was collected from the peritoneal cavity. Weight was expressed in gram (g).
Tumor volume
The volume of ascitic fluid (in ml) was calculated by collecting the ascitic fluid into a graduated centrifuge tube.
Tumor cell count
The tumor cell count was assessed by trypan blue exclusion assay. Briefly, a drop of the ascitic fluid in PBS of was placed on a Neubauer's hemocytometer, and the total number of cells present in the 64 squares was calculated. On staining with trypan blue dye (0.4% v/v in normal saline), cells that were viable did not take up the stain whereas the dead cells were stained blue.
Change in body weight
The weight of all animals was recorded on the day of EAC induction (day 0) and once every 2 days subsequently. The percentage increase in body weight was evaluated by the formula:
Percentage of increase in body weight = (Body weight on the respective day/Body weight on day 0) −1 × 100.
Hematological parameters
Blood was withdrawn at the end of the investigational period to estimate parameters such as hemoglobin (Hb) content, white blood cell (WBC) count, and red blood cell (RBC) count as per standard guidelines.[15]
Percentage increase in lifespan and mean survival time
The percentage increase in lifespan and mean survival time of the animals in different groups were assessed depending on the mortality of the experimental mice.
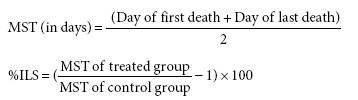
High-performance thin layer chromatography fingerprinting
Preparation of extract and standard
Sample preparation
Two milligrams per milliliter stock solution of A. carnosus aqueous and ethanol extracts were prepared by weighing 2 mg of each extract and dissolving in 1 ml methanol.
Standard preparation
One milligram luteolin was weighed and dissolved in 10 ml methanol (analytical grade) to obtain a stock solution having a concentration of 100 µg/ml.
Quantification of luteolin in different extracts/fractions by validated HPTLC method.[16]
HPTLC analysis was performed using Linomat V and CAMAG HPTLC software. Ten microliters of each sample were drawn in a Camag 100 µl syringe and applied onto an HPTLC precoated silica gel G60 F254 plate (20 cm × 10 cm) using Linomat V semi-automatic applicator. The chromatogram was developed in a Camag twin trough chamber which was presaturated for 20 min at room temperature with mobile phase constituting toluene: Ethyl acetate: Formic acid in the ratio 10:9:1, v/v/v. The plate was subsequently air-dried and scanned at a scanning speed of 20 mm/s in the absorbance mode at a wavelength of 254 nm and 366 nm using Camag TLC scanner III. The slit dimension was maintained at 6.0 mm × 0.45 mm. The peak areas were recorded, and the percentage of luteolin content in each sample was estimated.
Statistical analysis
Microsoft Office 2010 Beta – Excel was used in graph preparation. GraphPad Prism version 5.0 software (GraphPad Software, Inc., California) was utilized to calculate the IC50 via the linear regression equation. Statistical significance (P value) was determined using one-way ANOVA followed by Dunnett's post-hoc test of significance between treated groups and EAC control group, where P < 0.05 was deemed to be significant. Results were denoted as mean ± standard error of the mean (n = 6 mice per group).
RESULT
Evaluation of anti-cancer activity
Effect on tumor volume
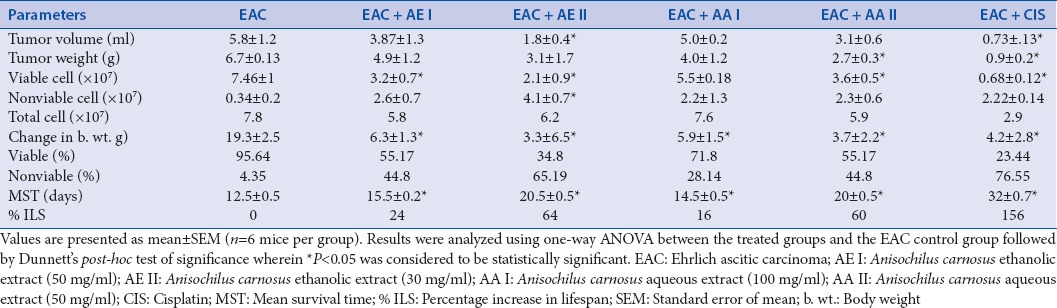
Tumor volume was estimated in the control and treated groups to gauge the effectiveness of the plant extract in reducing the tumor growth. Several reports have indicated that ascitic fluid is capable of providing nourishment and nutrition to the tumor cells and is vital for their growth and proliferation.[17] Accordingly, in the current study, we noted a build-up in tumor volume in the EAC bearing mice in the control group. However, there was a significant (P < 0.05) lowering in tumor volume in the group treated with cisplatin [Table 1]. Likewise, i.p administration of AE II was also able to significantly (P < 0.05) cause a decline in tumor volume in Group 3 animals. Similarly, animals in all other treated groups showed comparatively less tumor volume than animals in EAC-induced group.
Table 1.
Effect of Anisochilus carnosus extracts on tumor growth response
Effect on tumor weight
EAC-induced group showed a considerable increase in body weight. Administration of cisplatin decreased the elevated body weight significantly (P < 0.05). Similarly, A. carnosus treatment also reduced the tumor weight in animals at all doses, with a significant (P < 0.05) reduction in tumor weight following AA II treatment [Table 1].
Effect on tumor cell count
In the tumor-induced group, we observed more viable and few nonviable cells after staining with trypan blue. In contrast, all treated groups showed a decrease in viable cells. In particular, a significant reduction (P < 0.05) was noted in the number of viable peritoneal EAC cells in the AE I, AE II, and AA II treated group [Table 1].
Effect on change in body weight
A substantial increase in body weight of EAC-induced group was noted with the progression of the experiment. There was comparatively less variation in the body weight (P < 0.05) in the standard group that received cisplatin treatment. Likewise, in all drug-treated groups, there was less alteration in body weight. A significant difference of P < 0.05 and P < 0.01was specially noted in AE II, AA I and AE I, AA II treated groups, respectively.
Effect on Hematological parameters
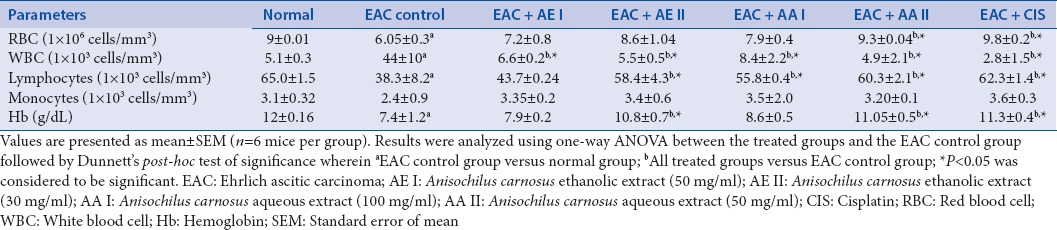
Hematological parameters of treated and untreated groups were evaluated. During tumor progression, there is a usual decline in the level of RBC and Hb[15] as was distinctly established in EAC-induced group [Table 2]. Additionally, a massive increase in the number of WBCs was noted in the same group. We observed that hematological parameters were brought back to almost normal levels in the treated groups.
Table 2.
Effect of Anisochilus carnosus extracts on hematological parameters
Effect on percentage increase in lifespan
Survival rate was higher in the treated group as compared to the animals in the EAC-induced group.
High-performance thin layer chromatography estimation
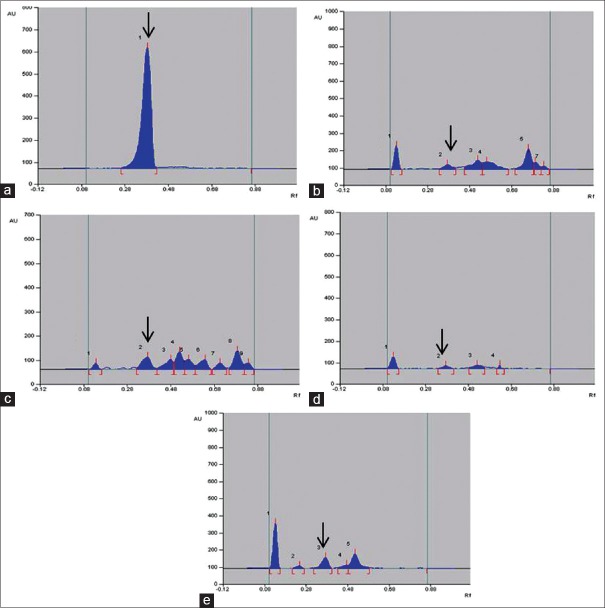
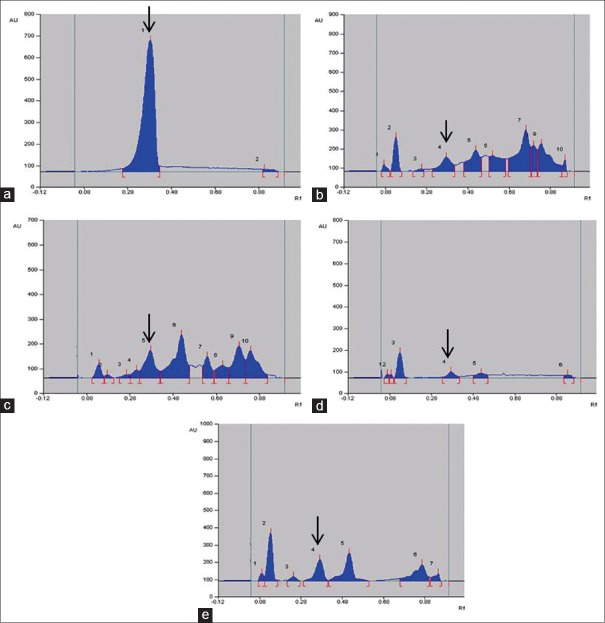
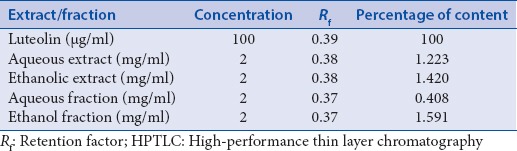
Quantity of luteolin present in different extracts of A. carnosus was estimated by HPTLC. The peak corresponding to luteolin in A. carnosus extracts/fractions was identified by comparing with the peak in luteolin reference compound at 254 and 366 nm. It was observed that luteolin eluted at Rf 0.38 at 254 [Figures 1 and 2] and 366 nm [Figures 3 and 4]. Further, we observed that ethanolic fraction contained the maximum concentration of luteolin (0.956% w/w at 254 nm and 1.591% w/w at 366 nm). Tables 3 and 4 list the concentration of luteolin present in different extracts and fractions of A. carnosus.
Figure 1.
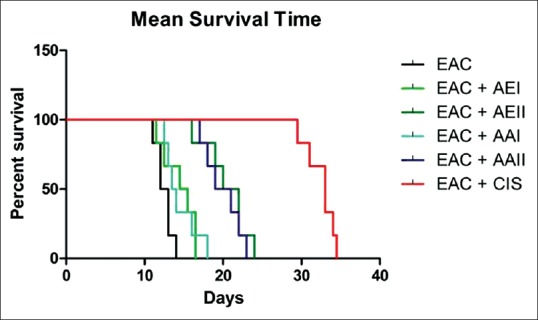
Kaplan–Meier survival plot depicting the mean survival time of Anisochilus carnosus extracts treatments
Figure 2.
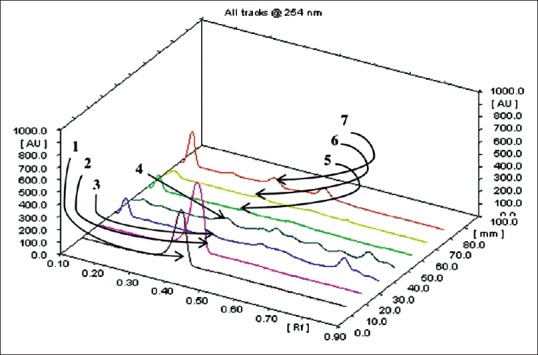
Three-dimensional overview of densitometric chromatogram of standard luteolin and various extracts and fractions of Anisochilus carnosus at 254 nm: (1 and 2) Luteolin; (3) Aqueous extract; (4) Ethanol extract; (5 and 6) Aqueous fraction; (7) Ethanol fraction
Figure 3.
High-performance thin layer chromatography chromatogram of different extracts and fractions of standard luteolin and Anisochilus carnosus at 254 nm: (a) Luteolin (100 μg/ml); (b) Aqueous extract (2 mg/ml); (c) Ethanol extract (2 mg/ml); (d) Aqueous fraction (2 mg/ml); (e) Ethanol fraction (2 mg/ml). The black arrow mark indicates the luteolin peak in Anisochilus carnosus extract/fraction
Figure 4.
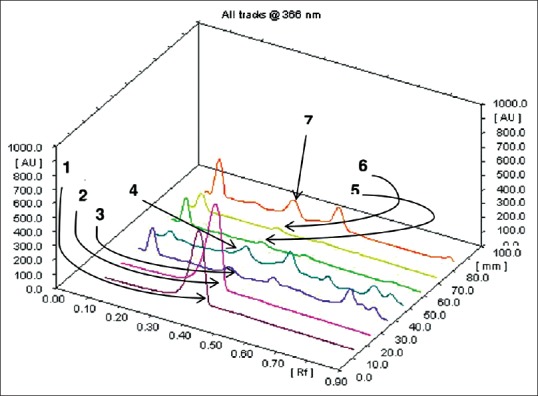
Three-dimensional overview of densitometric chromatogram of various extracts and fractions of Anisochilus carnosus at 366 nm: (1 and 2) Luteolin; (3) Aqueous extract; (4) Ethanol extract; (5 and 6) Aqueous fraction; (7) Ethanol fraction
Table 3.
Estimation of luteolin content in Anisochilus carnosus by HPTLC at 254 nm
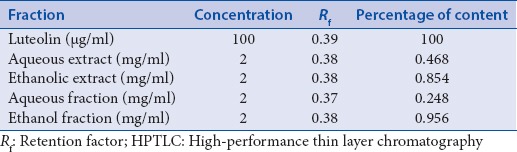
Table 4.
Estimation of luteolin content in Anisochilus carnosus by HPTLC at 366 nm
DISCUSSION
EAC originally appeared in the form of murine breast cancer with highly aggressive growth.[18] These cells exhibit traits such as undifferentiation, rapid proliferation, high vascular permeability, and cellular migration which has made it a suitable choice as an experimental model for human tumor.[19] This model is further unique as it can form liquid tumor if the EAC cells are injected intraperitoneally in the mouse and solid tumor if injected subcutaneously.[20] In the present study, various factors such as tumor volume, viable/nonviable tumor cell count, tumor weight, hematological parameters, prolongation of lifespan, and change in body weight were evaluated in animals injected intraperitoneally with EAC cells.
We noted that pharmacotherapy with A. carnosus extract significantly reduced the tumor volume in AE I treated group. A substantial increase was observed in the body weight of EAC-induced mice. In contrast, EAC mice that were treated with A. carnosus had sufficiently less weight. There was also significant lowering in the viable cell count after treatment with A. carnosus. Bone marrow suppression and anemia are identified to be the foremost side effects of chemotherapy.[21] We evaluated the effect of A. carnosus extract on hematological parameters of treated and untreated groups. A decline in the level of RBC and Hb usually occurs during tumor progression;[15] we observed a distinct decrease in RBC level in the EAC-induced group [Table 2]. This has been noted and validated in earlier studies.[22] Additionally, a massive proliferation of WBC was noted in the same group. Remarkably, treatment with A. carnosus extracts restored the levels of RBC, Hb, WBC, lymphocytes, and monocytes to normal, indicating its protective effect on the hematopoietic system [Table 2]. The ability of an anti-cancer drug to prolong the lifespan of the host is considered to be one of the most dependable benchmarks for evaluating its effectiveness against cancer.[23] Per se, we observed a higher survival rate in the treated group as compared to animals in EAC-induced group [Figure 1 and Table 1]. The highlight of the current study is that groups treated with A. carnosus extracts revealed remarkable anti-tumor activity even at low doses such as 30, 50, and 100 mg/kg b. wt. In the past, several studies[24,25,26,27] have reported good anti-tumor activity in vivo with EAC model although with comparatively higher doses of the anti-cancer agent. Also, previous studies on A. carnosus have revealed the plant to possess strong anti-tumor activity with its ethanolic and petroleum ether extract exhibiting potent anti-tumor activity in vitro against cancer cell lines such as BT-549 (human mammary ductal carcinoma)[28] and A-549 (human alveolar adenocarcinoma).[29]
Traditional medicine has a crucial role to play in cancer therapy, with a multitude of the current anti-cancer drugs being either of natural origin or derived therefrom.[30] Plants are rich in phytoconstituents such as flavonoids, phytosterols, and tannins all of which have exceptional cancer-protective effects. Several clinical trials have indicated flavonoids to be excellent chemopreventive agents.[31] One such naturally occurring flavonoid is luteolin which is commonly found in vegetables such as celery, broccoli, thyme, parsley, carrot, etc.[32] Luteolin is reported to show exceptional anti-cancer activity against several carcinogenic agents such as 7,12-dimethylbenz (a) anthracene, dimethylhydrazine in vivo.[33,34] It also exerts potent anticancer activity in vitro against numerous malignant cell lines such as MCF-7 (human breast adenocarcinoma), HepG2 (human liver carcinoma cell line), HT-29 (human colorectal adenocarcinoma), OCM-1 (human uveal melanoma) and A375 (human malignant melanoma) etc.[35] Besides, molecular mechanistic studies have indicated that luteolin can bring about the cessation of cell cycle and apoptosis in malignant cells via intrinsic and extrinsic pathways.[36] Since luteolin has been credited with such phenomenal anti-tumor potential, we deemed it worthwhile to assess the concentration of luteolin in A. carnosus by HPTLC fingerprinting and probe for any possible correlation between its occurrence and anticancer activity. Luteolin content was estimated in various fractions and extracts of A. carnosus by HPTLC. The luteolin peak in A. carnosus extract/fractions and luteolin reference compound was observed at 254 nm and 366 nm. It was observed that luteolin eluted at Rf 0.38 at 254 nm [Figures 2 and 3] and 366 nm [Figures 4 and 5]. Further, it was noted that ethanol fraction contained the maximum concentration of luteolin (0.956% [w/w] at 254 nm and 1.591% [w/w] at 366 nm) [Figures 2–5, Tables 3 and 4]. Tables 3 and 4 list the % content of luteolin detected in the different extracts and fractions of A. carnosus.
Figure 5.
High-performance thin layer chromatography chromatogram of standard luteolin and different extracts and fractions of Anisochilus carnosus at 366 nm: (a) Luteolin (100 μg/ml); (b) Aqueous extract (2 mg/ml); (c) Ethanol extract (2 mg/ml); (d) Aqueous fraction (2 mg/ml); (e) Ethanol fraction (2 mg/ml). The black arrow mark indicates the luteolin peak in Anisochilus carnosus extract/fraction
CONCLUSION
Considerable increase in lifespan, decrease in tumor volume, tumor weight, and restoration of the hematological parameters to near normal level in A. carnosus treated groups in the host could possibly be attributed to the innumerable phytoconstituents present in the plant such as flavonoids, phytosterols, saponins, phenols, tannins, and volatile oil. HPTLC fingerprinting confirmed the presence of luteolin in various extracts and fractions of A. carnosus. As such, luteolin in A. carnosus may have a crucial role to play in tumor suppression of EAC. Efforts toward identification and isolation of other compounds from A. carnosus specifically responsible for anti-tumor activity would be worthwhile.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Dr. Richard Lobo
Dr. Richard Lobo is an alumnus of Manipal College of Pharmaceutical Sciences (MCOPS), Manipal University. He has published more than 45 research and review articles in reputed national and international Journals and has contributed chapters in two books. He is an Associate editor for BMC Complementary and Alternative medicine and is an editorial board member for Journal of Pharmaceutical Crops, Current Traditional Medicine (Bentham Science) and Journal of Bioequivalence Studies (Annex publishers). He is presently working as Associate Professor in the Department of Pharmacognosy, MCOPS, Manipal University.

Ms. Nimmy Kumar
Ms. Nimmy Kumar is currently pursuing PhD in Manipal College of Pharmaceutical Sciences, Manipal University. Her area of interest includes cancer biology, phytochemistry and isolation of constituents.
Acknowledgments
The authors would like to express their sincere thanks to Manipal University and Manipal College of Pharmaceutical Sciences, Manipal, Karnataka, India for providing the necessary facilities to carry out this work.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran P, Govindarajan R. Cancer – An ayurvedic perspective. Pharmacol Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: The influence of 5HT3 antagonists. Br J Cancer. 1997;76:1055–61. doi: 10.1038/bjc.1997.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondal S, Bandyopadhyay S, Ghosh MK, Mukhopadhyay S, Roy S, Mandal C. Natural products: Promising resources for cancer drug discovery. Anticancer Agents Med Chem. 2012;12:49–75. doi: 10.2174/187152012798764697. [DOI] [PubMed] [Google Scholar]
- 5.Kamble SY, Patil SR, Sawant PS, Sawant S, Pawar SG, Singh EA. Studies on plants used in traditional medicine by Bhilla tribe of Maharashtra. Indian J Tradit Knowl. 2010;9:591–8. [Google Scholar]
- 6.Ignacimuthu S, Ayyanar M, Sivaraman KS. Ethnobotanical investigations among tribes in Madurai District of Tamil Nadu (India) J Ethnobiol Ethnomed. 2006;2:25. doi: 10.1186/1746-4269-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesan S, Suresh N, Kesaven L. Ethnomedicinal survey of lower Palni hills of Tamil Nadu. Indian J Tradit Knowl. 2004;3:299–304. [Google Scholar]
- 8.Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. New Delhi: Springer; 2007. p. 52. [Google Scholar]
- 9.Lobo R, Bhagat J, Ballal M, Gupta N. Histo-anatomical study of Anisochilus carnosus (L.f.) Wall: An Indian Habitant. Res J Med. 2012;6:456–60. [Google Scholar]
- 10.Senatore F, Lentini F, Venza F, Bruno M, Napolitano F. Composition and antibacterial activity of the essential oil of Anisochilus carnosus (Linn. fil.) Benth, a Tamil plant acclimatized in Sicily. Flavour Fragr J. 2003;18:202–4. [Google Scholar]
- 11.Nepka C, Sivridis E, Antonoglou O, Kortsaris A, Georgellis A, Taitzoglou I, et al. Chemopreventive activity of very low dose dietary tannic acid administration in hepatoma bearing C3H male mice. Cancer Lett. 1999;141:57–62. doi: 10.1016/s0304-3835(99)00145-7. [DOI] [PubMed] [Google Scholar]
- 12.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: Promising anticancer agents. Med Res Rev. 2003;23:519–34. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 13.Batra P, Sharma AK. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech. 2013;3:439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paris: Organisation for Economic Co-Operation and Development Publishing; 2008. OECD. Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. [Google Scholar]
- 15.Price VE, Greenfield RE. Anemia in cancer. In: Grenstein JP, Haddow A, editors. Advance in Cancer Research. New York: Academic Press; 1958. pp. 199–200. [DOI] [PubMed] [Google Scholar]
- 16.Kukkar M, Kukkar R, Saluja A. Validation of HPTLC method for the analysis of luteolin in Cardiospermum halicacabum Linn. Int J Green Pharm. 2014;8:252–6. [Google Scholar]
- 17.Prasad SB, Giri A. Antitumor effect of cisplatin against murine ascites Dalton's lymphoma. Indian J Exp Biol. 1994;32:155–62. [PubMed] [Google Scholar]
- 18.Ozaslan M, Karagoz ID, Kilic IH, Guldur ME. Ehrlich ascites carcinoma. Afr J Biotechnol. 2011;10:2375–8. [Google Scholar]
- 19.Dolai N, Karmakar I, Suresh Kumar RB, Kar B, Bala A, Haldar PK. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J Ethnopharmacol. 2012;142:865–70. doi: 10.1016/j.jep.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 20.Wójciak E, Korohoda W. Ehrlich ascites tumour cells show tissue-specific adherence and modify their shape upon contact with embryonic fibroblasts and myotubes. J Cell Sci. 1990;97(Pt 3):433–8. doi: 10.1242/jcs.97.3.433. [DOI] [PubMed] [Google Scholar]
- 21.Hoagland HC. Hematologic complications of cancer chemotherapy. Semin Oncol. 1982;9:95–102. [PubMed] [Google Scholar]
- 22.Bala A, Kar B, Haldar PK, Mazumder UK, Bera S. Evaluation of anticancer activity of Cleome gynandra on Ehrlich's ascites carcinoma treated mice. J Ethnopharmacol. 2010;129:131–4. doi: 10.1016/j.jep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Oberling C, Guerin M. The role of viruses in the production of cancer. In: Grenstein JP, Haddow A, editors. Advances in Cancer Research. New York: Academic Press; 1954. pp. 353–423. [DOI] [PubMed] [Google Scholar]
- 24.Gupta M, Mazumder UK, Rath N, Mukhopadhyay DK. Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J Ethnopharmacol. 2000;72:151–6. doi: 10.1016/s0378-8741(00)00227-0. [DOI] [PubMed] [Google Scholar]
- 25.Hazra B, Sarkar R, Bhattacharyya S, Roy P. Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia. 2002;73:730–3. doi: 10.1016/s0367-326x(02)00232-0. [DOI] [PubMed] [Google Scholar]
- 26.Senthil Kumar R, Rajkapoor B, Perumal P, Dhanasekaran T, Alvin Jose M, Jothimanivannan C. Antitumor activity of Prosopis glandulosa Torr. on Ehrlich ascites carcinoma (EAC) tumor bearing mice. Iran J Pharm Res. 2011;10:505–10. [PMC free article] [PubMed] [Google Scholar]
- 27.Brandao EM, Brandão PH, Souza IA, Paiva GS, de C Carvalho M, Lacerda CM. Antineoplasic effect of aqueous extract of Plectranthus amboinicus in Ehrlich ascites carcinoma. J Cancer. 2013;4:573–6. doi: 10.7150/jca.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagat J, Lobo R, Kumar N, Mathew JE, Pai A. Cytotoxic potential of Anisochilus carnosus (L.f.) wall and estimation of luteolin content by HPLC. BMC Complement Altern Med. 2014;14:421. doi: 10.1186/1472-6882-14-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekar S, Kiruthiga N. Anticancer activity of ethanol extracts of Anisochilus carnosus in human lung cancer A549 cell lines. Int J Curr Res. 2014;6:10033–7. [Google Scholar]
- 30.da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;1:364–9. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 31.Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki N, Toda T, Kaneko T, Baba N, Matsuo M. Protective effects of flavonoids on the cytotoxicity of linoleic acid hydroperoxide toward rat pheochromocytoma PC12 cells. Chem Biol Interact. 2003;145:101–16. doi: 10.1016/s0009-2797(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 33.Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz (a) anthracene-induced mammary tumors in rats. Chem Biol Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Manju V, Nalini N. Chemopreventive potential of luteolin during colon carcinogenesis induced by 1,2-dimethylhydrazine. Ital J Biochem. 2005;54:268–75. [PubMed] [Google Scholar]
- 35.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–51. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]