Abstract
Background:
Roscoea purpurea or Roscoea procera Wall. (Zingiberaceae) is traditionally used for nutrition and in the treatment of various ailments.
Objective:
Simultaneous reversed-phase high-performance liquid chromatography-ultraviolet (RP-HPLC) photodiode array detector identification of phenolic acids (PA's) was carried out in whole extract of tuber and their cytotoxic potential was estimated along with radical scavenging action. Bioactivity guided fractionation was also done to check the response potential against the same assay.
Materials and Methods:
Identification and method validation was performed on RP-HPLC column and in vitro assays were used for bioactivity.
Results:
Protocatechuic acid, syringic acid, ferulic acid, rutin, apigenin, and kaempferol were quantified as 0.774%, 0.064%, 0.265%, 1.125%, 0.128%, and 0.528%, respectively. Validated method for simultaneous determination of PA's was found to be accurate, reproducible, and linearity was observed between peak area response and concentration. Recovery of identified PA's was within the acceptable limit of 97.40–104.05%. Significant pharmacological response was observed in whole extract against in vitro cytotoxic assay, that is, Sulforhodamine B assay, however, fractionation results in decreased action potential. Similar pattern of results were observed in the antioxidant assay, as total phenolic content and total flavonoid content were highest in whole extract and decreases with fractionation. Radical scavenging activity was prominent in chloroform fraction, exhibiting IC50 at 0.25 mg/mL.
Conclusion:
Study, thus, reveals that R. purpurea exhibit significant efficacy in cytotoxic activity with the potentiality of scavenging free radicals due the presence of PA's as reported through RP-HPLC.
SUMMARY
Proto-catechuic acid, syringic acid, ferulic acid, rutin, apigenin and kaempferol were quantified as 0.774, 0.064, 0.265, 1.125, 0.128 and 0.528 %
Preliminary cytotoxic activity revealed that whole extract of R. purpurea exhibit promising effect and after fractionation the potentiation of action reduces
The radical scavenging potential of whole extract and fractions are well reflected by TPC, TFC and DPPH assay.
Keywords: Cytotoxic activity, phenolic acids, radical scavenging activity, reversed-phase high-performance liquid chromatography, Roscoea purpurea
INTRODUCTION
Roscoea purpurea or Roscoea procera Wall. is a perennial herb of family Zingiberaceae. The species is locally known as Kakoli, Red Gurkha, Dhawanksholika, Karnika, Ksheera, Madhura, Shukla, Svadumansi, Vayasoli and Vaysasha also. R. purpurea is widely available in The Himalayas and is native of Nepal and grown on steepy, grassy hillsides, damp gullies, and stony slopes. R. purpurea is cultivated as an ornamental plant and traditionally in Northern India; fleshy roots are used for the treatment of malaria and urinary infection. In ethnobotanical practice, leaves, roots, and flowers are used for the treatment of diabetic, hypertension, diarrhea, fever, and inflammation. In Nepal, tubers are boiled for edible purpose and also used in traditional veterinary medicine.[1,2] Tubers of Roscoea are major constituent of polyherbal Ayurvedic formulation, “Ashtavarga,” which according to Nighandu Samhita and Indian Metria Medica is like chawanprash having, antioxidant, anti-aging effect, and elevates overall health status of a well-being.[3] Pharmacological reports support the immunomodulatory[4] and antidiabetic activity[5] of R. purpurea tubers.
Natural phenolic acids (PA's) are the class of biologically active compounds possessing one or more ring structure with a variable number of the hydroxyl group. They are broadly diversified in plants and are one of the major secondary metabolites. PA's include a class of compounds (flavonoids, flavones, flavanones, tannins, and lignans, etc.), among which flavonoids are the most beneficial and richly available polyphenolic acids/phenolic acids in our diet. Classically, polyphenolic acids have been used as biologically active antioxidants; however, these compounds play several other remarkable activities also, for example antiallergic, anti-inflammatory, antimutagenic, and modulation of enzyme activities. Several workers have proven the efficacy of PA's as potent chemotherapeutic and chemoprotective agents.[6,7,8] Isolated PA's, that is, quercetin, kaempferol, caffeic acid, apigenin, (±) catechin, and naringenin, etc., have well established and prominent anti-cancerous activities.[9,10,11] Hence, chemoprevention or chemotherapy via a natural source with fortified radical scavenging activity is always welcomed.
As state of art suggested that tubers are effective as anti-aging, anti-inflammatory, and have immunomodulatory action, and if focused over the underlying cause of these disorders, free radicals were found as common agents. In addition to this, its traditional use as potential antioxidant further suspect for the presence of bioactive PA's. Hence, an attempt was made to identify, characterize, and quantify the PA's present in R. purpurea though reverse phase-high performance liquid chromatography (RP-HPLC). As stated above, polyphenolics are emerging source of chemotherapeutic agents, thus, the cytotoxic potential of R. purpurea tubers were evaluated for which scientific data is still lacking. In addition to this activity, guided fractionation was also carried out to determine the potentiation of action on targeted activity. Biologically active nature of identified PA's was further supported by radical scavenging assay.
In summary, the study was conducted with two major objectives, firstly for identification and quantification of PA's through validated RP-HPLC-ultraviolet (UV) photodiode array detector (PDA) developed method in whole extract (A) of species (tuber) and evaluated for preliminary cytotoxic activity. On the basis of results obtained, work was further extended with second objective of activity guided fractionation from tubers with solvents of differential polarity, that is, petroleum ether (B), chloroform (C), acetone (D), alcohol (E), and water (F) fractions as shown in Figure 1 and subjected to in vitro cytotoxic activity. The bioactive nature of identified PA's was further validated by in vitro antioxidant assay's, that is, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and determination of total PA and flavonoid content.
Figure 1.
Diagrammatic representation of experimental study
MATERIALS AND METHODS
Chemicals
Reference standards of protocatechuic acid (97%), syringic acid (≥97%), ferulic acid (≥99%), rutin (≥95%), apigenin (≥95%), kaempferol (≥97%), ascorbic acid (≥97%), quercetin (≥97%), 1-1-diphenyl-2-picrylhydrazyl (≥99%, DPPH) and Vinblastine were purchased from Sigma-Aldrich. HPLC grade solvents viz., acetonitrile, methanol, water, and all other solvents/chemicals (AR grade) were purchased from Merck, Mumbai, India. Ham's Nutrient Mixtures F-12 medium, Roswell Park Memorial Institute-1640 (RPMI-1640), Dulbecco's modified Eagle medium (DMEM), heat-inactivated fetal bovine serum, and antibiotic antimycotic (Ref. No. 15240-062) were purchased from Invitrogen Bio Services India Pvt. Ltd.
Plant material
Fresh tubers of R. purpurea were collected in the month of October to November from the nearby area of Dhanaulti (Phytogeographical zone: Western Himalayas, Altitude: 1705 meter, latitude: 30° 21’ 34.4”, Longitude: 78° 23’ 15.3”), Uttarakhand, India. It was authenticated, and a voucher specimen was deposited in institute's herbarium (LWG No. 254028).
Preparation of whole extract and bioactivity guided fractions
Collected sample was washed, shade dried, and coarsely powdered (40 mesh). About 5 g of defatted material (with petroleum ether) was macerated with ethyl alcohol at 30 ± 2°C for 24 h, filtered (Whatman No. 1 filter paper) and again macerated with fresh solvent. Extraction was repeated thrice, pooled, and concentrated in a vacuum with rotator evaporator (Buchi Labortechnik, Switzerland). The concentrated extracts were finally lyophilized to obtain ethanol soluble extractive yield (whole extract, A) of 41.85% of dry weight. For activity guided fractionation, about 5 g of powder was successively extracted through maceration (same conditions as above) with solvents in increasing order of polarity, that is, petroleum ether (B), chloroform (C), acetone (D), ethanol (E) and aqueous (F) having fraction yield (%) of 3.38%, 1.47%, 1.23%, 1.90% and 63.15%, respectively.
High-performance liquid chromatography method development and validation for phenolic acids in whole ethanol extract of Roscoea purpurea tuber
Preparation of standards and high-performance liquid chromatography conditions
The standard stock solutions (1 mg/mL) of protocatechuic acid, syringic acid, ferulic acid, rutin, apigenin and kaempferol were prepared in HPLC grade methanol and stored at 4°C, until used. Working solutions of lower concentration (0.1 mg/mL) were prepared by appropriate dilution of the stock solutions in methanol.
Waters RP-HPLC-PDA-2996 (Waters Corporation, Milford, MA, USA) was used for the qualitative and quantitative analysis of analytes. Chromatographic separation was performed on Supelco C18 column (4.6 mm × 50 mm, 5.0 µm,) with C18 guard column in gradient mode with binary mobile phase, duly filtered through 0.22 µm Millipore filter and degassed ultrasonically for 15 min before delivered to column for separation. Data acquisitions were performed using Empower software version 2 (Waters, Milford, MA 01757, USA). The injection volume was 20 µL for standard and sample. Detection was observed at a maximum wavelength of λ285 nm.
Optimization of method
A RP-HPLC-PDA-2996 (Waters, USA) was used for separation of reference compound on Chromatopak C18 column AQ (4.6 mm × 50 mm, 5.0 µm,) using a gradient mode with 0.1% formic acid in water (A) and pure acetonitrile (B) as mobile phases. Separation of analytes was carried out at 27°C using 20 µL of sample/standard injection volume. Total HPLC running time was 55 min and standards were eluted at their corresponding retention time viz.: 6.19, 10.56, 15.17, 18.51, 32.25, and 39.92 min for protocatechuic acid, syringic acid, ferulic acid, rutin, apigenin, and kaempferol, respectively. Peak areas versus reference standard concentration were subjected to regression analysis, and the slope, intercept, and correlation coefficient for the calibration curve was determined at 5 concentrations and quantified.
Validation of method
The HPLC method for quantification of PA's viz., protocatechuic acid, syringic acid, ferulic acid, rutin, apigenin, and kaempferol were determined by selectivity, linearity, precision, sensitivity, and accuracy.
Selectivity
A standard solution of six PA's was dissolved in 1 mL methanol, and 10 µL of the standard mixture was injected into the column, analyzed as per the method described above and peak purity was also accessed.
Linearity
Reference standards at the concentration range of 0.25–2.0 µg were injected into the HPLC system and calibration curves were plotted by linear regression of the peak area ratio (y) of each PA versus concentration (x) in µg/mL. Stability of the standard calibration mixture and extract was determined by injecting them repeatedly over a 24 h period. The percent relative standard deviation (% RSD) and percentage of change over the period were also determined for each compound.
Sensitivity
Sensitivity of method was determined with respect to limit of detection (LOD) and limit of quantification (LOQ), was calculated from the standard deviation (SD) of the response and slope of the calibration curve. The formulae used for LOD (3.3 σ/S) and LOQ (10 σ/S) were calculated with SD of the response (σ) and slope of the calibration curve (S).
Precision
Precision is a test for the distribution of concentrations measured. Two different concentrations of the stock solutions were exposed to five determinations on the same day. Repeatability of analysis was performed at five different concentrations ranging from 0.25 to 2.0 µg/mL for standards. All RSD values were below 3%, which is considered to be acceptable. To determine the precision of developed method, each standard and extract was analyzed six times on the same day to determine the intra-day precision. The same procedure was repeated over 3 days and compared by calculating the % RSD for the average values for each day to determine the inter-day precision.
Recovery
Accuracy of the method was analyzed by recovery studies, five different concentrations diluted from the stock solution were added to an extract with a known content of standards and the recovery of respective constituent was calculated.
Robustness
Precision is a test for the distribution of concentrations measured. Two different concentrations of the stock solutions were exposed to five determinations on the same day. Repeatability of analysis was performed in three concentrations of all standards. All RSD values were below 1%, which is considered to be acceptable to perform this test. The deviations were calculated on the basis of peak areas with parameters 0.8–1.2 mL/min and 27°C as reference values, which is the middle value of flow rate and temperature, respectively. Mean deviation is <3% which is acceptable for analysis.
Cytotoxic activity
Cell culture lumen lung carcinoma (A549), human cervical cancer (SiHa), Chinese hamster ovary cells (CHOK1), and rat glioma (C-6) cells were obtained from National Centre for Cell Science, Pune. A549 and CHOK1 cells were grown in Ham's F-12 medium, SiHa cells were cultured in RPMI-1640, and C-6 cells were grown in DMEM, supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotic antimycotic. The cells were maintained at 37°C in a 5% CO2 humidified atmosphere.[12,13]
Sulforhodamine B cytotoxic activity
The cell cultures were trypsinized and washed twice with phosphate buffer saline by centrifugation and incubated at a density of 20,000 cells/well in 96-well plates in 100 μl of complete medium. Several dilutions (10, 50, 100, and 150 μg/mL) of the whole extract and fractions were added in 100 μL of complete medium. Cell culture alone supplemented with the complete medium was used as negative control whereas; Vinblastine (1 μM) used as positive control for the assay. The plates were incubated at 37°C for 48 h in the CO2 incubator. After 48 h, 50 μl of 50% trichloroacetic acid was added, and the plates were kept at 4°C for 1 h. The plates were flicked and washed 5–6 times with tap water and then air-dried. Subsequently, 100 μL of the sulforhodamine B solution (0.4% in 0.1% glacial acetic acid w/v) was added and incubated for 30 min at room temperature. After incubation, plates were washed rapidly 5–6 times with 1% acetic acid and air dried. 100 μL of 10 mM tris base was added to the wells. The absorbance was measured using microplate reader (BioTeK Synergy H1 Hybrid Reader) at a wavelength of 540 nm.[14]
Antioxidant activity
Total flavonoids[15] and phenolics[16] were expressed in terms of mg/g of quercetin equivalent (QE) and g/g gallic acid equivalent (GAE) based on calibration curve of quercetin and gallic acid as standard. The radical scavenging potential[17] of whole extracts (A) and fractions viz., B, C, D, E, and F of Roscoea tubers were analyzed using DPPH.
Statistical analysis
For HPLC interpretation, results were reported as means ± SD of at least three replicates of the whole extract. Data were subjected to one-way analysis of variance (ANOVA) and the least significant difference between the extracts at P < 0.01 was calculated by post-hoc comparison test (SPSS 11.5 SPSS Inc. Chicago, USA).
In the antioxidant activity, results were expressed as mean ± SD. Linear regressions analysis was carried out for standards to calculate TPC and TFC, and GraphPad Prism 5 (San Diego, CA, USA) software was used to calculate the IC50 values. One-way ANOVA followed by Student's t-test (P < 0.01) was used to find the significance of standard and sample.
RESULTS
High-performance liquid chromatography method validation
Method development began with the optimization of chromatographic conditions, including mobile phase composition and column type. Versatility, suitability, and robustness of method were checked with several C18 columns of various manufacturers (data not shown) and it was found that chromatographic resolution, selectivity, and sensitivity were good with Supelco C18 column (4.6 mm × 50 mm, 5.0 µm). Feasibility of various mixtures (s) of solvents such as ammonium acetate, acetic acid, and formic acid with variable pH range of 0.4–6.6, along with altered flow-rates (in the range of 0.4–1.5 mL/min) was tested for complete chromatographic resolution of PA's. Finally, mobile phase A comprising 0.1% formic acid in water and B was pure acetonitrile were selected, and conditions were as follows: Eluent A; eluent B; gradient, 0–30 min (5–25% B) at 0.80 mL/min flow rate, 30–40 min (25–45% B) at 0.90 mL/min flow rate, 45–50 min (50–40% B) at 0.90 mL/min, 45–50 min (40–20% B) at 1.0 mL/min 50–55 min (20–5% B) at 1.2 mL/min flow rate, and then equilibrated with 8% B for 10 min at 1.0 mL/min flow rate.
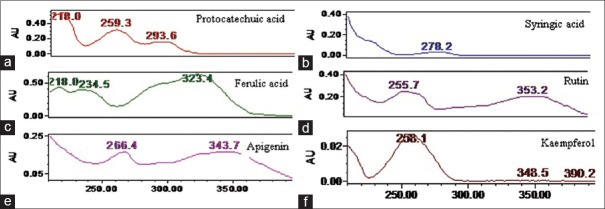
A typical HPLC chromatogram of the standard and extract is shown in Figures 2 and 3. The identification of PA's was based on a comparison of retention times and UV spectra of reference standards with the corresponding peaks in the extract [Figure 4]. The retention time (Rt) for standards were obtained according to the optimized method as described in experimental section. Peak purity was accessed by PDA data at λ285 nm and purity factor obtained for extract reveals the presence of pure peak without contamination of other co-eluting compounds. Quantification of PA compounds ranges from 0.064% to 0.528% [Table 1] in triplicate.
Figure 2.
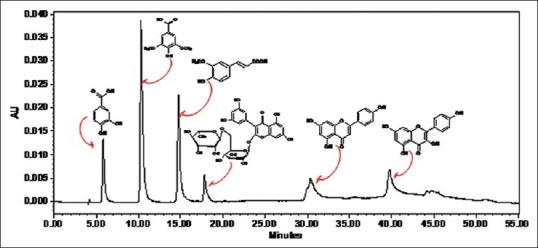
High-performance liquid chromatography-ultraviolet photodiode array detector chromatograms of standards
Figure 3.
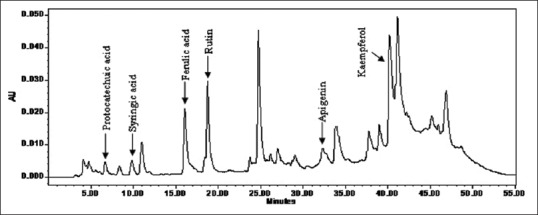
High-performance liquid chromatography-ultraviolet photodiode array detector chromatograms of whole extract of Roscoea purpurea rhizome
Figure 4.
Ultraviolet spectra of reference standard (a) protocatechuic acid (b) syringic acid (c) ferulic acid (d) rutin (e) apigenin, and (f) kaempferol in whole extract of Roscoea purpurea rhizome
Table 1.
RP-HPLC quantification of phenol acids in whole extract of Roscoea purpurea rhizomes
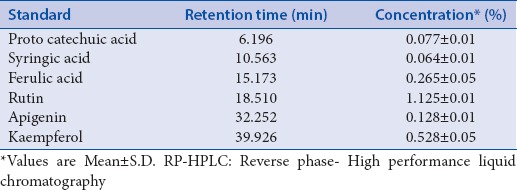
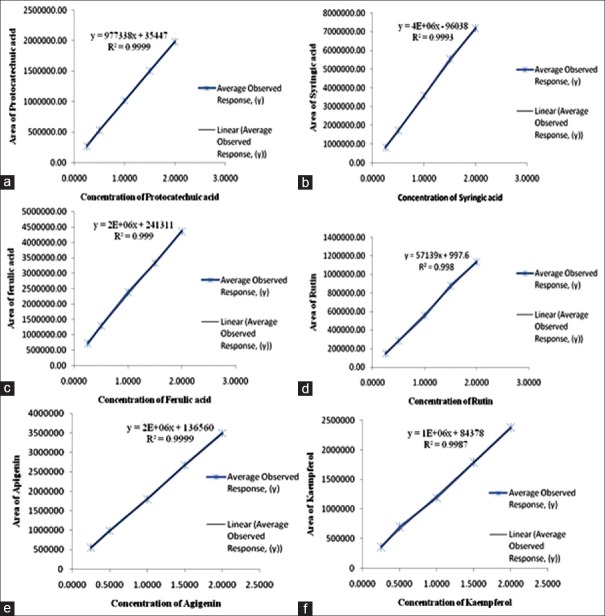
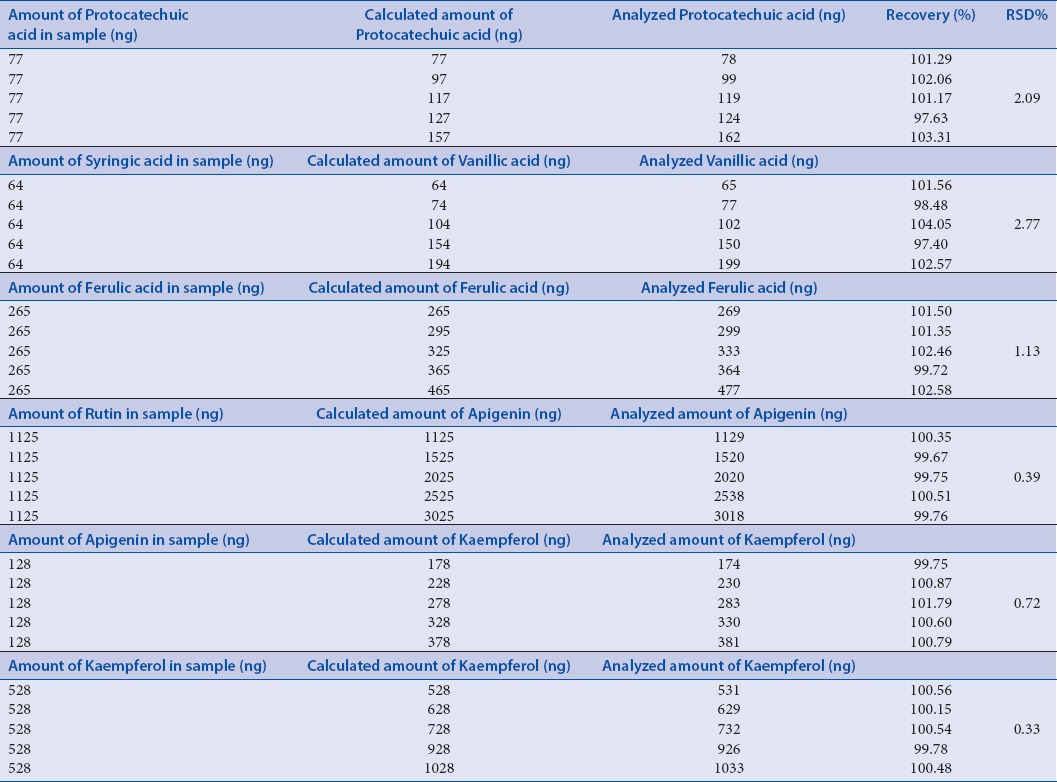
The HPLC quantification method for PA's was validated, and all the included parameters are within the specified limit [Table 2]. The selectivity validation of method is well demonstrated by the excellent separation of analytes in reference mixture and as explained above, the peak purity was also good; it indicates that there is no merging of any unidentified peak with known identified PA (analyte), and thus confirms the selectivity of method. Standard solutions of PA's in methanol showed a linear correlation between the peak area and concentration, correlation coefficient was found to be satisfactory while plotting of calibration for standards. To estimate the stability, % RSD of standard mixture and extract was calculated and results (not more than 3%) shows that the analytical solutions are stable for duration of 24 h at a temperature of 4°C in autosampler unit. Value of correlation coefficient for protocatechuic acid, vanillic acid, ferulic acid, apigenin, and kaempferol indicates good linearity [Figure 5]. LOD and LOQ values depict that method is sensitive [Table 2]. A fixed concentration of extract was used to access the precision and accuracy of method, inter and intra-day RSD (%) indicate the values within the limit range (not more than 5%) as shown in Table 3. Recovery studies of PA's, as shown in Table 4, vary from 97.40% to 104.05%, respectively. The observed values are within the specified limit (95–105%) and hence the method was found to be accurate, precise, and reproducible. To the best of our knowledge and available literature, this is first reporting on the development of a simple, rapid, and sensitive method on quantification of protocatechuic acid, vanillic acid, ferulic acid, rutin, apigenin, and kaempferol in whole extract of R. purpurea tubers. The outcome supports the evidence that developed method is accurate and reproducible in specified conditions for quantification of targeted PA's.
Table 2.
Validation parameters for HPLC analysis of Protocatechuic acid, Syringic acid, Ferulic acid, Rutin, Apigenin and Kaempferol
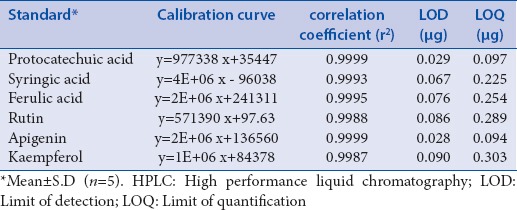
Figure 5.
Calibration curve of phenolic acids (a) protocatechuic acid (b) syringic acid (c) ferulic acid (d) rutin (e) apigenin, and (f) kaempferol in whole extract of Roscoea purpurea rhizome
Table 3.
Inter day and Intraday precision table of standard compounds
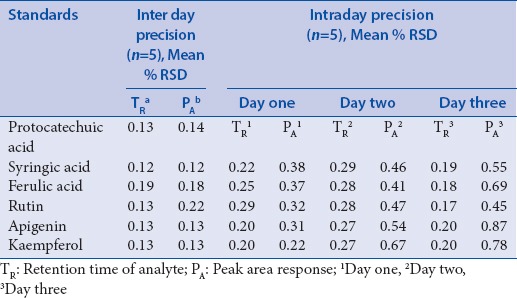
Table 4.
Recovery studies (%) of phenol acids in Roscoea purpurea
Cytotoxic activity of whole extract and fraction (s)
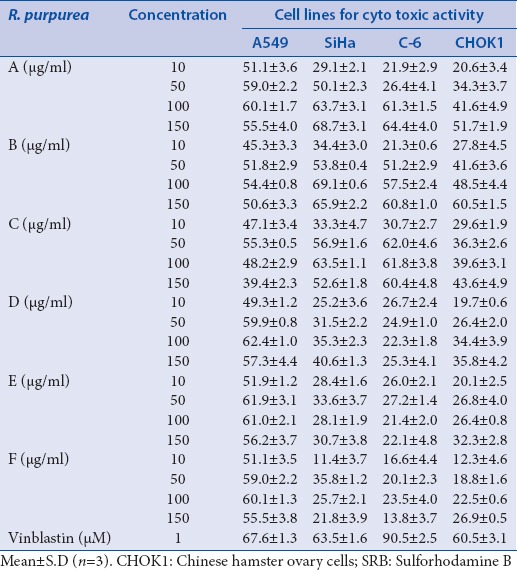
In vitro cytotoxicity was carried out against four different cell lines viz., A549, SiHa, CHOK1, and C-6 cells at 10, 50, 100, and 150 µg/mL, respectively. The percentage of cellular growth inhibition was calculated by measuring the absorbance of respective incubated cells [Table 5]. Potential activity was observed in the whole extracts against the tested cell lines exhibiting IC50 value of <10, 49.5, 83.8, and 141.6 µg/mL against A549, SiHa, C-6, and CHOK1, respectively. These interesting results, lead to the idea of fractionating the phytomolecules present in the whole extract on the basis of solvent polarity for identification of the nature of molecules responsible for targeted bioactivity. Data reveals that all the tested fractions exhibit dose-dependent cytotoxicity against the cell lines. Among them fraction B exhibit the highest activity (69.1 ± 0.6 and 60.5 ± 1.5) on SiHa and CHOK-1 cells at concentration of 100 and 150 μg/mL, respectively, and C showed the highest activity (62.4 ± 1.0) on A549 cells at a concentration of 100 μg/mL. However, the activity against C-6 cells is similar in B and C fractions [Table 5].
Table 5.
Cytotoxicity exhibited by whole extract and fractions of R. purpurea (rhizomes) against different cells by SRB assay
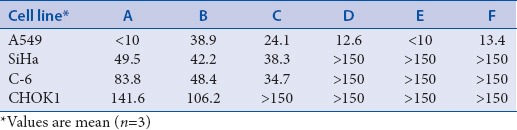
The IC50 of tested fractions ranges between < 10 and > 150 μg/mL [Table 6]. In a nutshell, from above preliminary cytotoxic activity, it was observed that whole extract of Roscoea exhibits promising effect and after fractionation, the potentiation of action reduces and variable responsible was observed. The underlying mechanism of action was not clearly known and may be suspected due to the synergistic effect of PA's with other bioactive metabolites in whole extract. Although fractionation results in segregation of metabolites, and thus the differential response was achieved. Previously, workers had reported the cytotoxic/anti-cancerous activity of pure PA's which are identified in the whole extract of Roscoea also. The preventive effect of apigenin in chemotherapy is also established.[18] Further studies in near future will reveal the precise cellular and molecular mechanisms induced by the extract. The inhibitory effect of natural PA's in carcinogenesis and tumor growth may be through two main mechanisms: (1) Modifying the redox status and (2) interfering with basic cellular functions (cell cycle, apoptosis, inflammation, angiogenesis, invasion, and metastasis) and both the action are mediated through their ability to scavenge the reactive oxygen species/free radicals at one or other steps of cancer pathology. The study, however, needs to be extended further on SAR and QSAR lineage to depict the mode of action of the compound responsible for same.[19]
Table 6.
IC50 value of whole extract (A) and fractions (B, C, D, E and F) of Roscoea rhizomes against A549, SiHa, C-6 and CHOK1 cells in μg/ml
Antioxidant potential
Presence of natural phenolic and flavonoid moiety in the plants serves as an indicator of their free radical scavenging activity and quantification of the same can be useful in accessing the antioxidant capacity of species. Total flavonoid content (TFC) and total phenolic content (TPC) were significantly (P < 0.01) rich in whole extract of Roscoea, that is, 26.78 mg/g QE and 3.03 g/g GAE, respectively. After fractionation, flavonoid content decreases in order of F fraction and then E, D, B, and C fractions having 9.15, 1.71, 1.39, 0.27, and 0.216 mg/g QE, respectively. Phenolic content was also highest in F (0.565 g/g GAE) fraction, followed by D (0.135 g/g GAE), E (0.106 g/g GAE), C (0.100 g/g GAE), and B (0.040 g/g GAE).
Bioactive PA's are the class of compounds that effectively inhibits free radicals because of their scavenging activity and therapeutically beneficial as they are common underlying cause of several disorders viz., cardiovascular, diabetes, aging, arthritis, cancer, and inflammatory disorders, etc.[20] Thus, in order to estimate the radical scavenging effect of the whole extract and fractions, DPPH radical was used. Data show that activity varied considerably among the whole extract and fractions of tubers [Figure 6] when compared to standard reference viz., ascorbic acid, quercetin, and rutin. In standards, maximum inhibition of free radicals was observed in ascorbic acid (77.57%, IC50: 3.86 ± 0.057 µg/mL), followed by quercetin (72.43%, IC50: 5.93 ± 0.115 µg/mL), and rutin (71.48%, IC50: 6.80 ± 0.173 µg/mL). Whole extract exhibits IC50 at 0.925 ± 0.005 mg/mL. Among the Roscoea fractions, inhibition of radicals varies from 5.2% to 83.21% [Figure 6]. Fraction C possesses significant IC50 value at 25 mg/mL [Table 7].
Figure 6.
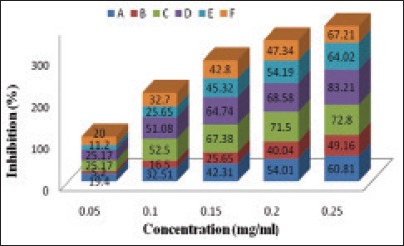
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of Roscoea whole extract and fractions
Table 7.
IC50 value of R. purpurea whole extracts and fractions in DPPH radical scavenging assay

CONCLUSION
HPLC method developed for simultaneous quantification of six PA's reveals the presence of protocatechuic acid, syringic acid, ferulic acid, rutin, apigenin, and kaempferol in a concentration range of 0.064–1.125%. It is evident that the developed protocol is accurate and reproducible under specified conditions and can be utilized as a quality control method for quantification of PA's marker in the species. On the basis of data from preliminary cytotoxic study of whole extract, significant results have been noticed against various cell lines viz., A549, SiHa, CHOK1, and C-6 cells, and thus may be explored as a source of cytotoxic agent. The observed above activity response of whole extracts may be suspected due to the synergistic effect of PA's with other phytomolecules present in the extracts. However, fractionation leads to segregation of phytochemicals, and thus the activity response reduced prominently. The radical scavenging potential of the whole extract and fractions are well reflected by TPC, TFC, and DPPH assays.
The work is novel as this is the first ever report on simultaneous identification and quantification of PA's in the species, in addition to this cytotoxic potential of identified PA's was not reported yet. we summarized that R. purpurea (whole extract and fractions) revealed significant efficacy in cytotoxic activity with potentiality of scavenging free radicals due the presence of six bioactive PA's as reported via RP-HPLC-UV PDA validated method.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Dr. Sharad Srivastava
Dr. Sharad Srivastava, is Principal Scientist in Pharmacognosy Division at CSIR-National Botanical Research Institute; Lucknow, INDIA. He has made significant contributions to quality control of crude drugs/products, chemotaxonomy, bio-prospection and natural product development and have developed quality parameters of single crude drugs (more than 70 medicinal plants) and also identified biomarkers for their quality control. He has contributed 30 monographs of single herbal drugs in Ayurvedic Pharmacopoeia of India. He has 110 publications in peer reviewed journals, 17 patents and developed some technologies/formulations, few has already been transferred to industry.

Ms. Ankita Misra
Ms. Ankita Misra is working as a Research Associate in Pharmacognosy Division at CSIR-National Botanical Research Institute; Lucknow, INDIA. She is working in the area of analytical chemistry on medicinal plants, chemotaxonomy, bio-prospection and natural product developemnt, handling major analytical instruments including HPLC, HPTLC, OPLC and column chromatography for quality control of herbal drugs. She has 11 publications in peer reviewed journals and 1 patent is also to her credit.
Acknowledgment
The authors thankfully acknowledge the Directors of CSIR-NBRI, Lucknow (UP) and CSIR-IHBT, Palampur, Himachal Pradesh, India, for continuous support and for providing necessary facilities during the course of the experiment.
REFERENCES
- 1.Handa N. Flora of India. 1980. [Last accessed on 2014 Oct 12]. Available from: http://www.arvindguptatoys.com/arvindgupta/nimrethanda.pdf .
- 2.Singh G, Rawat GS. Ethnomedicinal survey of Kedarnath wildlife sanctuary in Western Himalaya, India. Indian J Fundam Appl Life Sci. 2011;1:35–6. [Google Scholar]
- 3.Singh AP. Ashtavarga – Rare medicinal plants. Ethnobotanical Lealf. 2006;10:104–8. [Google Scholar]
- 4.Sahu MS, Mali PY, Waikar SB, Rangari VD. Evaluation of immunomodulatory potential of ethanolic extract of Roscoea procera rhizomes in mice. J Pharm Bioallied Sci. 2010;2:346–9. doi: 10.4103/0975-7406.72138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bairwa R, Basyal D, Srivastav B. Study of antidiabetic and hypolipidemic activity of Roscoea purpurea (Zingiberaceae) Int J Inst Pharm Life Sci. 2012;2:130–7. [Google Scholar]
- 6.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 7.Galati G, Teng S, Moridani MY, Chan TS, O’Brien PJ. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol Drug Interact. 2000;17:311–49. doi: 10.1515/dmdi.2000.17.1-4.311. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2000;2:381–6. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 9.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro . J Agric Food Chem. 2006;54:9329–39. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 10.Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, et al. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–41. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, et al. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37:223–33. doi: 10.1207/S15327914NC372_16. [DOI] [PubMed] [Google Scholar]
- 12.Walia M, Mann TS, Kumar D, Agnihotri VK, Singh B. Chemical composition and in vitro cytotoxic activity of essential oil of leaves of Malus domestica growing in Western Himalaya (India) Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/649727. 649727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parashar G, Parashar NC, Capalash N. Curcumin causes promoter hypomethylation and increased expression of FANCF gene in SiHa cell line. Mol Cell Biochem. 2012;365:29–35. doi: 10.1007/s11010-012-1240-z. [DOI] [PubMed] [Google Scholar]
- 14.Katoch D, Kumar D, Sharma U, Kumar N, Padwad YS, Lal B, et al. Zephgrabetaine: A new betaine-type amaryllidaceae alkaloid from Zephyranthes grandiflora. Nat Prod Commun. 2013;8:161–4. [PubMed] [Google Scholar]
- 15.Ordonez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) swart extracts. Food Chem. 2006;97:452–8. [Google Scholar]
- 16.Bray HG, Thorpe WV. Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal. 1954;1:27–52. doi: 10.1002/9780470110171.ch2. [DOI] [PubMed] [Google Scholar]
- 17.Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–40. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 18.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (review) Int J Oncol. 2007;30:233–45. [PubMed] [Google Scholar]
- 19.Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi KD. 4th ed (reprint) India: Jaypee Brothers Medical Publishers (P) Ltd; 2004. Essentials of Medical Pharmacology. [Google Scholar]