Abstract
Purpose of review
To describe the potential contribution of immune activation in the pathogenesis of HIV-associated cardiovascular disease (CVD)—a leading cause of morbidity and mortality among HIV positive persons with access to antiretroviral therapy (ART).
Recent findings
We review recent literature that suggests abnormalities in both adaptive and innate immunity contributes to CVD risk among persons with HIV infection. In particular, potentially atherogenic T-cell mechanisms include persistent high-level T-cell activation (and associated pro-inflammatory mechanisms), as well as the presence of co-pathogens (e.g., CMV) providing an ongoing stimulus for cytotoxic T-cell responses. More recent data has then emphasized the potential impact of monocyte/macrophage-mediated inflammation and injury within atherosclerotic lesions. The pathology driving innate immune activation many not fully reverse with ART treatment, highlighting the need for interventions that target inflammation as a CVD prevention strategy.
Summary
Premature CVD among persons with HIV infection is due, in part, to persistent abnormalities in immune activation and systemic inflammation despite viral suppression. Prevention strategies for persons with HIV infection include those that target traditional CVD risk factors as well as newer candidate treatments with potential immunomodulatory benefits.
Keywords: HIV, cardiovascular disease, Immune activation
Introduction
Soon after effective antiretroviral therapy (ART) became available in the mid-1990's, the first case reports of acute myocardial infarction in young HIV-infected men on combination ART were published in the Lancet[1]. In the nearly two decades since, we have developed a greater understanding of the epidemiology and mechanisms of cardiovascular disease (CVD) among those with HIV infection. Recent reviews in this journal have summarized this evolving field in terms of the epidemiology and risk factors for CVD in HIV[2] and whether early ART might reduce CVD risk[3].
Currently, it is now recognized that chronic HIV infection is associated with higher risk for a broad spectrum of cardiovascular diseases that includes not only myocardial infarction[4], but also stroke[5], heart failure[6], and sudden cardiac death[7]. Furthermore, the HIV-associated risk appears to be at least as high in women as it is in men[8]. Pathogenesis studies of subclinical CVD suggest that individuals with HIV-infection may have a greater prevalence of high-risk features within coronary artery plaques,[9, 10] and potentially significant myocardial steatosis and fibrosis[11, 12]. Despite the relative increased CVD risk, absolute event rates remain low in this relatively young population—approximately 0.5 CVD deaths per 1000 person years among 65,000 patients in Europe and North America[13]. Some reports have even described event rates that approach HIV-negative cohorts, possibly due to heightened awareness and more aggressive risk factor modification[14, 15]. Ultimately, CVD event rates are likely to increase, potentially more rapidly, as the treated HIV-infected population continues to age[2].
Traditional risk factors remain important mediators of CVD in the HIV-infected population. For example, smoking is highly prevalent and is associated with a higher risk of MI in HIV infection compared to an uninfected control population[14]. Cardiometabolic risk factors such as hypertension, dyslipidemia, and diabetes are also common in HIV infection, partly attributable to certain antiretroviral medications, and associated with cardiovascular events.[4, 16-18] However, newer ART drugs have more favorable cardiometabolic profiles[19], and some data suggest ART initiation with modern regimens may reduce risk for the metabolic syndrome[17]. Ultimately, with availability of well-established approaches to target traditional risk factor modification, an important unmet need in the field remains understanding and targeting non-traditional risk factors. In particular, chronic inflammation and immune activation persist despite effective ART and appear to contribute to subclinical disease and CVD events, similar to what is seen in patients with inflammatory autoimmune diseases such as rheumatoid arthritis or psoriasis. In this review, we will explore recent evidence of how adaptive and innate immune mechanisms relate to CVD among individuals with HIV infection, as well as review potential strategies to mitigate CVD risk by reducing immune activation.
Adaptive Immune Mechanisms
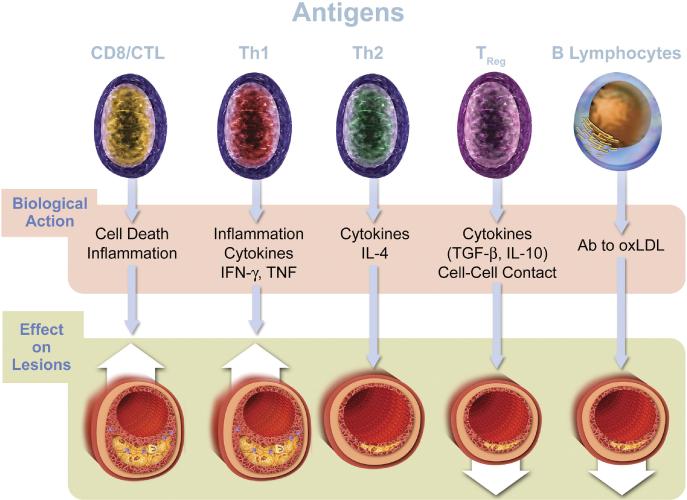
CD4+ T-cell depletion and dysfunction, and chronic CD8+ T-cell activation, are hallmarks of HIV infection. Greater T-cell proliferation and turnover among HIV infected when individuals is a central components of AIDS pathogenesis[20]. Recent research has focused increasingly on understanding the implications of changes in adaptive immunity for CVD and other end-organ disease risk. Figure 1 provides schematic context for how the cellular arms of adaptive immunity can have differential effects on atherogenesis.
FIGURE 1. Adaptive immunity in atherosclerosis.
T and B lymphocytes may positively or negatively influence atherosclerotic plaque through direct action on lesions or through inflammatory cytokine production. Emerging observational data have linked CD8+ and CD4+ T-cell activation to subclinical vascular disease and adverse outcomes among those with HIV infection; relatively less is known about the role of TReg or B lymphocytes in the context of HIV disease. Reproduced with permission from Libby et al.[39]
Associations between CD4+ T-cell depletion and cardiovascular risk have been widely reported on [9, 21-28]. In the HIV Outpatient Study cohort, CD4 < 500 cells/mm3 was an independent risk factor for cardiovascular disease events that was as dangerous as smoking or high LDL[21]. Similar data show associations between lower nadir CD4 and other measures of preclinical CVD including higher left ventricular mass, increasing intima–media thickness, and lower brachial artery flow-mediated dilation[9, 22]; however, the D:A:D (Data collection on Adverse events of Anti-HIV Drugs) study failed to show a strong linear relationship between current CD4 count and a broad composite of CVD outcomes, although most events were less frequent among those who did not experience immune depletion [23]. Data from the Kaiser Permanente System are consistent with this, showing that MI rates for HIV+ persons with CD4 counts >500 cells/mm3 were similar for HIV infected and uninfected persons [24]. In summary, while severe immune depletion (e.g., at CD4 counts <200 cells/mm3) may contribute to premature CVD, more recent literature suggests this relationship may be less clinically relevant among those with immune preservation. In support of this notion, the Strategic Timing of AntiRetroviral Therapy (START) trial failed to show that immediate ART treatment specifically reduced CVD events among ART-naïve HIV positive patients with CD4 counts >500 cells/mm3, despite reductions in a number of other AIDS and non-AIDS clinical events [29].
Another potentially important contributor to CVD pathogenesis relates to activation of the adaptive immune response. Concurrent with CD4 depletion, high-level and persistent CD8+ T-cell activation is a key feature of the natural history of HIV infection [30]. Both a higher absolute CD8+ T-cell count as well as a lower CD4:CD8 ratio, have been associated with a greater degree of coronary plaque by CT angiography and an increased risk of MI among HIV-infected individuals[28, 31]. In sentinel data by Kaplan et al, higher frequencies of immune activation and senescent CD8+ T-cells phenotypes were associated with higher prevalence of carotid artery lesions, even among patients on ART with effective viral suppression[32]. Mechanisms accounting for persistent CD8+ T-cell activation despite clinically undetectable HIV viral loads, may include both a persistent anti-HIV response as well as stimulation from other prevalent co-pathogens such a cytomegalovirus (CMV) [33, 34]. In a cross-sectional comparison, Hsue et. al. reported higher carotid intima-media thickness (CIMT), inflammation, and T-cell activation among HIV infected versus uninfected persons, though only CMV-specific T-cell responses were independently associated with CIMT in adjusted analyses [35]. The potential importance of CMV-specific T-cell activation for HIV-associated CVD pathogenesis has since been supported by data demonstrating that CMV IgG titers predict greater carotid artery lesions, and that CMV-specific CD4+CX3CR1+ T-cells may contribute to greater CIMT progression via mechanisms that induce arterial wall inflammation [36, 37].
Cumulatively, these data emphasize that ongoing abnormalities in adaptive immune likely contribute to increased atherosclerosis during chronic HIV infection. Research has largely focused on T-cell activation, associated pro-inflammatory mechanisms, and the potential influence of co-pathogens (e.g., CMV) providing an ongoing stimulus for cytotoxic T-cell responses (CTL; Figure 1). To date, the potential loss of anti-atherogenic adaptive immune responses (e.g., T-regulatory and B-cell responses) has not been extensively studied as a potential driver of HIV-associated CVD risk. Although one recent study from sub-Saharan Africa demonstrated that subjects with HIV have a profile of natural auto-antibodies that has been associated with increased risk of cardiovascular disease in the general population and patients with auto-immune disorders.[38] Further research is needed to both explore these underlying mechanisms, as well as to identify treatment strategies that further improve immune recovery and normalize immune activation.
Innate Immune Mechanisms
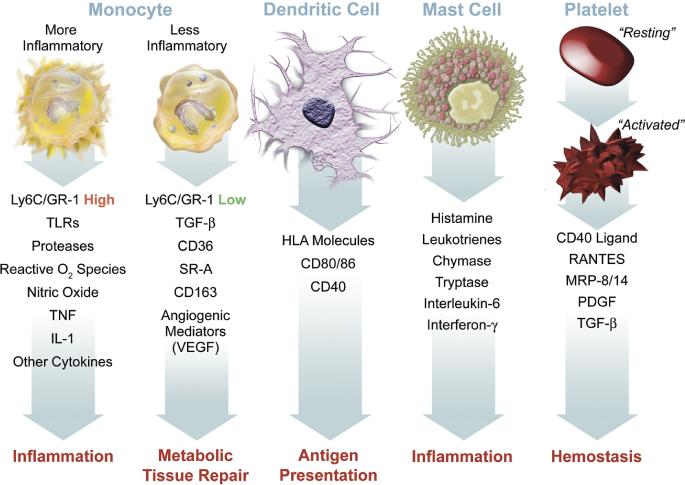
Innate, or non-specific, immunity is well known to be a central feature of atherosclerosis pathogenesis (Figure 2).[39] The general sequence involves adherence of circulating monocytes to vessel walls with infiltration into nascent plaques, where, as tissue macrophages, they may perpetuate tissue injury via release of pro-inflammatory cytokines and/or evolve into cholesterol rich ‘foam cells’. Recent data have demonstrated that the spectrum of immunologic abnormalities during HIV infection, including treated disease, also includes activation of innate immunity (e.g., monocytes) and associated consequences for systemic inflammation and the development of premature CVD.[40-45]
FIGURE 2. Innate immunity in atherosclerosis.
Elements of the innate immune system, particularly monocytes and macrophages, drive atherosclerosis and the inflammation that leads to unstable atherothrombotic syndromes. Current literature has demonstrated that HIV infection activates monocytes, impairs reverse cholesterol transport from foam cells, and promotes thrombus formation through platelet-monocyte interactions. Reproduced with permission from Libby et al.[39]
Multiple studies have now demonstrated that HIV infection is associated with higher frequencies of activated monocyte subsets; i.e., so called intermediate (or ‘pro-inflammatory’, CD14+CD16+) and non-classical (or ‘patrolling’, CD14dimCD16+) monocytes.[43-48] CD16+ monocyte subsets may also be more permissive to infection by HIV.[49] Funderburg et. al. demonstrated that the frequency of pro-inflammatory intermediate monocytes among HIV+ persons were correlated with viral load, CD8+ T-cell activation, CD4+ T-cell declines, and interleukin-6 levels.[44] Compared to HIV uninfected persons, the frequency of patrolling non-classical monocytes were also elevated among HIV infected persons, but also persisted among those with viral suppression.[44] In this study, the frequency of both intermediate and non-classical monocytes was similar between HIV viremic patients and those without HIV infection but who presented for cardiac catheterization with acute coronary syndrome (ACS).[44] Consistent with these data, higher frequencies of CD16+ monocytes (intermediate and non-classical phenotypes) among 436 HIV+ patients (SUN Study) were associated with greater subsequent progression of coronary artery calcium (CAC), independent of traditional and HIV risk factors.[50]
Although the functional characteristics of CD16+ (intermediate and non-classical) monocyte phenotypes remain controversial, these subsets exhibit properties that promote atherogenesis [48, 51-53]. Specifically, the patrolling non-classical phenotype may act as a ‘patrolling’ subset that has greater affinity for vascular surfaces and migration into atherosclerotic lesions [51, 52]. The intermediate monocyte phenotype appears to be functionally more pro-inflammatory, with greater cytokine release after stimulation.[48, 51, 53] CD16+ monocytes then localize to tissues sites of inflammation and fibrosis, with transendothelial migration facilitated by CX3CL1.[54] Epidemiologic data from HIV-uninfected participants at risk for CVD (n=951) also demonstrates that the intermediate monocyte phenotype independently predicts higher risk for subsequent CVD events (i.e., myocardial infarction, stroke or CVD-death).[55] Elevated levels of CD16+ monocytes among those with HIV infection, and their associations with CVD risk measures, suggests that innate immune activation may function largely to amplify key aspects of atherogenesis.
Numerous recent HIV studies of soluble plasma biomarkers have supported the notion that monocyte-related inflammation has CVD consequences for HIV+ patients.[42, 56-61] Soluble CD14 and CD163 levels, both reflecting monocyte activation, have been associated with greater carotic intima media thickness,[56, 57] calcified and non-calcified coronary plaque,[57-60] and arterial wall inflammation (estimated via FDG-PET imaging) among HIV-positive patients.[61] Recent non-human primate data using SIV infection further implicate monocyte/macrophage activation by demonstrating that cardiac pathology and myocardial fibrosis was associated with greater numbers of CD163+ monocytes as well as mycocardial macrophages overall.[62]
In summary, the current literature supports the notion that monocyte/macrophage-mediated inflammation and injury within atherosclerotic lesions are amplified in the context of HIV infection. Additional HIV associated monocyte abnormalities, related to impaired cholesterol efflux by foam cells and pro-coagulant effects (e.g., related to platelet-monocyte complexes), then further contribute to CVD risk.[63-65] For example, monocytes from HIV+ subjects are more likely to become foam cells, have decreased expression of the cholesterol transporter ABCA1, and show impaired cholesterol efflux[66]. Dysfunctional HDL appears to further impair reverse cholesterol transport from macrophages, but this improves with ART[64]. The pathology driving innate immune activation and other monocyte abnormalities are likely related to both indirect effects from an ongoing HIV immune response, as well as due to permanent damage to the immune system—e.g., damage to mucosal lymphatic tissue and epithelial integrity leading to greater translocation of microbial products that drive innate immune responses.[44, 67] In this context, treatment interventions that target non-specific innate inflammation broadly may be more effective as a CVD prevention strategy.
Immune-based Strategies to Mitigate Risk
The accumulating observational evidence that immune activation is associated with CVD risk in persons with treated HIV infection suggests that therapies targeting these mechanisms—whether immunomodulatory drugs or proven CVD prevention therapies with anti-inflammatory effects—may have a cardiovascular benefit. Table 1 lists a number of completed and ongoing trials of immune targeted therapies that have included measures of subclinical vascular disease as surrogate outcomes of cardiovascular risk. In addition to the studies in Table 1, there are other trials that aim to improve outcomes such as immune function and tissue fibrosis, which would also be expected to influence cardiovascular risk.
Table 1. Clinical trials to reduce immune activation and vascular risk.
This table of completed and ongoing trials of immunomodulatory therapies or cardiovascular risk modifying drugs with anti-inflammatory effects includes only trials with subclinical vascular outcome measures. Trials of anti-inflammatory and anti-fibrotic drugs without vascular outcome measures are not included in this table but are discussed in the text.
| Intervention | Identifier | Population | Design | Size | Subclinical Vascular Outcome(s) | Status |
|---|---|---|---|---|---|---|
| Immunomodulatory Therapies | ||||||
| Methotrexate | NCT01949116 | >40yrs on ART with mod-high CVD risk | Placebo RCT | 200 | FMD | Recruiting |
| Tocilizumab (IL-6 receptor Ab) | NCT02049437 | 18-60yrs on ART with CD4 200-500 | Placebo crossover RCT | 36 | FMD; hyperemic VTI | Recruiting |
| Canakinumab (IL-1β Ab) | NCT02272946 | >40yrs on ART with high CVD risk | Placebo RCT | 110 | FMD; Aortic TBR | Not yet open |
| Pentoxifylline | NCT00864916 | >18yrs; all started ART at study entry | Placebo RCT | 26 | FMD | Completed[68] |
| Salsalate 4gm | NCT01046682 | >18yrs; on ART | Open label RCT | 40 | FMD | Completed[69] |
| Salsalate 3gm | N/A | >18yrs: NOT on ART | Single arm Open label | 11 | FMD | Completed[70] |
| CVD Risk Modifying Drugs with Anti-Inflammatory Effects | ||||||
| Rosuvastatin 10mg | NCT01218802 | >18yrs; on ART with LDL<130 and elevated hsCRP or CD8+ activation | Placebo RCT | 147 | Carotid IMT; FMD; CAC | Completed |
| Rosuvastatin 10mg | NCT02234492 | >40yrs; On ART with 10-yr CVD risk 10-20% and LDL <155 | Open-label RCT | 82 | Coronary flow reserve; Aortic TBR | Recruiting |
| Rosuvastatin 20mg | NCT01813357 | >18yrs; On ART with 10-15% 10-yr CVD risk | Placebo RCT | 102 | Carotid IMT; FMD | Recruiting |
| Rosuvastatin | NCT01881971 | 18-80yrs; On ART with COPD | Placebo RCT | 30 | Carotid IMT; FMD | Ongoing; not recruiting |
| Atorvastatin 20/40mg | NCT00965185 | 18-60; on ART with coronary plaque and high aortic TBR | Placebo RCT | 40 | Coronary plaque volume and high risk features by CT; Aortic TBR | Completed |
| Pitavastatin 4mg | NCT02344290 | 40-75yrs; on ART with <7.5% 10-yr ASCVD risk | Placebo RCT | 6500 | MACE; Coronary plaque volume and high risk features | Recruiting |
| ASA 81mg vs. Atorvastatin 40mg | NCT02081638 | >18yrs; elite controller compared to persons with >4yrs of ART | RCT | 80 | Carotid MRI | Recruiting |
| ASA 325mg | NCT02401269 | 18-70yrs; on ART without diabetes or heart disease | Placebo RCT | 100 | RHI and FMD | Recruiting |
| ASA 100 or 300mg | NCT02155985 | >18yrs; on ART | Placebo RCT | 121 | FMD | Ongoing; not recruiting |
| Telmisartan 80mg | NCT01578772 | >50yrs; on ART with one or more risk factors for CVD | Placebo RCT | 17 | FMD | Completed; not published |
| Losartan 100mg +/− switch to RAL | NCT01529749 | >18yrs; on ART as tenofovir DF, emtricitabine, and efavirenz | Placebo RCT; 2×2 factorial | 48 | Carotid IMT | Recruiting |
| Metformin 1000mg | NCT02383563 | >45yrs; on ART | Open label RCT | 12 | Total coronary plaques by CT | Recruiting |
| Lovaza (OM-3 FA) 2gm | NCT01001767 | 18-70yrs; on ART | Placebo RCT | 35 | FMD | Completed[71] |
CVD, cardiovascular disease; yrs, years; ART, antiretroviral therapy; RCT, randomized controlled trial; FMD, flow-mediated dilation of the brachial artery; IL, interleukin; Ab, antibody; VTI, velocity time integral; TBR, target to background ratio; LDL, low-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IMT, intima-media thickness; CAC, coronary artery calcium; COPD, chronic obstructive pulmonary disease; ASCVD, atherosclerotic cardiovascular disease; MACE, major adverse cardiovascular events; ASA, aspirin; MRI, magnetic resonance imaging; RHI, reactive hyperemic index; RAL, raltegravir; DF, disoproxil fumarate; OM-3 FA, omega-3 fatty acids.
Methotrexate (MTX) is a competitive dihydrofolate reductase inhibitor that modifies progression of rheumatoid arthritis primarily by reducing T-cell activation, but it also appears to have a beneficial effect on mortality in this population[72]. In light of this observation, the Cardiovascular Inflammation Reduction Trial (CIRT, www.thecirt.org) was designed to test whether low-dose MTX will prevent CVD events in the general population. Similarly, a pilot study of 200 HIV-infected participants (NCT01949116) will evaluate the safety of low-dose MTX and its effect on endothelial function—results are anticipated in 2016. Other “biologic” agents (i.e. monoclonal antibodies that block cytokines or cytokine receptors) approved for the treatment of autoimmune disease are being tested in HIV-infected patients. Such agents include the interleukin-6 receptor antagonist tocilizumab (NCT02049437) and the interleukin-1 antagonist canakinumab (NCT02272946). Although dramatic reductions in immune activation are expected, whether the benefits will outweigh the significant risks is unclear. A worrisome precedent is that the tumor necrosis factor-α (TNF-α) antagonists etanercept and infliximab were associated with worse outcomes in trials to improve advanced heart failure [73, 74]. In contrast, interleukin-1 receptor antagonism improved heart failure outcomes in smaller studies[75, 76]. Less potent anti-inflammatory drugs salsalate and pentoxifylline have been poorly tolerated and/or have no meaningful effect on endothelial function in small trials of HIV positive participants[68-70].
Many traditional CVD prevention treatments also have ‘off-target’ anti-inflammatory effects that may partly mediate their CVD benefits. Chief among these are the statins, whose ‘pleiotropic’ anti-inflammatory properties are highly touted. Recent studies have reported on subclinical vascular outcomes of statins in the HIV-infected population and the potential effect modifying and mediating roles of inflammation and immune activation[77, 78]. Atorvastatin 20mg titrated to 40mg after 3 months reduced non-calcified plaque volume and high-risk coronary plaque features compared to placebo in a trial of 40 HIV positive participants on ART, who had evidence of subclinical coronary atherosclerosis at baseline and elevated vascular inflammation measured by PET-CT[77]. In the SATURN-HIV trial, rosuvastatin 10mg slowed progression of carotid intima-media thickness among 147 subjects with elevated hs-CRP and/or heightened CD8+ T-cell activation. Similar to HIV studies of atorvastatin[79-82], rosuvastatin reduced T-cell activation and exhaustion markers over 48 weeks in SATURN-HIV[83]. Markers of innate immune activation such as soluble CD14 and the proportion of tissue factor-positive non-classical (CD14dimCD16+) monocytes were also reduced with rosuvastatin[83]. Furthermore, baseline interleukin-6 levels and CD14dimCD16+ monocytes were inversely associated with IMT change in the statin group, and the statin benefit on IMT progression was greatest among subjects with higher levels of baseline markers of inflammation and immune activation[78].
Despite being at high risk, recent data suggest that nearly 75% of HIV infected subjects with high risk plaque features on coronary CTA would not be recommended to receive statin therapy by current guidelines[84]. Thus, the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE; reprievetrial.org) recently began enrolling 6500 HIV positive participants without a current indication for a statin (10-year ACC/AHA risk <7.5%) in an outcomes trial of pitavastatin vs. placebo. Results are expected in 2020.
Other drugs used for CVD prevention may also have beneficial effects on inflammation. Aspirin reduces relative risk of CVD events by about 10-20% in the general population[85], and also appears to rapidly reduce immune activation in subjects on ART[86]. Ongoing trials of aspirin will further explore its immune and vascular effects in HIV-infected populations (Table 1), but whether the reduction in CVD and other (e.g. cancer) events as primary prevention will outweigh the risks of bleeding remains unclear. Omega-3 fatty acids modestly improve markers of systemic inflammation, but do not appear to have any effect on endothelial function in one small study[71].
Finally, drugs that act to reduce activation of the renin-angiotensin-aldosterone system (RAAS) may reduce CVD events through multiple pathways including reduction of inflammation and tissue fibrosis. RAAS activation may be worsened by protease inhibitors[87] and is associated with visceral adiposity and insulin resistance in HIV-infected subjects[88], but few clinical trials of RAAS blockade have been conducted in this population. Lisinopril reduced hsCRP and TNF-α despite sub-optimal adherence in one small pilot trial[89]. Telmisartan may ameliorate visceral adiposity in subjects on ART[90], but did not improve FMD in another underpowered pilot (NCT01578772). Telmisartan's effect on tissue fibrosis and inflammation is being tested in a 48-week open label trial of 44 participants on ART (NCT01928927). Two ongoing larger randomized placebo-controlled trials (NCT02049307 and NCT01852942) will evaluate similar inflammation and tissue fibrosis outcomes for losartan; another will test the effect of losartan on carotid IMT progression (NCT01529749).
Summary and Conclusions
Chronic HIV infection is characterized by persistent abnormalities in both adaptive and innate immunity, despite clinically suppressed viral load with effective ART. Drivers of this high-level immune activation remain diverse and may differ between individuals, though the consequences appear to fuel systemic inflammation and amplify well-described pro-atherogenic mechanisms. Treatment strategies with potential to mitigate HIV-associated CVD risk are currently an area of active investigation. Candidate interventions undergoing clinical trials include those that target traditional CVD risk factors, that have broad non-specific anti-inflammatory properties, and/or that target a specific underlying mechanism driving HIV-associated immune activation.
Key Points.
HIV infection is associated with excess CVD due to multiple factors, some of which are immunologic in nature.
Alterations in adaptive immunity, reflected in T-cell activation and dysfunction, likely contribute to HIV-associated CVD.
The CVD consequences of persistent abnormalities in innate immunity, commonly assessed via measures of monocyte activation, may be particularly relevant during treated HIV disease when adaptive immunity has recovered to some degree.
While effective treatment exists for traditional risk factor modification, CVD prevention strategies that target inflammation and immune activation are lacking though a number of promising candidates are under investigation.
Acknowledgements
None
Financial support and sponsorship: This work was supported in part by the National Institutes of Health (K23 HL123341 to CTL; R01 AG045032 and R01 HL126542 to JVB).
Footnotes
Conflict of Interests: CTL has received research funding from Bristol-Myers Squibb and Medtronic Philanthropy. CS and JVB have no conflicts.
Off-Label Use: This review discusses the ongoing investigation of the following drugs for off-label indication of decreasing inflammation and immune activation in patients with HIV infection: methotrexate, tocilizumab, canakinumab, pentoxifylline, salsalate, rosuvastatin, atorvastatin, pitavastatin, aspirin, telmisartan, losartan, metformin, Omega-3 fatty acids.
References
- 1.Henry K, Melroe H, Huebsch J, et al. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351:1328. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 2.So-Armah K, Freiberg MS. Cardiovascular disease risk in an aging HIV population: not just a question of biology. Curr Opin HIV AIDS. 2014;9:346–354. doi: 10.1097/COH.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–1940. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. doi: 10.1161/JAHA.114.001035. [Epidemiologic studies documenting a 1.5 to 2-fold increased risk of CVD in patients with HIV have historically included mostly male subjects. This study from the Veterans Aging Cohort Study (VACS) confirmed the hazard for CVD in women (HR=2.8) is at least as great if not greater than in men.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanni MV, Abbara S, Lo J, et al. Increased Coronary Atherosclerotic Plaque Vulnerability by Coronary Computed Tomography Angiography in HIV-Infected Men. Aids. 2013 doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiara DK, Liu CY, Raman F, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015 doi: 10.1093/infdis/jiv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 13.Ingle SM, May MT, Gill MJ, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–297. doi: 10.1093/cid/ciu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen LD, Helleberg M, May MT, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60:1415–1423. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 15.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60:1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 16.Armah KA, Chang CC, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58:121–129. doi: 10.1093/cid/cit652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan S, Schouten JT, Atkinson B, et al. Changes in metabolic syndrome status after initiation of antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:73–80. doi: 10.1097/QAI.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61:334–340. doi: 10.1097/QAI.0b013e31826bfc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. European journal of endocrinology / European Federation of Endocrine Societies. 2014;170:R185–202. doi: 10.1530/EJE-13-0967. [DOI] [PubMed] [Google Scholar]
- 20.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. Aids. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 22.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabin CA, Ryom L, De Wit S, et al. Associations between immune depression and cardiovascular events in HIV infection. Aids. 2013 doi: 10.1097/01.aids.0000432457.91228.f3. [DOI] [PubMed] [Google Scholar]
- 24**.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–166. doi: 10.1097/QAI.0000000000000009. [In the Kaiser Permanente system, risk of myocardial infarction was similar in HIV-infected subjects with a CD4+ T-cell count >500 cells/ml when compared to HIV-uninfected subjects.] [DOI] [PubMed] [Google Scholar]
- 25.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. Aids. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triant VA, Regan S, Lee H, et al. Association of Immunologic and Virologic Factors With Myocardial Infarction Rates in a US Healthcare System. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang S, Mary-Krause M, Simon A, et al. HIV Replication and Immune Status Are Independent Predictors of the Risk of Myocardial Infarction in HIV-Infected Individuals. Clin Infect Dis. 2012 doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 29**.Group ISS. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1506816. [Early initiation of ART at CD4+ count >500 cells/ml led to lower risk of serious non-AIDS events overall compared to initiation at <350 cells/ml, although CVD events were few (total and not different between groups [HR(95%CI) 0.84 (0.39-1.81)].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papagno L, Spina CA, Marchant A, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. Aids. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan RC, Sinclair E, Landay AL, et al. T Cell Activation and Senescence Predict Subclinical Carotid Artery Disease in HIV-Infected Women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 36.Parrinello CM, Sinclair E, Landay AL, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacre K, Hunt PW, Hsue PY, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. Aids. 2012;26:805–814. doi: 10.1097/QAD.0b013e328351f780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huck DM, Okello E, Mirembe G, et al. American Heart Association Scientific Sessions. Orland, FL, USA: 2015. Role of Natural Auto-antibodies among Ugandans with Rheumatic Heart Disease and HIV. [Google Scholar]
- 39.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhaus J, Jacobs DR, Jr., Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armah KA, McGinnis K, Baker J, et al. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clin Infect Dis. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowe SM, Westhorpe CL, Mukhamedova N, et al. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87:589–598. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin GE, Gouillou M, Hearps AC, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hearps AC, Maisa A, Cheng WJ, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. Aids. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 46.Scherberich JE, Nockher WA. CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin Chem Lab Med. 1999;37:209–213. doi: 10.1515/CCLM.1999.039. [DOI] [PubMed] [Google Scholar]
- 47.Wilson EM, Singh A, Hullsiek KH, et al. Monocyte Activation Phenotypes Are Associated with Biomarkers of Inflammation and Coagulation in Chronic HIV Infection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 49.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 50**.Baker JV, Huppler Hullsiek K, Singh A, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. Aids. 2014;28 doi: 10.1097/QAD.0000000000000145. [Demonstrated that greater frequency of activated monocytes (e.g., CD16+) predicted subsequent progression of coronary artery calcium among n=436 HIV positive persons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 52.Ancuta P, Rao R, Moses A, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. The Journal of experimental medicine. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Veerdonk FL, Netea MG. Diversity: a hallmark of monocyte society. Immunity. 2010;33:289–291. doi: 10.1016/j.immuni.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Aspinall AI, Curbishley SM, Lalor PF, et al. CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium. Hepatology. 2010;51:2030–2039. doi: 10.1002/hep.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. Aids. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [Soluble CD14, a biomarker of monocyte activation, was associated with subclinical vascular disease (coronary calcification, carotid intima-media thickening, and endothelial dysfunction) among n=147 subjects on ART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified Coronary Atherosclerotic Plaque and Immune Activation in HIV-Infected Women. J Infect Dis. 2013 doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594. [Soluble CD14 and CD163, another biomarker of moncyte activation, were both associated with subclinical coronary plaque in the Multicenter AIDS Cohort Study.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker JA, Sulciner ML, Nowicki KD, et al. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS research and human retroviruses. 2014;30:685–694. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feeney ER, McAuley N, Halloran JA, et al. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intracellular cholesterol accumulation. J Infect Dis. 2013;207:628–637. doi: 10.1093/infdis/jis723. [DOI] [PubMed] [Google Scholar]
- 64.Lo J, Rosenberg ES, Fitzgerald ML, et al. High-density lipoprotein-mediated cholesterol efflux capacity is improved by treatment with antiretroviral therapy in acute human immunodeficiency virus infection. Open forum infectious diseases. 2014;1:ofu108. doi: 10.1093/ofid/ofu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Liang H, Duan Z, Li D, et al. Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cellular & molecular immunology. 2015;12:435–443. doi: 10.1038/cmi.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Maisa A, Hearps AC, Angelovich TA, et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. Aids. 2015;29:1445–1457. doi: 10.1097/QAD.0000000000000739. [In this novel in-vitro model of atherosclerosis, HIV-infected monocytes were more likely to form foam cells and demonstrated impaired reverse cholesterol transport.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 68.Gupta SK, Mi D, Dube MP, et al. Pentoxifylline, inflammation, and endothelial function in HIV-infected persons: a randomized, placebo-controlled trial. PLoS One. 2013;8:e60852. doi: 10.1371/journal.pone.0060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hileman CO, Carman TL, Gripshover BM, et al. Salsalate is poorly tolerated and fails to improve endothelial function in virologically suppressed HIV-infected adults. Aids. 2010;24:1958–1961. doi: 10.1097/QAD.0b013e32833c3251. [DOI] [PubMed] [Google Scholar]
- 70.Gupta SK, Johnson RM, Saha C, et al. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. Aids. 2008;22:653–655. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 71.Hileman CO, Carman TL, Storer NJ, et al. Omega-3 fatty acids do not improve endothelial function in virologically suppressed HIV-infected men: a randomized placebo-controlled trial. AIDS Res Hum Retroviruses. 2012;28:649–655. doi: 10.1089/aid.2011.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wasko MC, Dasgupta A, Hubert H, et al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis and rheumatism. 2013;65:334–342. doi: 10.1002/art.37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 74.Chung ES, Packer M, Lo KH, et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 75.Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCUART2 pilot studies). The American journal of cardiology. 2015;115:288–292. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). The American journal of cardiology. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [Among 40 HIV+ subjects on ART with evidence of subclinical coronary atherosclerosis and elevated vascular inflammation at baseline, atorvastatin 20mg titrated to 40mg decreased non-calcified coronary plaque volume and high risk features compared to placebo, but did not reduce vascular inflammation by PET/CT.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Longenecker CT, Jiang Y, Debanne SM, et al. Rosuvastatin Arrests Progression of Carotid Intima-Media Thickness in Treated HIV.. 22nd Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA, USA. 2015. [Google Scholar]
- 79.Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203:756–764. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakanjako D, Ssinabulya I, Nabatanzi R, et al. Atorvastatin reduces T-cell activation and exhaustion among HIV-infected cART-treated suboptimal immune responders in Uganda: a randomised crossover placebo-controlled trial. Tropical medicine & international health : TM & IH. 2015;20:380–390. doi: 10.1111/tmi.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Overton ET, Sterrett S, Westfall AO, et al. Effects of atorvastatin and pravastatin on immune activation and T-cell function in antiretroviral therapy-suppressed HIV-1-infected patients. Aids. 2014;28:2627–2631. doi: 10.1097/QAD.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Wit S, Delforge M, Necsoi CV, Clumeck N. Downregulation of CD38 activation markers by atorvastatin in HIV patients with undetectable viral load. Aids. 2011;25:1332–1333. doi: 10.1097/QAD.0b013e328347c083. [DOI] [PubMed] [Google Scholar]
- 83*.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin Reduces Vascular Inflammation and T-cell and Monocyte Activation in HIV-Infected Subjects on Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [A 48-week interim analysis of SATURN-HIV demonstrated reductions in T-cell and monocyte activation with 10mg of daily rosuvastatin. These findings expand on previous studies showing reductions in T-cell activation with atorvastatin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Zanni MV, Fitch KV, Feldpausch M, et al. American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. Aids. 2013;2014;28:2061–2070. doi: 10.1097/QAD.0000000000000360. [Nearly 75% of patients with HIV infection who had subclinical features of “high-risk” coronary plaque would not be recommended for statin therapy under the most recent 2013 American College of Cardiology and American Heart Association guidelines on the treatment of dyslipidemia for the prevention of CVD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 86.O'Brien M, Montenont E, Hu L, et al. Aspirin Attenuates Platelet Activation and Immune Activation in HIV-1-Infected Subjects on Antiretroviral Therapy: A Pilot Study. J Acquir Immune Defic Syndr. 2013;63:280–288. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boccara F, Auclair M, Cohen A, et al. HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2010;15:363–375. doi: 10.3851/IMP1533. [DOI] [PubMed] [Google Scholar]
- 88.Srinivasa S, Fitch KV, Wong K, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. J Clin Endocrinol Metab. 2015;100:2873–2882. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baker JV, Huppler Hullsiek K, Prosser R, et al. Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One. 2012;7:e46894. doi: 10.1371/journal.pone.0046894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lake JE, Tseng CH, Currier JS. A pilot study of telmisartan for visceral adiposity in HIV infection: the metabolic abnormalities, telmisartan, and HIV infection (MATH) trial. PLoS One. 2013;8:e58135. doi: 10.1371/journal.pone.0058135. [DOI] [PMC free article] [PubMed] [Google Scholar]