Abstract
Background
An acute ethanol challenge prior to fear conditioning typically disrupts fear retention to contextual cues to a greater degree than fear retention to a discrete tone cue, and adolescent rats are less sensitive than adults to these ethanol-induced disruptions of context fear memory. Given that some research suggests that repeated ethanol exposure during adolescence may “lock-in” adolescent-typical ethanol sensitivity into adulthood, the purpose of this study was to determine whether adults exposed to ethanol as adolescents would be less sensitive to ethanol-induced disruptions of context fear.
Methods
Male Sprague-Dawley rats were given 4 g/kg i.g. ethanol (25%) or water every 48 hours for a total of 11 exposures during adolescence [Postnatal day (P) 28–48] or adulthood (P70-90). After a 22 day non-ethanol period, animals were acutely challenged with 1 g/kg i.p. ethanol or saline 10 minutes prior to tone or context (non-cued) fear conditioning. Tone and context fear retention were subsequently examined.
Results
Regardless of age or exposure history, typical deficits in context fear retention were evident after ethanol challenge during conditioning. Similarly, tone fear retention was disrupted in all animals that were trained in the presence of ethanol, which was somewhat surprising given the relative resistance of tone fear retention to an acute ethanol challenge.
Conclusion
These results do not support the notion of a “lock-in” of adolescent-typical ethanol sensitivity since there was no influence of exposure age on sensitivity to the disruptive effects of an acute ethanol challenge. Thus, it appears that not all adolescent-like ethanol sensitivities persist into adulthood after prior ethanol exposure during adolescence.
Keywords: Ethanol Exposure, Adolescent, Fear conditioning, Sprague-Dawley rats, Male
Introduction
A number of studies in rodents have supported the hypothesis of a “lock-in” effect that posits retention of adolescent-typical ethanol sensitivities into adulthood following a history of adolescent alcohol exposure (see Fleming et al., 2011 for discussion). For instance, adolescents are less sensitive to several acute ethanol effects, such as ethanol-induced motor impairment (Broadwater et al., 2011a; Ramirez & Spear, 2010; White et al., 2002b) and conditioned taste aversion (CTA) (Anderson et al, 2010; Schramm-Sapyta et al, 2010), factors that may contribute to adolescents’ ability and/or propensity to consume large amounts of alcohol relative to their more mature counterparts (e.g., Brunell & Spear, 2005; Doremus et al., 2005; Vetter et al., 2007; Vetter-O’Hagen et al., 2009). Interestingly, these adolescent-typical attenuations in sensitivity to both the motor impairing (White et al, 2002a) and aversive (Diaz-Granados & Graham, 2007; Sherrill et al, 2011) effects of ethanol have been found to persist into adulthood after exposure to alcohol during adolescence. Such maintenance of adolescent-like attenuations in ethanol sensitivity could potentially promote and/or allow greater ethanol consumption in adulthood. Indeed, there is a correlation between early age of alcohol initiation and increased susceptibility for alcohol use disorders (AUDs) in adulthood in humans (Grant & Dawson, 1997), as well as some preclinical studies reporting increased voluntary ethanol intake in adulthood after adolescent alcohol exposure (Alaux-Cantin et al., 2013; Maldanado-Devincci, 2010; Pascual, 2009). Whether maintenance of other adolescent-typical ethanol sensitivities would persist into adulthood as a result of alcohol exposure during adolescence is a question currently under investigation in the field of developmental alcohol research.
In terms of fear conditioning, adolescent rats are less sensitive than adults to disruption of context fear retention by an acute challenge with 1 g/kg ethanol during conditioning (Broadwater & Spear, 2013a; Land & Spear, 2004). Ethanol challenge, however, did not affect tone fear retention at either age (Broadwater & Spear, 2013a), consistent with previous studies in adults of greater disruption of context fear retention than tone by acute ethanol (Gould, 2003; Melia et al., 1996). Given that context fear conditioning is a relatively hippocampal-dependent task (Kim et al., 1993; Maren & Fanselow, 1997; Antoniadis & McDonald, 2000), whereas tone conditioning appears to be more reliant on the amygdala (Davis, 1992; LeDoux, 2000; Maren & Quirk, 2004; Fanselow & Poulos, 2005), these data suggest that the hippocampus may be particularly susceptible to perturbations of context fear memory by acute ethanol, with adolescents being less sensitive to these effects than adults. However, effects of an acute ethanol challenge on fear retention have yet to be examined in animals with a history of chronic ethanol exposure in adolescence or adulthood. Given that adolescent ethanol exposure may “lock-in” adolescent-like ethanol sensitivity in adulthood, the purpose of this study was to examine if an acute 1 g/kg ethanol challenge would influence context and tone conditioning and retention in adulthood after adolescent (P28-48) or adult (P70-90) ethanol exposure.
Methods
Subjects & Design
A total of 156 adolescent and adult male Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in this experiment. On the day after birth, postnatal day (P) 1, litters were culled to 8–10 pups, with a sex ratio of 6 males and 4 females retained whenever possible. Pups were housed with their mother in a standard clear plastic tub with shavings until being pair-housed with a same-sexed littermate at the time of weaning (P21). Animals were maintained in a temperature-controlled vivarium on a 12:12-h light: dark cycle (lights on 0700), with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. All animals were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health (8th Ed), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Design
A 2 exposure (water [H20]; ethanol [EtOH]) × 2 exposure age (adolescent: P28-48; adult: P70-90) × 2 conditioning stimulus (tone; context) × 2 (acute challenge: EtOH; saline [SAL]) factorial design was used with, an n=8–10/group. Pair-housed littermates were randomly assigned to the same age, exposure and challenge conditions, with one animal of the pair assigned to tone conditioning and the other animal assigned to context conditioning.
Exposure
Animals at each age were given 4 g/kg (25% v/v) EtOH or an equivalent volume of H20 intragastrically (i.g.) every other day throughout the 20 day exposure period for a total of 11 intubations. All intubations were given between 1000 and 1200 hrs. After the exposure period, animals were not disturbed aside from routine animal care (i.e., cage changing, etc.) for 22 days. In these experiments, post exposure period was held constant between the age groups rather than testing age in adulthood, given that the length of the drug-free period post-exposure may impact the nature of the adaptations observed.
Fear Conditioning Methods
Apparatus
All behavioral assessments were conducted in 8 identical fear conditioning chambers (32 × 25 × 25 cm, Med Associates). Each conditioning chamber was made of clear polycarbonate (top, front walls), white acrylic (back wall), and stainless steel (sides, shock grids, drop pan) material, and equipped with a speaker in the side wall. The grid floors consisted of 19 parallel 4.8 mm diameter rods situated 1 cm apart. At the time of the test for tone retention/extinction (Day 3), the context was modified by the addition of a smooth floor covering made of white plastic and an A-frame ( ) made of black acrylic that fit tightly in the chamber (height:17.5cm, side length: 23.5cm). Chambers and inserts were cleaned with 6% hydrogen peroxide after each session. Each chamber was located within a sound-attenuated wood box (63.5 cm wide, 35.5 cm high, 76 cm deep) affixed with an overhead LED-based light source (Med Associates NIR-100) and a ventilation exhaust fan that provided background noise (65 dB). All behavioral sessions were video recorded by a camera in each conditioning chamber that was connected to a computer in the room. Percent time spent freezing was calculated at 30 frames per second by the Med Associates VideoFreeze system, a validated method for automated assessment of Pavlovian conditioned freezing behavior (Anagnostaras et al., 2010).
Procedure
All manipulations throughout the fear conditioning procedure took place between the hours of 1100 and 1400 hrs. For 3 days prior to conditioning, animals were transported to a room adjacent to the conditioning room to be weighed and handled once daily.
Conditioning (Day 1)
Eight animals at a time were transported in their homecages to a room adjacent to the conditioning room where they were weighed and injected intraperitoneally (i.p.) with 1 g/kg ethanol (20% v/v) or an equivalent volume of saline, then placed back in their homecage. After ten minutes, animals were transported to the conditioning room and placed in the conditioning chambers. The conditioning context was the same for both tone and context conditioning, and consisted of a grid floor delivering footshock, and white light illuminating the chambers. All animals were given a 2 minute habituation period in the conditioning chambers followed by a ~6 minute conditioning period, and a 2 minute interval following the final footshock. The animal in each housing pair that was assigned to tone conditioning received 3 CS-US pairings of a 10 second (s) tone (80 dB, 2000 Hz) coterminated with a 1 s footshock (0.5 mA) presented on a 110 s variable ITI. The animal in the context conditioning group in each housing pair received 3 presentations of 1s footshock (0.5mA) at the same time intervals as for tone conditioning, but without any tone presentations. After conditioning, animals were immediately placed in their home cage and returned to the colony room.
Context Fear Retention Test and/or Extinction (Day 2)
Approximately 24 hrs after tone and context conditioning, all animals were placed in the original conditioning context, with identical pre- and post-test procedures as on conditioning day, except animals did not receive injections on this day. Context fear retention (i.e., freezing during the first 2 min of exposure to the original conditioning context) was assessed for context conditioning animals. On this day, tone conditioned animals were given a 12 min context extinction session in the training context to reduce pre-CS freezing to the test context during the tone fear retention test on Day 3 (see Broadwater & Spear, 2013a, for further discussion).
Tone Fear Retention Test (Day 3)
Tone conditioned animals were placed in a novel context created by the addition of a smooth floor and an A-frame to the original conditioning chamber. After a 2 min acclimation period, animals were given 6 presentations of the 10 s tone alone, with an ITI of 10 s.
Data Analysis
Statistics
The percentage of time spent freezing on the conditioning days were separated into time bins for each type of conditioning. Tone conditioning data were separated into 5 bins: 2 minutes prior to the first tone, the 9 second duration of each of the three tones prior to footshock and the 2 minutes following the final CS-US pairing. Context conditioning data were separated into 4 bins: 2 minutes prior to the first footshock, the two time periods between footshock exposures (1st–2nd and 2nd–3rd), as well as the 2 minutes following the final footshock. Repeated measures ANOVAs over time bin were used to analyze acquisition data separately at each age, with Fisher’s LSD planned comparisons used to investigate significant effects involving time bin. Factorial ANOVAs were used to analyze baseline freezing prior to the tone fear retention test in tone conditioned animals, as well as context and tone fear retention data, with age included as a between subjects factor. Tukey’s HSD post hocs were used to assess the locus of significant effects in these analyses.
Exclusion
For tone conditioning, animals were excluded if they showed baseline freezing of >50% during the 2 min period prior to the tone fear retention test, given that high baseline freezing can make interpretation of CS freezing difficult (Jacobs et al., 2010). This resulted in the exclusion of one adolescent ETOH-exposed, EtOH challenged animal. Percent freezing during the tone and context fear retention test was checked for outliers at each age, with scores > 2 standard deviations from the mean of each experimental condition excluded from analysis. A total of 4 animals that were context conditioned were excluded as statistical outliers during the context fear retention test: 1 adolescent (H20-exposed, EtOH challenged) and 3 adults (1 H20-exposed, SAL challenged; 1 EtOH-exposed, SAL challenged; 1 EtOH-exposed, ETOH challenged).
Results
Tone Conditioning
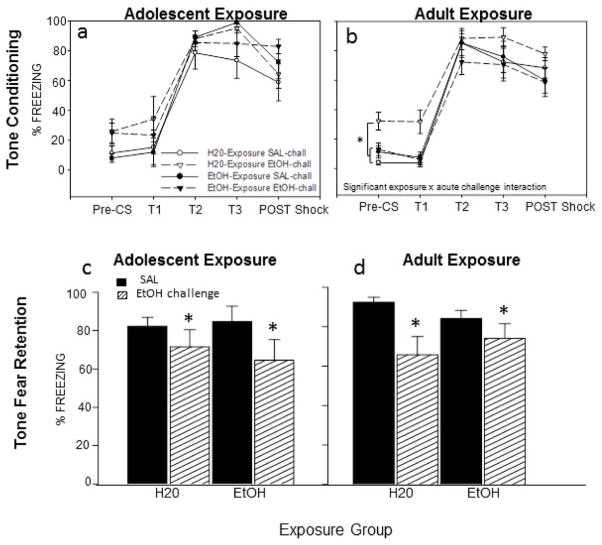
Acute ethanol challenge prior to tone conditioning disrupted tone fear retention regardless of exposure type (H20; EtOH) or exposure age (see Fig 1c and d).
Figure 1.
Tone conditioning (a–b) and tone fear retention test (c–d) in adult animals that were exposed to H20 or EtOH as adolescents (left panel) or adults (right panel) and given either an acute EtOH challenge or SAL during conditioning. (a) Acquisition of tone conditioning was observed in all adolescent exposure groups, but no effect of prior exposure or challenge emerged. (b) Among the adult exposure groups, H20-exposed, EtOH-challenged animals showed significantly more freezing overall than all other groups (see *). (c–d) Animals challenged with EtOH during conditioning showed disrupted tone fear retention relative to their counterparts challenged with SAL (see *), regardless of prior exposure or exposure age.
Conditioning
No effects involving prior exposure to or acute challenge with ethanol emerged in the analysis of the adolescent-exposure data (see Fig. 1a). The 2 (exposure: H20; EtOH) × 2 (challenge: SAL; EtOH) × 5 (time bin) repeated measure ANOVA of the adult exposure data revealed a significant exposure x acute challenge interaction [F(1,37)= 5.86, p<.05], with H20-exposed, EtOH-challenged animals showing significantly more freezing overall than all other adult groups (see Fig. 1b). Elevated freezing early in conditioning (prior to footshock) indicates motor impairing effects of the acute ethanol challenge in H20-exposed adults, an effect that was not evident in adults with a history of repeated ethanol exposure.
Baseline (Pre-CS) freezing on test day (Day 3)
Freezing to the test context did not significantly differ among groups according to the 2 (exposure age: adolescent; adult) × 2 (exposure: H20; EtOH) × 2 (acute challenge: SAL; EtOH) factorial ANOVA, although there was a tendency for EtOH-exposed adolescents to show more baseline freezing than their H20-exposed counterparts (see Table 1). Thus, after extinction to the training context on Day 2, the groups did not differ significantly in freezing to the context prior to the tone fear retention test.
Table 1.
Baseline freezing (%) prior to tone testing
| challenge | Adolescent Exposure | Adult Exposure | ||
|---|---|---|---|---|
|
| ||||
| H20 | EtOH | H20 | EtOH | |
| SAL i.p. | 9 ± 3.1 | 22 ± 7.2 | 25 ± 6.0 | 17 ± 7.4 |
| EtOH i.p. | 3 ± 1.4 | 14 ± 3.2 | 20 ± 4.8 | 14 ± 3.3 |
Tone fear retention on test day (Day 3)
A 2 (exposure age: adolescent; adult) × 2 (exposure: H20; EtOH) × 2 (acute challenge: SAL; EtOH) factorial ANOVA conducted on the tone fear retention data revealed a significant main effect of acute challenge [F(1,67)= 10.35, p< .05], with animals challenged with EtOH during conditioning freezing significantly less to the tone CS than those exposed to SAL during conditioning, indicating an unexpected disruption of tone fear retention by EtOH challenge at the time of conditioning, regardless of exposure history or age (see Fig 1c).
Context Conditioning
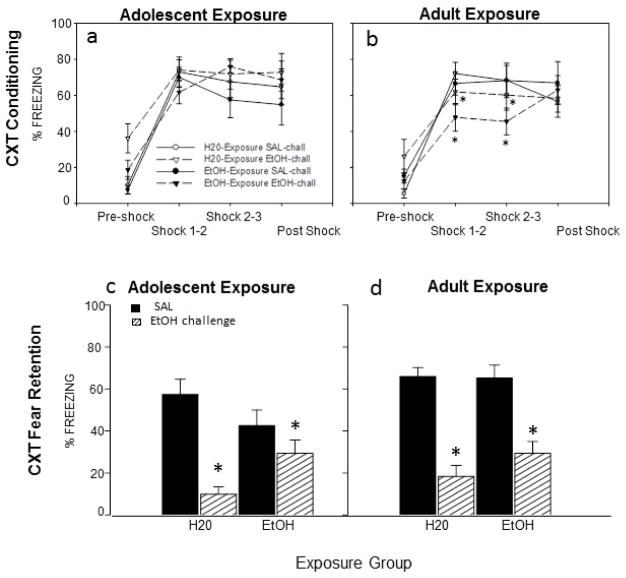
Animals exposed to H20 as adolescents and both adult exposure groups showed typical deficits in context fear retention after an acute EtOH challenge during conditioning; this effect tended to be less pronounced in animals with a history of adolescent ethanol exposure (see Fig 2c and d).
Figure 2.
Context conditioning (a–b) and context fear retention (c–d) in adult animals that were exposed to H20 or EtOH as adolescents (left panel) or adults (right panel) and given either an acute EtOH challenge or SAL during conditioning. (a) All adolescent-exposed animals showed acquisition of context fear, but no effects of exposure or challenge emerged during conditioning. (b) Adult-exposed animals also showed acquisition of context fear. However, acute EtOH-challenged adults showed significantly less freezing than SAL challenged animals between shocks 1 & 2 and 2 & 3 (see *’s), an effect that tended to be more pronounced in animals with a history of EtOH exposure. (c–d) An acute EtOH challenge during conditioning disrupted context fear retention in all exposure groups (see*), an effect that tended to be attenuated in animals exposed to EtOH as adolescence.
Conditioning
No effects of exposure or challenge emerged in the analysis of the adolescent-exposure data (see Fig. 2a). A 2 (exposure: H20; EtOH) × 2 (acute challenge: SAL; EtOH) × 4 (time bin) repeated measure ANOVA of the adult exposure conditioning data revealed a significant bin x acute challenge interaction [F(3,105)= 3.60, p<.05], with EtOH-challenged adults showing significantly less freezing than SAL challenged animals between shocks 1 & 2 and 2 & 3, regardless of exposure group (see Fig. 2b); this effect tended to be more pronounced in animals with a history of EtOH exposure.
Context fear retention on test day (Day 2)
A 2 (exposure: H20; EtOH) × 2 (exposure age: adolescent; adult) × 2 (acute challenge: SAL; EtOH) factorial ANOVA revealed a significant main effect of acute challenge [F(1,66)= 73.92, p< .05], as well as an interaction of acute challenge x exposure [F(1,66) = 7.54, p<.05], with subsequent post-hoc tests revealing that animals from each exposure group challenged with EtOH during context fear conditioning froze significantly less than animals challenged with SAL during conditioning, regardless of age (see Fig. 2c). A significant main effect of exposure age [F(1,66)= 5.62, p< .05] also emerged, with adolescent-exposed animals showing significantly less freezing overall (35% ± 4) than the adult exposure groups (44% ± 4); this effect appeared to be driven by SAL-challenged animals that had been exposed to EtOH during adolescence, although the 3-way interaction involving age, exposure and acute challenge did not reach significance [F (1, 66)= 2.27, p= .14].
Discussion
The purpose of this experiment was to determine whether an acute ethanol challenge influences tone and context conditioning differently in animals exposed to ethanol during adolescence versus adulthood. Acute ethanol challenge during context conditioning resulted in typical disruptions of context fear retention in animals from both exposure ages. Context fear retention also tended to be attenuated in SAL challenged animals with a history of ethanol exposure during early adolescence. Tone fear retention, like context retention, was disrupted by acute ethanol challenge during conditioning regardless of exposure group or age. This finding was somewhat unexpected given that delay tone conditioning, where the CS and US overlap as in the current study, is thought to be more resistant to acute ethanol challenge than context conditioning (Broadwater & Spear, 2013; Hunt et al., 2009; Melia et al., 1996; Weitemier & Ryabinin, 2003). Since the amygdala is important for tone conditioning (Davis, 1992; LeDoux, 2000; Maren & Quirk, 2004; Fanselow & Poulos, 2005), disruptions seen after acute ethanol challenge in animals with prior H20 or ethanol exposure potentially reflect enhancement of sensitivity of amygdala processing to ethanol challenge, regardless of the age at which the perturbation occurred. Unfortunately, a non-manipulated control group was not included to allow for assessment of the effects of the chronic perturbation itself (i.e., repeated H20 intubations) on the dependent measures. Hence, these suggestions remain speculative. Furthermore, given that the amygdala is also important for context fear conditioning (e.g., Akirav & Richter-Levin, 2006; Maren, 2008), it is possible that changes in ethanol sensitivity of the amygdala may have also influenced our results of ethanol-induced disruptions of context fear retention.
Acquisition of tone and context fear did not differ among adolescent-exposure groups, suggesting that fear learning in adulthood was not disrupted by prior adolescent ethanol exposure, which is consistent with our previous report (Broadwater & Spear, 2013b). Also, disruption in tone and context fear retention after ethanol challenge was most likely not attributable to learning deficits. Among adult exposure groups, however, two effects of ethanol challenge emerged during acquisition. During tone conditioning, H20-exposed adults acutely challenged with ethanol during conditioning showed signs of ethanol-induced motor impairment, with significantly more freezing than saline challenged animals early in conditioning (i.e., prior to footshock). This effect was not evident in ethanol-challenged adults with a history of ethanol exposure, thus indicating tolerance to the motor impairing effects of acute ethanol challenge in this group. Given that this effect was not observed in adolescent ethanol-exposed adults, these data are reminiscent of greater chronic tolerance (CT) to the sedative effects of ethanol in adults than adolescents when tested immediately following ethanol exposure (Broadwater et al., 2011b; Linsenbardt et al., 2009; Matthews et al., 2008; although see Swartzwelder et al., 1998), and suggests that these potential age differences in CT expression may be persistent. Tolerance to ethanol-related motor impairment was not evident during context conditioning at any age. This inconsistency after adult ethanol exposure may be due to the differences in amount of time, and hence opportunity, to observe the motor impairing effects of an ethanol challenge, with tone conditioning including the 2 min habituation period and the first tone (29 s), whereas during context conditioning only the 2 min habituation period served as an index of freezing prior to footshock.
The other effect observed during acquisition among the adult exposure groups was the attenuated acquisition of context freezing induced by acute ethanol challenge regardless of whether animals were exposed to water or ethanol. Although freezing did not differ among challenge groups by the end of the conditioning session, a slower acquisition rate could have contributed to the decreased context fear retention observed the following day in these animals. Given that ethanol-induced deficits of context fear retention have been previously observed in adults without evidence of disruptions in acquisition (Broadwater & Spear, 2013a), it is likely that the attenuated freezing seen here during the context retention test in animals challenged with ethanol during conditioning is due to retention deficits. However, ethanol-induced disruptions of learning cannot be ruled out as a contributor to these context retention deficits seen in adult exposure groups after acute challenge with ethanol.
These data suggest that, other than the modest motor impairing effects of ethanol challenge seen during acquisition in adult, but not adolescent exposure groups, there were minimal differences between adolescent and adult exposure groups in terms of disruption of learning and memory after an acute ethanol challenge. Lack of age differences in long-term effects in the current study may be in part related to exposing animals at each age to the same ethanol dose during the exposure period, given that adolescents show decreased sensitivity to many (albeit not all) effects of ethanol than do adults (see Spear & Varlinskaya, 2005 for review). However, in our previous study using the same exposure regimen as in the current study (4 g/kg i.g. every 48 hrs for 11 exposures), we found that, despite the reduced ethanol sensitivity (indexed via body weight gains and intoxication rating following each dosing) shown by adolescents during the exposure period, the adolescent ethanol-exposed animals showed deficits in context fear retention that were not evident after ethanol exposure during adulthood (Broadwater & Spear, 2013b). These data support the suggestion that the exposure regimen used in the current study was sufficient to induce long-term changes after adolescent exposure, although not in terms of evidence of long-term alterations in ethanol sensitivity after adolescent (or adult) ethanol exposure.
In line with this, no evidence of a “lock-in” effect of adolescent-like ethanol sensitivity was seen in the current study after adolescent exposure in terms of resistance to ethanol-induced disruptions of context fear retention, since acute ethanol disrupted fear retention similarly across exposure age. That is, given previous reports that adolescents are less sensitive than adults to the disruption of context fear retention associated with a 1 g/kg EtOH challenge during fear conditioning (Broadwater & Spear, 2013a; Land & Spear, 2004), a “lock-in” like effect would likewise be defined as significantly more fear (i.e., more freezing) during the retention test after EtOH challenge among animals repeatedly exposed to ethanol during adolescence than adulthood. Yet, levels of freezing between these two groups in the current study (see Fig 2c and d) were almost identical. It should be noted, however, that in the present study, animals exposed to ethanol as adolescents and that received only SAL during conditioning tended to show less context freezing when compared with all other exposure groups that received SAL during conditioning. This tendency for a disruption in context fear memory as a consequence of adolescent ethanol exposure per se is reminiscent of findings of Broadwater & Spear (2013b), and could potentially have influenced the ability to detect further disruptions in context fear after ethanol exposure during conditioning in these animals. Thus, although the present data suggest that not all adolescent-typical ethanol sensitivities may be retained into adulthood after repeated ethanol exposure during adolescence, it is nevertheless possible that initially low levels of context freezing after adolescent ethanol exposure per se may have limited our ability to detect differences in sensitivity to an acute ethanol challenge.
It is also possible that the chronic intragastric exposure to H20 may have influenced ethanol sensitivity in the current study, masking differences in ethanol sensitivity across groups, as in previous work where repeated intraperitoneal injections of saline in adulthood (but not adolescence) were found to attenuate sensitivity to acute EtOH challenge (see Broadwater et al., 2011a, b). This possibility appears unlikely, however, given that in the current study we found that water-exposed adults were more sensitive than EtOH exposed adults to the apparent motor impairing effects of an EtOH challenge early in the tone conditioning session, suggesting that the chronic H20 intubations did not notably reduce ethanol sensitivity, at least when tested > 3 wks after the intubation period. Nonetheless, although non-manipulated control groups are rarely included in studies examining effects of repeated drug exposures, where feasible, inclusion of these controls would be valuable for detecting possible effects of the repeated administration process per se that could potentially mask or exacerbate effects of the chronic drug exposure per se.
Another limitation of the current study is that only one ethanol dose was examined, the dose at which previous studies reported an age differences in sensitivity to ethanol-induced disruptions of context fear retention (Broadwater & Spear, 2013; Land & Spear, 2004). It is possible that differences in ethanol sensitivity might have been detected with analysis of a more extensive dose range. Future studies examining ethanol sensitivity after adolescent ethanol exposure should consider incorporating multiple ethanol challenge doses. Furthermore, more studies across several species utilizing different ethanol exposure models and tests of ethanol sensitivity would aid in our understanding of the potential for a “lock-in” of adolescent-typical ethanol sensitivity as a consequence of repeated ethanol exposure during adolescence.
Acknowledgments
The research presented in this paper was supported by NIAAA grants NIAAA grants R01AA018026 and U01AA019972-NADIA Project
References
- Akirav I, Richter-Levin G. Factors that determine the non-linear amygdala influence on hippocampus-dependent memory. Dose-Response: A Publication Of International Hormesis Society. 2006;4(1):22–37. doi: 10.2203/dose-response.004.01.003.Akirav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, Zurn JB, Sage JR, Herrera GM. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Frontiers In Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcoholism, Clinical And Experimental Research. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning to context. Behavioural Brain Research. 2000;108(1):1–19. doi: 10.1016/s0166-4328(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Age differences in fear retention and extinction in male Sprague-Dawley rats: Effects of ethanol challenge during conditioning. Behavioural Brain Research. 2013a;252:377–387. doi: 10.1016/j.bbr.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behavioural Brain Research. 2013b;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Different chronic ethanol exposure regimens in adolescent and adult male rats: effects on tolerance to ethanol-induced motor impairment. Behavioural Brain Research. 2011a;225(1):358–362. doi: 10.1016/j.bbr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcoholism, Clinical And Experimental Research. 2011b;35(8):1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2005;29(9):1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Davis M. The Role of the Amygdala in Fear and Anxiety. Annual Review Of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcoholism Clinical and Experimental Research. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, Clinical And Experimental Research. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review Of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcoholism, Clinical And Experimental Research. 2012;36(2):279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. Journal Of Psychopharmacology (Oxford, England) 2003;17(1):77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal Of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Levillain ME, Spector BM, Kostelnik LA. Post-training ethanol disrupts trace conditioned fear in rats: effects of timing of ethanol, dose and trace interval duration. Neurobiology of Learning and Memory. 2009;91(1):73–80. doi: 10.1016/j.nlm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. Journal Of Neuroscience Methods. 2010;190(2):235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience. 1993;107(6):1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. International Journal Of Developmental Neuroscience: The Official Journal Of The International Society For Developmental Neuroscience. 2004;22(5–6):355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review Of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcoholism, Clinical And Experimental Research. 2009;33(3):464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacology, Biochemistry, And Behavior. 2010;96(4):476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JA. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42(8):617–621. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. The European Journal Of Neuroscience. 2008;28(8):1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of Learning and Memory. 1997;67(2):142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74(2):313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal Of Neurochemistry. 2009;108(4):920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacology, Biochemistry, And Behavior. 2010;95(2):242–248. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcoholism, Clinical And Experimental Research. 2010;34(12):2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural Brain Research. 2011;225(1):104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments In Alcoholism: An Official Publication Of The American Medical Society On Alcoholism, The Research Society On Alcoholism, And The National Council On Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15(4):311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time Course of Elevated Ethanol Intake in Adolescent Relative to Adult Rats Under Continuous, Voluntary-Access Conditions. Alcoholism: Clinical and Experimental Research. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol And Alcoholism (Oxford, Oxfordshire) 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13(3):305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcoholism, Clinical And Experimental Research. 2002a;26(7):960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: A unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004;1021:206–220. doi: 10.1196/annals.1308.026. Adolescent Brain Development: Vulnerabilities and Opportunities. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology, Biochemistry, And Behavior. 2002b;73(3):673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]