Abstract
Planar cell polarity (PCP) or tissue polarity describes a coordinated polarity at the plane of the tissue where most or all cells within a tissue are polarized in one direction. It is perpendicular to the apical–basal polarity of the cell. PCP is manifested readily in the Drosophila wing and cuticle bristles, Drosophila eye ommatidia, and mammalian hair and inner ear hair bundles, and less evidently, in cellular processes such as in the coordinated, directional cell movements, and oriented cell divisions that are important for tissue morphogenesis. Several distinct molecular and cellular processes have been implicated in the regulation of PCP. Here, we review potential roles for PCP during mouse kidney development and maintenance, including ureteric bud branching morphogenesis, renal medulla elongation, tubule diameter establishment/maintenance, glomerulogenesis, and response to injury. The potential mechanisms underlying these processes, including oriented cell division and coordinated cell migration/cell intercalation, are discussed. In addition, we discuss some unaddressed research topics related to PCP in the kidney that we hope will spur further discussion and investigation.
1. INTRODUCTION
Although it is well known that most cells show polarity along an apical/basal (A/B) axis, it has become increasingly evident that cells are also polarized along a second axis perpendicular to the A/B axis. This is easy to visualize for migrating cells as the leading and lagging edges. However, even epithelial cells can show this second axis of polarity, and when all or most of the cells within the tissue coordinate their polarity in one direction, it is referred to as tissue or planar cell polarity (PCP).
Although intensively studied in the fly for many years, PCP was largely ignored by vertebrate biologists. However, over the past 10 years, mutagenesis of vertebrate orthologs of genes necessary for PCP establishment in flies has revealed multiple requirements during normal development and tissue maintenance. In some tissues, the connection between the PCP genes and the actual PCP is clear, while in others, they are a complete mystery.
In this review, we characterize a number of processes that are thought to be regulated by PCP during kidney development and maintenance. We review what is known about the molecular regulators and the cellular processes controlled.
2. PLANAR CELL POLARITY
PCP describes polarity within the plane of a tissue, perpendicular to the apical–basal polarity of the cells. It can manifest itself externally, for example, in the directional alignment of Drosophila wing hairs and cuticular bristles (all point in the same direction), the orientation of mammalian body hair, or the orientation of stereociliary bundles of the inner ear. Less readily identifiable but essential examples are the organization of the ommatidia of the Drosophila eye or the uniform cellular orientation, coordinated directional cell migration, and oriented cell division that drive tissue morphogenesis. It is important to emphasize that mere asymmetry alone is not equivalent to PCP. Individual cells can show asymmetry, but if that asymmetry is not coordinated between all (or most) cells within a tissue, it is not PCP.
2.1. The Fat/Dachsous pathway
Mutagenesis screens in Drosophila have identified a number of genes necessary for establishing PCP in all or most tissues. These genes are broken up into distinct pathways based on where and when they act and also their relationship to one another. One such pathway is referred to as the Fat/Dachsous (Ds) pathway (Thomas & Strutt, 2012). Fat and Dachsous are atypical cadherins that interact heterotypically between adjacent cells (Matakatsu & Blair, 2004). Members of the Fat/Ds group show signs of subcellular localization (Ambegaonkar, Pan, Mani, Feng, & Irvine, 2012; Bosveld et al., 2012; Brittle, Thomas, & Strutt, 2012). The Fat and Dachsous proteins are enriched on one side or the other. Although the subcellular localization is not as strict as is seen for other PCP determinants (see below), its strength is reinforced by Fat/Ds intercellular dimerization as well as processes that regulate the activity of these two proteins (Ambegaonkar et al., 2012; Brittle et al., 2012).
Four-jointed (Fj) is a Golgi-associated kinase that phosphorylates the extra-cellular domain of Fat and Ds (Simon, Xu, Ishikawa, & Irvine, 2010). Phosphorylation of Fat by Fj increases its affinity for Ds, while phosphorylation of Ds decreases its affinity for Fat (Brittle, Repiso, Casal, Lawrence, & Strutt, 2010; Simon et al., 2010). In many tissues, Fj is expressed in a graded pattern, which results in graded activity of Fat and Ds (Simon, 2004). The net result of the action of Fj and the polarized distribution of Fat and Ds is that, although the proteins may be distributed throughout the cell, the active forms will segregate to opposite sides. In this way, the proteins establish PCP. Although there are some exceptions, neither does the polarization of active Fat and Ds require the activity of the core determinants (see below) nor does the Fat/Ds pathway affect core protein localization in individual cells (it does affect global polarization) (Casal, Lawrence, & Struhl, 2006). Thus, it appears that for the most part the Fat/Ds and core pathways act in parallel to each other rather than in a simple linear pathway (Lawrence, Struhl, & Casal, 2007).
2.2. The core pathway
A second major pathway identified in Drosophila is referred to as the “core” pathway (Gray, Roszko, & Solnica-Krezel, 2011; Maung & Jenny, 2011). It is composed of the seven-pass transmembrane receptor Frizzled (Fz), the cytoplasmic PDZ domain-containing protein Disheveled (Dsh), the atypical cadherin Flamingo/Starry night (Fmi/Stan), the tetramembrane-spanning protein Van gogh/strabismus (Vang/Stbm), the Lim and Pet domain-containing cytoplasmic protein Prickle (Pk), and the ankyrin domain-containing protein Diego (Dgo).
Although PCP affects polarity over an entire tissue, it is established at the level of individual cells. Within a cell, PCP results in the polarized localization of proteins, macromolecules, and organelles. Indeed, in some tissues, many of the PCP determinants are themselves localized within the plane of the cell and are required for the subcellular localization of other pathway determinants (Fig. 8.1). For instance, during PCP establishment in the fly wing, Fz and Dsh are localized on the distal side of the cell, while Vang and Pk are localized on the proximal side (Axelrod, 2001; Bastock, Strutt, & Strutt, 2003; Das, Jenny, Klein, Eaton, & Mlodzik, 2004; Jenny, Darken, Wilson, & Mlodzik, 2003; Shimada, Usui, Yanagawa, Takeichi, & Uemura, 2001; Tree, Shulman, et al., 2002). The precise mechanisms regulating the planar-polarized positions of each protein are still not perfectly clear although they appear to be reinforced by positive and negative interactions with other core proteins. It is still not clear if the polarized localization of the core or Fat/Ds determinants is maintained in all vertebrate tissues manifesting PCP. Ultimately, the Fz/PCP and Ft/Ds pathways regulate the polarization of the cytoskeleton (Tree, Ma, & Axelrod, 2002).
Figure 8.1.
Schematic illustration of planar cell polarity (PCP). PCP is perpendicular to the apico-basal polarity of the epithelial tissue. PCP proteins are asymmetrically localized at the cell junctions between neighboring cells. This asymmetry defines the tissue polarity.
2.3. The Wnt pathway
Wnt ligands play a crucial role in establishing PCP in vertebrates. Wnts signal through an ever-expanding group of receptors and coreceptors (Buechling & Boutros, 2011). Nearly all Wnt signaling depends on interaction of the ligand with a Fz receptor. Note that this is the same Fz molecule that is a component of the core PCP group. Dsh also appears to play a dual role as a core PCP determinant and a component of the Wnt pathway. These observations led many to speculate that perhaps Wnts established PCP. However, there is no evidence that Wnt ligands have a role in PCP in flies.
Depending on the receptor complex and intracellular environment within the receiving cell, the Wnt signal is transduced down one of several different pathways (Cadigan & Liu, 2006). If the ligand binds a Fz and a low-density lipoprotein receptor, it activates the “canonical pathway” (Wehrli et al., 2000). Canonical activity results in stabilization of β-catenin and the formation of a β-catenin/Lef/Tcf transcriptional complex. If the Wnt interacts with a Fz and Ror1/2, then a noncanonical/β-catenin-independent cascade is activated (Green, Inoue, & Sternberg, 2008; Mikels & Nusse, 2006; Oishi et al., 2003). The noncanonical transduction cascade is still not well understood, although, in some instances, it may involve activation of Rho GTPases and Jnk (Boutros, Paricio, Strutt, & Mlodzik, 1998; Habas, Dawid, & He, 2003; Kohn & Moon, 2005; Oishi et al., 2003). Ultimately, activation of the noncanonical pathway results in polarization of the cytoskeleton and PCP. The two pathways appear to antagonize each other so that in one particular cell at one particular moment, only one pathway may be active (Green et al., 2008; Liao et al., 2006; Mikels & Nusse, 2006).
Although it would be attractive to think that gradients of Wnt proteins play a role in establishing PCP, there is little evidence that this is the case (Strutt, 2009). In some instances, it appears that Wnts play permissive roles in PCP while in others they are instructive (Gao et al., 2011; Goldstein, Takeshita, Mizumoto, & Sawa, 2006; Gong, Mo, & Fraser, 2004; Green et al., 2008; Heisenberg et al., 2000; Matsui et al., 2005; Prasad & Clark, 2006; Schlesinger, Shelton, Maloof, Meneghini, & Bowerman, 1999; Ulrich et al., 2003; Witze, Litman, Argast, Moon, & Ahn, 2008). How the activation of the Wnt pathway affects planar polarity is still not clear.
2.4. The primary cilium
The primary cilium is a small projection present on the apical side of most cells, which consists of membrane surrounding a cytoskeletal structure known as the axoneme. The axoneme is anchored within the basal bodies that further act as anchoring or nucleation sites for other cytoskeletal components and various cytosolic proteins as well as forming a part of the centrioles during cell division (Pan, Wang, & Snell, 2005). In some tissues, the cilia show planar-polarized localization within the apical membrane (Bayly & Axelrod, 2011; Ganner et al., 2009; Guirao et al., 2010; Park, Mitchell, Abitua, Kintner, & Wallingford, 2008).
Several recent studies have suggested that the cilium regulates Wnt pathway usage and PCP. This is an extremely complicated issue that cannot be dealt with appropriately here (for a more detailed discussion, see Wallingford & Mitchell, 2011). However, we briefly discuss this issue here, as it is an important topic.
Mice carrying mutations in genes that are necessary for ciliogenesis show pathologies that have been linked to PCP defects including cystic kidney tubules (Hildebrandt, Attanasio, & Otto, 2009; Lin et al., 2003; Patel, Chowdhury, & Igarashi, 2009; Sattar & Gleeson, 2011; Sharma, Berbari, & Yoder, 2008; Waters & Beales, 2011). Although there is strong evidence that PCP signaling is necessary for the planar-polarized location of the cilia and the polarized beating of the cilia in some tissues, neither of these situations appears to be relevant to the mammalian kidney. Kidney cilia are nonmotile and do not show obvious planar polarization.
Ciliary signaling has been linked to oriented cell divisions, a form of PCP, and activation of the noncanonical Wnt pathway. However, at this point, the mechanisms connecting the cilia, Wnt signaling, and PCP signaling (if there is such a connection) are still unclear. It is important to point out that the mere presence of a defect in PCP in a mutant background does not necessarily indicate that the mutated gene or structure plays a role in PCP. It may simply be necessary for the cell type-specific effect of PCP. Further, characterization will be required to gain a greater understanding of the cilia to Wnt signaling and PCP.
2.5. Junctional remodeling
One of the processes affected by PCP are directed cell movements that occur during morphogenesis (Aigouy et al., 2010; Carreira-Barbosa et al., 2009; Phillips, Murdoch, Chaudhry, Copp, & Henderson, 2005; Shnitsar & Borchers, 2008; Skoglund & Keller, 2010; Takeuchi et al., 2003; Ulrich et al., 2003; Veeman, Slusarski, Kaykas, Louie, & Moon, 2003; Wallingford & Harland, 2002). Although such movements take place during axis elongation in Drosophila, they do not appear to be dependent on any of the “canonical” PCP determinants discussed above. Instead, these cell movements appear to be regulated by the spatial expression of factors along the anterior/posterior axis of the embryo (Blankenship, Backovic, Sanny, Weitz, & Zallen, 2006; Zallen & Blankenship, 2008). A/P patterning regulates cell adhesion and junctional remodeling that leads to changes in cell geometry, resulting in the formation of multicellular rosette structures. The rosettes resolve themselves along the A/P axis, resulting in tissue thinning and elongation, a process that can broadly be described as convergent extension (CE) (Blankenship et al., 2006).
A distinct set of factors that are localized to the adherens junctions such as the Par3, Scribbled, and nonmuscle myosin homologs are required for CE during Drosophila germband extension (Blankenship et al., 2006; Djiane, Yogev, & Mlodzik, 2005; Gray et al., 2011; Montcouquiol et al., 2003; Zallen & Blankenship, 2008). Interestingly, although these factors show planar-polarized expression within cells similar to what is observed for the core PCP proteins, mutations in Fz and Dsh do not affect these morphogenetic movements. These studies emphasize that not all PCP is equivalent. Multiple independent pathways most likely regulate PCP in tissue-specific manners. Therefore, defects in seemingly unrelated cellular processes can lead to similar effects on the entire tissue. This is an important point, especially when one begins to consider all the pathologies that have been attributed to PCP defects in the kidney.
3. KIDNEY DEVELOPMENT
The formation of the mammalian permanent kidney, or metanephros, is initiated when an epithelial bud (the ureteric bud) forms from the Wolffian duct and invades the surrounding mesenchyme (known as the metanephric mesenchyme, MM). The MM consists of at least two cell types. Cells that lie closest to and surround the ureteric bud are collectively referred to as the cap mesenchyme. A second population of cells lies distal to the ureteric bud and adjacent to the cap mesenchyme and is referred to as the cortical interstitium. Following its invasion into the mesenchyme, the ureteric bud epithelium undergoes branching morphogenesis forming a highly complex, tree-like system of tubes that make up the renal collecting duct system of the mature kidney.
The cap mesenchyme contains a self-renewing, multipotent progenitor cell population that proliferates with the branching ureteric buds so that each new bud tip is covered by cap mesenchyme (Boyle et al., 2008; Kobayashi et al., 2008). After each round of branching, a subpopulation of the cap mesenchyme will undergo a mesenchymal-to-epithelial transition just below the ureteric bud tips forming a pretubular aggregate and then a primitive epithelial structure known as the renal vesicle. The renal vesicle will undergo extensive morphogenesis to form the nonvascular portion of the nephron.
The cortical interstitium also consists of a stem/progenitor cell that gives rise to renal interstitial cells including the pericytes, smooth muscle, and the mesangial cells within and about the renal corpuscles (Humphreys et al., 2010). Signals provided by both the cap mesenchyme and the interstitium regulate the branching morphogenesis of the ureteric bud and potentially the patterning of the nephron and collecting ducts (Costantini & Kopan, 2010; Yang et al., 2002).
The epithelial components of the kidney undergo extensive growth during the embryonic period and continue postnatally. Morphogenesis establishes the ultimate diameter, length, and shape of the kidney tubules, which are absolutely essential for normal organ function. The cellular and molecular processes regulating this morphogenesis are still poorly understood although studies over the past several years have provided some insights.
Karner et al. (2009) found that, during the embryonic period, orientation of the mitotic spindle of most of the epithelial cells appears to be random relative to the proximal–distal axis of the tubule. Without some sort of compensatory mechanism, one would then predict that tubule diameter (or at least the number of cells within the cross-sectional circumference of the tubule) would increase during the embryonic period. It does not. In fact, it was found that the number of cells within the circumferential wall decreased as the tubule elongated (Karner et al., 2009). How can this be? One possibility is that a significant numbers of epithelial cells are culled by apoptosis. However, several groups have reported that the rate of epithelial cell apoptosis is negligible during the embryonic period. A second possibility is that some sort of cellular rearrangement is occurring that causes the tubules to thin and contributes to their elongation. Indeed, cellular rearrangements of this type have been observed during gastrulation and neurulation in multiple species (Axelrod & McNeill, 2002; Concha & Adams, 1998; Darken et al., 2002; Goto & Keller, 2002; Heisenberg et al., 2000; Honda, Nagai, & Tanemura, 2008; Nikolaidou & Barrett, 2005; Torban, Kor, & Gros, 2004; Wallingford, Fraser, & Harland, 2002; Wallingford et al., 2000; Wang et al., 2006; Winklbauer, 2009). Although the mechanism regulating tissue elongation and thinning varies, the phenotypic outcome is essentially the same and these processes are collectively referred to as CE.
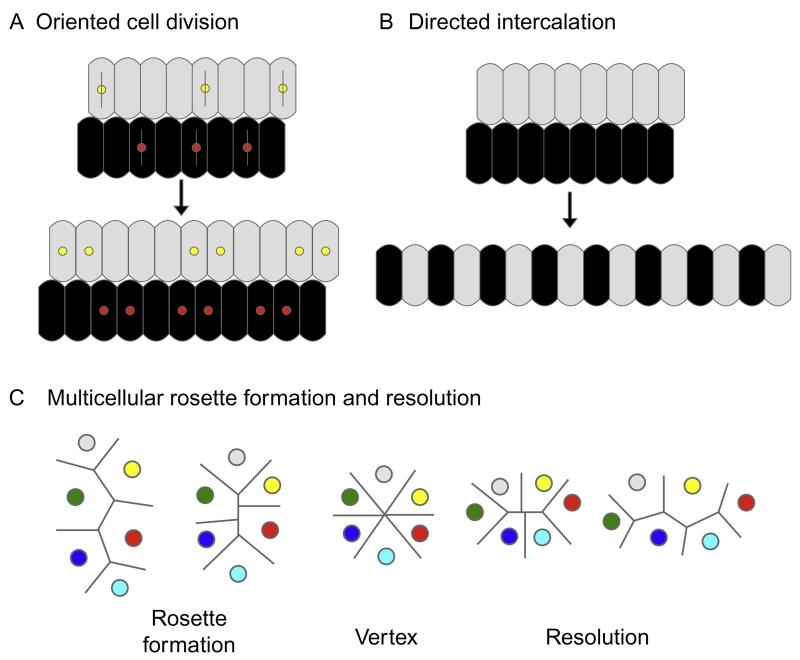
Several distinct types of cell behavior have now been described that lead to CE movements including mediolateral intercalation and multicellular rosette formation and resolution (Fig. 8.2). Each of these processes has distinct, defining characteristics that can indicate whether they are occurring. For example, cells undergoing intercalation frequently show mediolateral elongation perpendicular to the axis of extension (a form of PCP) (Bertet, Sulak, & Lecuit, 2004; Nikolaidou & Barrett, 2005; Shih & Keller, 1992; Skoglund & Keller, 2010; Wallingford et al., 2000; Wang et al., 2005; Zallen & Blankenship, 2008). Kidney epithelial cells show such morphology. However, kidney epithelia also form multicellular rosettes (C.M. Karner & T.C. Carroll, unpublished observations). Unfortunately, the issue of which (if any) type of movement is taking place during tubule formation can only be conclusively demonstrated using live imaging, which has not been accomplished in a vertebrate as of yet. However, CE-like movements would explain how a proliferating epithelium with randomly oriented cell division does not increase its diameter.
Figure 8.2.
Processes regulating tubule elongation. (A) Oriented cell division leads to lengthening of the tubule, while the diameter stays the same. (B) Directed intercalation leads to the tubule elongating while it also becomes thinner. (C) Multicellular rosette formation and resolution (adapted from Zallen & Blankenship, 2008) describe the changes in cell morphology and adhesiveness that result in a transient rosette with a vertex forming. The rosette resolves resulting in the tubule becoming longer and thinner. Only oriented cell division has been demonstrated in the kidney. Panels (B) and (C) require live imaging to confirm.
Cell intercalation/rearrangement appears to be a conserved mechanism in regulating renal tubule size. During Drosophila Malpighian tubule formation, a distinct phase of cell intercalation and cell rearrangement is observed that drives the renal tubule elongation and narrows the renal tubule diameter from 6–10 cells to 2 cells surrounding the lumen (Jung, Denholm, Skaer, & Affolter, 2005 and references therein). Though the molecular machinery is not well characterized, nonmuscle myosin II heavy chain, a gene involved in multicellular rosette formation and resolution during Drosophila germband extension, has been implicated in this process.
After birth and for the first several weeks of life, Fischer et al. (2006) reported that the tubules of the kidney (both the nephrons and the collecting ducts) continue to elongate, while the number of cells in the circumference remains fairly constant. By following the progeny of individually labeled postnatal tubule cells over a period of 5 weeks, they noticed little divergent cell migration or nonclonal cell intercalation. They concluded that, at least in a normal postnatal tubule, CE/cellular intercalation is unlikely to be involved in maintenance of tube diameter. However, measurement of mitotic angles in outer medullary Aqp2+ (collecting ducts), uromodulin+ (ascending limb of loops-of-Henle), or Lotus tetragonolobus (LTL)+ (proximal tubules) tubules identified rigorously oriented cell division (Fig. 8.2) along the proximal/distal axis of tubules such that 95% of the mitotic angles were within 34° and the average is 11°. This finding suggests that oriented cell division drives tubule elongation and diameter establishment during postnatal tubular development. Yu et al. (2009) found a similar process was involved in the growth of the renal papilla. Thus, oriented cell divisions appear to be essential for much of the growth that drives the ultimate shape of the kidney.
Interestingly, Luyten et al. (2010) found that the average number of cells in the circumference of the collecting duct continues to decrease postnatally, suggesting that some intercalation continues after birth. The apparent discrepancy in the involvement of cell intercalation in postnatal collecting ducts in regard with the findings of Fischer et al. and Luyten et al. remains to be resolved. Although the observations of Fischer et al. could be explained if cell intercalation in collecting ducts is clonal, this explanation cannot explain a decrease in circumference. Pkd1 mutant cells displayed reduced rates in scattered cell migration in the in vitro wound healing assay, suggesting that defective cell intercalation may contribute to cyst formation in Pkd1 mutants and PC1 plays a role in regulating cell intercalation (Luyten et al., 2010). Thus, it appears that the ultimate length and diameter of the kidney tubules depend on a combination of directed cell movements and oriented cell divisions.
4. PCP IN KIDNEY DEVELOPMENT
Many of the processes proposed to regulate kidney development such as directed cell migration during UB branching and potentially nephron formation, CE movements during tubule diameter establishment, and oriented cell division during elongation depend on PCP. Several orthologs of the PCP determinants are expressed in the developing kidney. Characterization of mouse kidneys mutated for these factors has uncovered a number of defects, some expected and some unexpected. In many cases, the defects have not been attributed to any specific deficit in PCP. On the other hand, several mutants have recently been found to have defects in some aspects of PCP. In many of these cases, it is not clear how the genes affected relate to the establishment of PCP. In this section, we describe our current understanding of the role of all types of PCP during kidney development.
4.1. The Fat/Ds group
4.1.1 Branching morphogenesis
There are four mammalian paralogs of Fat (Fat1-4) and two orthologs of Ds (Dchs1 and Dchs2). Although Fat4 has the highest overall degree of similarity to Drosophila Fat, the other three paralogs show conservation in important functional regions (Matakatsu & Blair, 2012).
Kidneys lacking Dchs1 show mild defects in early UB branching morphogenesis, resulting in kidneys that are reduced in size (Mao et al., 2011). Fat4 mutants show a very similar phenotype, suggesting that the interaction between Fat and Dachsous proteins is conserved in mice (Mao et al., 2011). Indeed, compound heterozygotes show phenotypes nearly identical to the individual homozygotes, further supporting an interaction (Mao et al., 2011).
The precise causes of the defects leading to reduced branching in these mutants are unclear. Both show increased rates of apoptosis within the ureteric bud and decreased rates of proliferation within the nephron progenitors and the UB. These sorts of changes on cell number are not typically associated with defects in PCP. Indeed, no specific defects in PCP were identified in mutants during branching morphogenesis although there are clear defects during tubule elongation (see below). Interestingly, Fat4 and Dchs1 proteins are present at high levels in the interstitium with lower levels in the nephron progenitors. Levels are very low to undetectable in the ureteric bud (Mao et al., 2011). Thus, it is possible that Fat4 and Dchs1 regulate an unknown PCP in the interstitium or nephron progenitors and the branching defects seen in mutants are indirect effects caused by disrupted communication between one of these cell types and the UB. However, definitive conclusions will require analysis of kidneys with tissue-specific ablation of these genes.
4.1.2 Tubule diameter establishment/maintenance
Dchs1 and Fat4 mutants show dilated distal nephron segments during embryogenesis (Mao et al., 2011). Fat4 mutants show defects in the orientation of cell division in postnatal collecting ducts (Saburi et al., 2008). Whether this is the cause of tubular dilation is not clear although orientation of cell division has been implicated in maintaining tubule diameter in other mouse models (Fischer et al., 2006).
Although Fat4 and Dchs1 mutants both show kidney defects, no defects are observed in kidneys lacking the only known Fj ortholog, Fjx1 (Saburi, Hester, Goodrich, & McNeill, 2012). Although this finding in itself is not all that surprising (mutation of Fj alone has a relatively mild phenotype in the fly wing), Fjx1 does not seem to genetically interact with Fat4 in the developing kidney (Saburi et al., 2012). There are a number of possible explanations for this finding. It is possible that there is another functional homolog of Fj in the mouse. A second possibility is that Fat4 and Dchs1 are not regulating PCP during kidney tubule morphogenesis. (Fat4 and Fjx1 do show strong genetic interaction in other tissues.) The final possibility is that the interaction between Fjx1 and Fat4 (and Dchs1?) is not conserved in mouse kidneys. Consistent with this last possibility, unlike Drosophila, in the mouse kidney, Fjx1 does not appear to be expressed in the same cells as Fat4 or Dchs1 (Carroll & Das, 2011; Mao et al., 2011). Further analysis will need to be performed to determine which, if any, of these scenarios is correct.
4.2. The core pathway
4.2.1 Branching morphogenesis
4.2.1.1 Vangl2
Although many of the core determinants are expressed in the developing kidney, there is relatively little known of their function. Yates et al. (2010) recently described the phenotype of the Loop-tail (Lp) mice that bear a point mutation in the Vangl2 gene (ortholog of the Drosophila core PCP determinant Vang/Stbm) that produces a potential dominant-negative mutation. Reduced ureteric bud branching morphogenesis was observed in mutant kidneys from E13.5. The mutant kidneys are most significantly reduced in size along their anterior–posterior axis.
Neither were significant changes noted in the expression of other core determinants, nor were the rates of proliferation or apoptosis affected. There was also no description of defects in any aspect of PCP in these mutants. During ureteric bud branching morphogenesis, dilated ampullae are resolved into two stalks with narrower diameter. This process may involve PCP-dependent cell rearrangements. Cell movements have been observed in ureteric bud tips during branching morphogenesis. It will be interesting to examine whether directional cell movements occur during branching morphogenesis and, if so, whether Wnt/PCP signaling affects this process cell autonomously in the ureteric bud epithelium. However, for now, the mechanism underlying this phenotype is unclear.
4.2.1.2 Fz4/8
Fz4 and Fz8 are expressed specifically in the ureteric bud epithelium during early kidney development and mice lacking both genes show mildly disrupted branching morphogenesis (Ye, Wang, Rattner, & Nathans, 2011). Fz receptors can act within the core PCP pathway, the canonical Wnt pathway, or the noncanonical Wnt pathway. In Fz4/8 mutants, it is not clear which aspect of Fz signaling is disrupted. The mutants show some similarities to Wnt11 mutants including reduced branching and decreased levels of the Ret receptor tyrosine kinase in the UB and its ligand Gdnf in the mesenchyme (Majumdar, Vainio, Kispert, McMahon, & McMahon, 2003). Indeed, Wnt11 is able to signal through Fz4 and Fz8 in vitro (Ye et al., 2011). However, Wnt11 can activate both canonical (Tao et al., 2005) and noncanonical (Garriock, D’Agostino, Pilcher, & Krieg, 2005; Marlow, Topczewski, Sepich, & Solnica-Krezel, 2002; Matsui et al., 2005; Tada & Smith, 2000; Takeuchi et al., 2003;Ye et al., 2011) pathways providing little insight into pathway usagein the kidney.
It is important to note that the Fz4/8 mutant UBs also show some similarity to kidneys that have had β-catenin removed from the UB (reduction of Ret/Gdnf expression and activity, reduced branching, and reduced proliferation rates) (Marose, Merkel, McMahon, & Carroll, 2008). Although the authors did not examine the expression of β-catenin targets such as Axin2 in Fz4/8 mutants, these data may support a canonical role for Fz4/8 in UB branching. However, at this point, it is impossible to rule out noncanonical or PCP roles for the Frizzleds.
4.2.1.3 Inversin
Inversin, encoded by the Nphp2 gene, was identified by positional cloning of the Inv mouse that was generated in a mouse mutagenesis screen (Morgan et al., 1998; Otto et al., 2003; Simons et al., 2005). Along with defects in left/right asymmetry, Inv mutants formed cystic kidneys. Sequencing of the Nphp2 gene revealed that it shared high homology with fly Dgo. Indeed, Invs and Dgo can substitute for each other functionally (Simons et al., 2005).
Inversin physically and functionally interacts with Dvl (Simons et al., 2005). Inversin has been proposed as a Wnt pathway switch, supporting noncanonical signaling while repressing canonical signaling. Whether this function is active during kidney development and contributes to the defects seen in mutant kidneys is still under debate. Analysis of the expression of a Lef/Tcf/β-catenin reporter in Inv mutants did not show any changes (Sugiyama, Tsukiyama, Yamaguchi, & Yokoyama, 2011). However, whether these reporters truly report all Wnt activity is still not clear. A more detailed analysis of cellular phenotypes or genetic studies would certainly shed light onto this question.
4.2.2 Tubule elongation
4.2.2.1 Vangl2
A possible direct role for PCP signaling in renal tubule morphogenesis comes from examination of the Lp mice. Renal medulla formation is defective in both heterozygous and homozygous mutants (Yates et al., 2010; Q. Ren and J. Yu unpublished observations).
In the developing renal medulla, Vangl2 is expressed at modest levels in both the ureteric bud epithelium and the interstitium and weakly in the loops-of-Henle (J. Yu, unpublished observations). The nature of the Lp mutation means that Vangl2 activity is compromised in all these cell types in Lp mutants. Thus, it is still unknown whether Wnt/PCP signaling is required cell autonomously within the renal tubules or within the interstitium. This question of where the PCP pathway is active and functioning needs to be addressed with cell type-specific ablation of Vangl2 or other Wnt/PCP signaling components. Interestingly, contrary to what is observed in flies, Vangl2 protein does not show obvious planar-polarized localization in any cell types in the kidney (T. J. Carroll & J. Yu, unpublished observations).
Interestingly, the presence of a single Lp allele on the Fat4 homozygous mutant background leads to an enhancement of tubular dilation (Saburi et al., 2012). Interpretation of this result is difficult. The simplest explanation is that both genes are regulating PCP but they function in separate, parallel pathways. Although reduction of Vangl2 activity alone does not lead to significant tubular dilation or cyst formation, its function is accentuated in the context of Fat4 loss. Once again, it will be interesting to assess PCP including cellular orientation in both individual and compound mutants as well as assessing the specific cell types in which these two gene products function.
4.2.2.2 Fz3
Fz3 has been implicated in PCP in other mouse organ systems. Fz3 expression levels were increased in cysts from human ADPKD kidneys as well as Pkd1 mutant kidneys, suggesting it may be involved in cyst progression (Luyten et al., 2010). However, no single or compound mutation of Frizzleds has been reported to result in cyst formation. Further, Fz3 levels were unchanged in precystic Pkd1 mutant tubules (Luyten et al., 2010), suggesting that, though it may be linked to cyst expansion, it is unlikely to cause the disease.
4.2.3 Glomerulogenesis
4.2.3.1 Vangl2
Planar polarity phenomenon has not been defined in renal corpuscles, but it is reported that its development was disrupted in Lp homozygotes by the criteria of the number of capillary lumens per glomerular tuft and glomerular tuft diameter at E18.5 (Yates et al., 2010). Only half of the Lp homozygous mutant renal corpuscles contain two capillary lumens, and a small fraction of glomeruli contain distorted capillary tufts, which are never observed in wild-type kidneys. The crenellated pattern of actin filaments in podocytes is less prominent in Lp mutants (Yates et al., 2010). Knockdown of Vangl2 in podocyte cells also leads to decreased number of cell projections, stress fibers and cell motility (Babayeva et al., 2011). This suggests that tissue polarity may be at play in some aspects of renal corpuscle maturation.
4.3. The Wnt pathway
4.3.1 Wnt7b
Wnt7b is expressed in the nonbranching/stalk region of the collecting ducts during the embryonic period (Yu et al., 2009). Ablation of Wnt7b results in a failure to form a renal medulla (Yu et al., 2009). Prospective medullary collecting ducts fail to elongate and instead become dilated. Orientation of cell division in this segment of a wild-type ureteric epithelium is biased toward longitudinal axis of the ducts, though the trend is not as tight as in postnatal collecting ducts. In mutants, the orientation of cell division tends to be perpendicular to the longitudinal axis of the duct (Fig. 8.3). This suggests that Wnt7b regulates oriented cell division and that this process is involved in medullary collecting duct elongation and renal medulla formation.
Figure 8.3.
Orientation of cell division in the prospective medullary collecting duct cells is disrupted in Wnt7b mutants. The cell division plane (red line in the schematic drawing) tends to bias toward perpendicular to the longitudinal axis of the prospective medullary collecting ducts (the arrow in the schematic drawing) in wild-type kidney, which favors elongation of the collecting ducts. In contrast, the plane of cell division tends to be parallel to the longitudinal axis of the prospective medullary collecting ducts in Wnt7b mutant kidneys, which favors an increase in the diameter of collecting ducts.
As mentioned, oriented cell division depends on PCP. Wnt7b has been shown to be capable of activating both canonical and noncanonical signaling in different cellular contexts (Tu et al., 2007). During kidney development, Wnt7b activates a β-catenin-dependent transcriptional program within the medullary interstitium. Interestingly, Wnt7b/β-catenin targets include Wnt11, Wnt4, and to a lesser extent, Wnt5a mRNAs (Yu et al., 2009). All three of these Wnts are thought to primarily signal through the noncanonical pathway. Thus, it appears that the regulation of PCP by Wnt7b within the context of the kidney collecting ducts may be indirect. A reasonable model based on the data is that Wnt7b, signaling through β-catenin, activates the expression of noncanonical ligands within the interstitium that signal back to the collecting ducts to regulate PCP and oriented cell division, thus driving medullary collecting duct extension.
4.3.2 Wnt9b
Like Wnt7b, Wnt9b is also expressed in the embryonic collecting ducts although Wnt9b expression extends more toward the branching tips. Mice carrying a germline null mutation of Wnt9b do not form renal vesicles or maintain their cap mesenchyme (Carroll, Park, Hayashi, Majumdar, & McMahon, 2005). However, mice that have had Wnt9b deleted in the nonbranching/stalk portion of the collecting ducts or mice carrying a hypomorphic allele of Wnt9b develop cysts (Karner et al., 2009). Interestingly, even though Wnt9b is expressed in the collecting ducts, cysts form in all nephron segments. In fact, cysts are first apparent in the proximal tubules.
From the earliest stages of development examined (E13.5 onward), Wnt9b mutants show a significant increase in the number of cells making up the cross-sectional circumference of the collecting ducts and proximal tubules compared to wild-type littermates (Karner et al., 2009). As mentioned, at these early stages, cell division is random and Wnt9b mutants have no significant change in the orientation of their mitotic spindles, suggesting that the cause of the tubular dilation in these mutants is defective cell movement/CE. Indeed, although the epithelial cells of mutants are elongated similar to what is seen in wild-type tubules, their orientation relative to the proximal–distal axis becomes randomized (Karner et al., 2009) (Fig. 8.4). This sort of defect in cell orientation would be predicted to lead to defects in mediolateral intercalation (if such a process is occurring), which could contribute to tubule dilation.
Figure 8.4.
Wnt9b mutants show defects in PCP. Wild-type (A and C) and Wnt9bneo/neo kidneys from E15.5 (A and B) and P30 kidneys (C and D). (A and B) Outlines of cells viewed from the apical side. The majority of wild-type cells are elongated, and their long axis lies between 45° and 90° relative to the proximal/distal axis of the tubule. This orientation becomes randomized in Wnt9b mutants. (A′ and B′) Outlines of the cells from (A) and (B) with their long axes indicated by arrows. Cells lying between 0° and 45° are black, while 45–90° are in white. (C and D) H and E sections of postnatal day 30 kidneys. Note that Wnt9b mutants are cystic.
Around the time of birth, cell division becomes tightly oriented throughout the collecting duct system (excluding the still branching UB tips). Interestingly, when noncystic collecting ducts were examined in postnatal Wnt9b mutants, it was found that the orientation of cell division was randomized (Karner et al., 2009). If the orientation of cell division is controlled in part by cellular orientation, this makes sense and the same cellular defect could be affecting both CE movements and oriented cell division in Wnt9b mutants. However, it is possible that Wnt9b regulates multiple aspects of PCP that regulate distinct cellular processes.
Although it has been shown that Wnt9b signals through β-catenin to the mesenchyme during the initial stages of mesenchymal-to-epithelial transition (Carroll et al., 2005; Karner et al., 2011), the target cell and signal transduction cascade used during tubule morphogenesis are still not clear. Wnt9b could be directly affecting PCP within the epithelia or it could be acting through the interstitium as Wnt7b does. Although Karner et al. (2009) found no defects in the expression of β-catenin targets in Wnt9b mutants, most of these are expressed in the epithelia. As the target cell of Wnt9b is unknown, the utility of epithelial targets is unknown. The authors did find decreased levels of GTP-bound (active) Rho and phosphorylated Jnk (targets of noncanonical Wnt signaling) in mutants, although these effects could also be indirect. Clearly, more work needs to be done in this field.
4.3.3 Wnt11
Wnt11 is traditionally thought of as a “noncanonical” Wnt although it clearly can activate the canonical pathway in some contexts (indeed, Wnt11 seems to be “the canonical Wnt” during Xenopus axis induction) (Tao et al., 2005). Although Wnt11 is expressed at the tips of the ureteric bud and within the medullary interstitium, it is not capable of inducing tubule formation in isolated mesenchyme, a process thought to be primarily mediated by β-catenin (Kispert, Vainio, & McMahon, 1998). This could indicate that Wnt11 signals noncanonically in the kidney or it does not signal to the cap mesenchyme. In cell culture, Wnt11 can signal through Fz4/8 receptors, which are normally expressed in the ureteric bud epithelium, and activate both canonical and noncanonical Wnt pathways (Ye et al., 2011), suggesting that Wnt11 acts cell autonomously on the ureteric bud epithelium. It remains to be determined which Wnt pathway Wnt11 activates in the UB cells.
Wnt11 mutants have mild branching defects similar to what is observed in Fat4, Dachs1, and Vangl2 mutants. Thus, it is tempting to speculate that Wnt11 works in the same pathway as one or more of these factors to regulate PCP. However, it is not clear how similar the defects viewed in these mutants are. Further, it is not clear how noncanonical Wnt signaling relates to the Fat/Ds or core pathways. In fact, the three pathways may function in parallel. However, Gao et al. recently showed that Wnt5a, signaling through the noncanonical receptor Ror2, mediated the phosphorylation and activation of Vangl2, thus demonstrating a direct connection between non-canonical Wnt and “core” PCP signaling (Gao et al., 2011). The authors suggested that the Vangl2 activity gradient was the result of a Wnt5a gradient and this process contributed to PCP. Ror2 is expressed at high levels in the developing kidney although kidney defects have not been described in Ror2 mutants (Al-Shawi, Ashton, Underwood, & Simons, 2001; Matsuda et al., 2001). It would be interesting to determine if a Wnt/Ror2/Vangl2 pathway is active in the kidney.
4.4. The primary cilium
Polycystic kidney disease (PKD) is a common genetic disorder characterized by cyst formation and overgrowth of the kidneys. Mutations in numerous genes can cause PKD. One commonality that connects several of the gene products is that they appear to be necessary for the formation and/or function of the primary cilia. This has led to PKD frequently being referred to as a ciliopathy (Hildebrandt et al., 2009; Patel et al., 2009; Sattar & Gleeson, 2011; Sharma et al., 2008; Waters & Beales, 2011).
Over the past several years, several reports have indicated that defects in PCP may contribute to PKD. Specifically, randomized orientation of cell division was observed prior to overt tubular dilation in ciliary mutants (Fischer et al., 2006). These data strongly suggest that the orientation in which a cell divides contributes to the formation of a cyst. However, misoriented cell division alone cannot be causal as most of the cell division that occurs in the embryo is randomly oriented and genetic lesions have been identified that lead to randomized cell division in tubules without cyst formation (Karner et al., 2009; Nishio et al., 2010). Indeed, if mechanisms for regulated cell movement and oriented cell division are both present in a growing tubule, it would seem that defects in both processes would have to occur to increase diameter.
As mentioned above, there is some evidence that the cilia can control PCP perhaps through regulating Wnt pathway usage. Thus, a simple model would be that loss of the cilia or ciliary signaling contributes to PKD in part by disrupting PCP, which then leads to defects in oriented cell division and CE/directed cell movements. However, other than analyzing the orientation of cell division and Wnt pathway activation, no other examination of PCP has been reported in ciliary mutants. It will be of great interest to see how ciliary mutants regulate cell elongation and orientation and otherwise compare to mice lacking PCP determinants. It will also be interesting to determine how defects in the core and Fat/Ds pathways affect ciliary signaling.
5. PCP AND TUBULAR REPAIR
Much of the discussion to this point has concerned the role of PCP in kidney development. A question arises as to the relevance of PCP to kidney diseases, such as autosomal dominant PKD, that do not appear to be congenital disorders. Recent studies suggest that tubular injury may be required for cysts to form (which may explain the high level of pathological heterogeneity in family members carrying the same mutation) (Happe et al., 2009; Patel et al., 2008; Takakura et al., 2009; Weimbs, 2006). After a cell dies within an epithelium, it must quickly be replaced without compromising the epithelial barrier function. This appears to be taken care of by a combination of oriented cell division and directed cell movement and/or junctional remodeling. How do cells know in which direction to move or divide in response to injury? In flies, members of both the core and the Fat/Ds PCP pathways are required (Li, Han, & Xi, 2010; Li, Zepeda-Orozco, et al., 2009). Growing evidence suggests that these processes may also be involved in proper response to injury in the kidney.
Patel et al. (2008) showed that injury of adult kidneys lacking cilia resulted in cyst formation. Further, they showed that, although cell division was oriented in wild-type tubules after injury, it was randomized in the ciliary mutants prior to overt signs of dilation or cystogenesis. Similar results have been reported in other mutant backgrounds (Bonnet et al., 2009; Fischer et al., 2006; Happe et al., 2009; Li, Kale, & Baker, 2009; Saburi et al., 2008; Simons & Walz, 2006; Sugiyama et al., 2011). However, Nishio et al. (2010) have found that defects in oriented cell division are neither necessary nor sufficient to cause cysts supporting the model that defective cell movement may also be involved.
Are the known PCP determinants involved in recovery from kidney injury? Li, Kale, et al. (2009) found that subcellular localization and levels of expression of Fz3, a Fz shown to mediate Wnt/PCP signaling, are altered in tubules with misoriented cell division following urinary tract obstruction, supporting an involvement of this pathway in epithelial repair. However, conclusive evidence of the role of PCP in repair will require assaying injured kidneys that have had PCP determinants mutated after development is complete.
6. CONCLUSION
PCP is currently a hot topic in the field of kidney research. However, as frequently happens in hot fields, some of the less exciting, descriptive work is forsaken in the attempt to be the first to publish. There are still numerous, basic questions about PCP in the kidney that have not been (but in our opinion should be) answered. Although there is increasing evidence that PCP plays an essential role in kidney development and disease, we still do not know what cellular processes it is controlling. Is it controlling cell elongation, orientation, movement, division, or all of the above? Are different PCP pathways affecting distinct cellular processes? Where does the signal that establishes the direction of PCP come from? When is it established? Does it depend on A/P patterning of the tubules? Given the expression of numerous PCP determinants in the interstitial fibroblasts, what is the role of this cell type in PCP? How do all the factors that affect PCP (especially, the cystogenic factors) relate to one another and the cellular processes regulated during PCP? Polarized, subcellular localization of many PCP determinants appears to be essential for their function and for PCP in flies and other vertebrate organ systems, but such a localization pattern has so far not been observed for any PCP proteins examined in renal tubules (Luyten et al., 2010; T. J. Carroll & J. Yu, unpublished observations). Is this aspect of PCP conserved, and if not, how is PCP established in the kidney? We speak of CE or directed cell movements regulating diameter, but there is actually no evidence that these movements occur and, if they do, what they look like. Although the process of directed intercalation/CE is perfectly plausible for a flat tissue, it does not work in a tubule as intercalation of cells toward the top of a tubule would lead to convergence and extension on the top side but divergence and retraction on the bottom. Clearly, a more complicated process must be involved.
There are still many questions to be answered in this exciting, growing field. Kidney researchers can take some solace in the fact that fly geneticists have been studying this question for decades and there are still many unanswered questions. Over the next several years, it is certain that the number of processes regulated by PCP will continue to grow and this area of investigation will expand our knowledge of kidney development and maintenance.
REFERENCES
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Development Genes and Evolution. 2001;211:161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of dachsous-fat planar cell polarity. Current Biology. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes & Development. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, McNeill H. Coupling planar cell polarity signaling to morphogenesis. The Scientific World Journal. 2002;2:434–454. doi: 10.1100/tsw.2002.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility and nephrin distribution in podocytes. Am. J. Physiol. Renal Physiol. 2011;300:F549–560. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bayly R, Axelrod JD. Pointing in the right direction: New developments in the field of planar cell polarity. Nature Reviews. Genetics. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Developmental Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Bonnet CS, Aldred M, von Ruhland C, Harris R, Sandford R, Cheadle JP. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Human Molecular Genetics. 2009;18:2166–2176. doi: 10.1093/hmg/ddp149. [DOI] [PubMed] [Google Scholar]
- Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, et al. Mechanical control of morphogenesis by fat/dachsous/four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Developmental Biology. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Current Biology. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Current Biology. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechling T, Boutros M. Wnt signaling signaling at and above the receptor level. Current Topics in Developmental Biology. 2011;97:21–53. doi: 10.1016/B978-0-12-385975-4.00008-5. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: Complexity at the surface. Journal of Cell Science. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, et al. Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development. 2009;136:383–392. doi: 10.1242/dev.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Das A. Planar cell polarity in kidney development and disease. Organogenesis. 2011;7:180–190. doi: 10.4161/org.7.3.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Developmental Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: A time-lapse analysis. Development. 1998;125:983–994. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Developmental Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. The EMBO Journal. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nature Genetics. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- Ganner A, Lienkamp S, Schafer T, Romaker D, Wegierski T, Park TJ, et al. Regulation of ciliary polarity by the APC/C. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17799–17804. doi: 10.1073/pnas.0909465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Developmental Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Developmental Biology. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Takeshita H, Mizumoto K, Sawa H. Wnt signals can function as positional cues in establishing cell polarity. Developmental Cell. 2006;10:391–396. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Developmental Biology. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: Coordinating morphogenetic cell behaviors with embryonic polarity. Developmental Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nature Cell Biology. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes & Development. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe H, Leonhard WN, Van der Wal A, Van de Water B, Lantinga-van Leeuwen IS, Breuning MH, et al. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Human Molecular Genetics. 2009;18:2532–2542. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: Disease mechanisms of a ciliopathy. Journal of the American Society of Nephrology. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Nagai T, Tanemura M. Two different mechanisms of planar cell intercalation leading to tissue elongation. Developmental Dynamics. 2008;237:1826–1836. doi: 10.1002/dvdy.21609. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American Journal of Pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. The EMBO Journal. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AC, Denholm B, Skaer H, Affolter M. Renal tubule development in Drosophila: A closer look at the cellular level. Journal of the American Society of Nephrology. 2005;16:322–328. doi: 10.1681/ASN.2004090729. [DOI] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nature Genetics. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: One or two pathways? Nature Reviews. Genetics. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han Y, Xi R. Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Genes & Development. 2010;24:933–946. doi: 10.1101/gad.1901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kale A, Baker NE. Oriented cell division as a response to cell death and cell competition. Current Biology. 2009;19:1821–1826. doi: 10.1016/j.cub.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zepeda-Orozco D, Patel V, Truong P, Karner CM, Carroll TJ, et al. Aberrant planar cell polarity induced by urinary tract obstruction. American Journal of Physiology. Renal Physiology. 2009;297:F1526–F1533. doi: 10.1152/ajprenal.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, et al. Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. Journal of the American Society of Nephrology. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Current Biology. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Developmental Biology. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development. 2012;139:1498–1508. doi: 10.1242/dev.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, et al. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mechanisms of Development. 2001;105:153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, et al. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes & Development. 2005;19:164–175. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung SM, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7:165–179. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biology. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, et al. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nature Genetics. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- Nikolaidou KK, Barrett K. Getting to know your neighbours; a new mechanism for cell intercalation. Trends in Genetics. 2005;21:70–73. doi: 10.1016/j.tig.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, et al. Loss of oriented cell division does not initiate cyst formation. Journal of the American Society of Nephrology. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nature Genetics. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Laboratory Investigation. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature Genetics. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Current Opinion in Nephrology and Hypertension. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Human Molecular Genetics. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circulation Research. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nature Genetics. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Goodrich L, McNeill H. Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development. 2012;139:1806–1820. doi: 10.1242/dev.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S, Gleeson JG. The ciliopathies in neuronal development: A clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Developmental Medicine and Child Neurology. 2011;53:793–798. doi: 10.1111/j.1469-8749.2011.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes & Development. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Current Topics in Developmental Biology. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Current Biology. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development. 2008;135:4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat: Dachsous binding by the cadherin domain kinase four-jointed. Current Biology. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Walz G. Polycystic kidney disease: Cell division without a c(l)ue? Kidney International. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Current Opinion in Cell Biology. 2010;22:589–596. doi: 10.1016/j.ceb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harbor Perspectives in Biology. 2009;1:a000489. doi: 10.1101/cshperspect.a000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney International. 2011;79:957–965. doi: 10.1038/ki.2010.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: Regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Human Molecular Genetics. 2009;18:2523–2531. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Current Biology. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Developmental Dynamics. 2012;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- Torban E, Kor C, Gros P. Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates. Trends in Genetics. 2004;20:570–577. doi: 10.1016/j.tig.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Seminars in Cell & Developmental Biology. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Developmental Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–5384. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Current Biology. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Developmental Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Mitchell B. Strange as it may seem: The many links between Wnt signaling, planar cell polarity, and cilia. Genes & Development. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nature Genetics. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: An expanding disease spectrum. Pediatric Nephrology. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, et al. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Weimbs T. Regulation of mTOR by polycystin-1: Is polycystic kidney disease a case of futile repair? Cell Cycle. 2006;5:2425–2429. doi: 10.4161/cc.5.21.3408. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. Cell adhesion in amphibian gastrulation. International Review of Cell and Molecular Biology. 2009;278:215–275. doi: 10.1016/S1937-6448(09)78005-0. [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Blum A, Novak T, Levinson R, Lai E, Barasch J. An epithelial precursor is regulated by the ureteric bud and by the renal stroma. Developmental Biology. 2002;246:296–310. doi: 10.1006/dbio.2002.0646. [DOI] [PubMed] [Google Scholar]
- Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Human Molecular Genetics. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138:1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Seminars in Cell & Developmental Biology. 2008;19:263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]