Abstract
Allergy diagnostics is being transformed by the advent of in vitro IgE testing using purified allergen molecules, combined with multiplex technology and biosensors, to deliver discriminating, sensitive, and high-throughput molecular diagnostics at the point of care. Essential elements of IgE molecular diagnostics are purified natural or recombinant allergens with defined purity and IgE reactivity, planar or bead-based multiplex systems to enable IgE to multiple allergens to be measured simultaneously, and, most recently, nanotechnology-based biosensors that facilitate rapid reaction rates and delivery of test results via mobile devices. Molecular diagnostics relies on measurement of IgE to purified allergens, the “active ingredients” of allergenic extracts. Typically, this involves measuring IgE to multiple allergens which is facilitated by multiplex technology and biosensors. The technology differentiates between clinically significant cross-reactive allergens (which could not be deduced by conventional IgE assays using allergenic extracts) and provides better diagnostic outcomes. Purified allergens are manufactured under good laboratory practice and validated using protein chemistry, mass spectrometry, and IgE antibody binding. Recently, multiple allergens (from dog) were expressed as a single molecule with high diagnostic efficacy. Challenges faced by molecular allergy diagnostic companies include generation of large panels of purified allergens with known diagnostic efficacy, access to flexible and robust array or sensor technology, and, importantly, access to well-defined serum panels form allergic patients for product development and validation. Innovations in IgE molecular diagnostics are rapidly being brought to market and will strengthen allergy testing at the point of care.
Keywords: Allergens, Allergy diagnostics, IgE testing, Molecular diagnostics, Component-resolved diagnostics
Introduction
In a comprehensive primer of human IgE antibody serology published in 2010, Hamilton and Williams succinctly made the point that the chemistry of in vitro IgE antibody assays had remained “essentially unchanged” since 1972 [1, 2]. Allergy diagnostics (in this context only referring to in vitro testing) is dominated by three test systems of similar specificity and sensitivity that are used in laboratories and clinics worldwide. All three systems are FDA-approved laboratory-based methods, using heterogeneous allergenic extracts for IgE antibody binding and enzyme-labeled anti-IgE for detection. The ImmunoCAP® system, the market leader developed by Phadia and now owned by Thermo Scientific, uses allergen extracts coupled to a high-capacity cellulose-activated particle (CAP) in a fluorescent enzyme immunoassay (FEIA). Large quantities of allergen can be directly coupled to the ImmunoCAP, creating a level of allergen excess which reduces potential interference by IgG antibodies. The ImmunoCAP has sufficient allergen capacity that it can also be used for IgG4 and IgA antibody measurements. The other market leaders are Siemens IMMULITE systems using “3gAllergy” liquid allergens and the HYCOR Biomedical HYTEC-288, an activated paper disc technology. Allergen extracts used for the IMMULITE are biotinylated and use streptavidin-coated beads to form the solid phase with chemiluminescent signal detection.
As noted by Hamilton and Williams, these IgE antibody assays have performance characteristics with coefficients of variation of <15 % [2]. However, results from the three assays are not interchangeable. Results of the Diagnostic Allergy SE Survey performed by the College of American Pathologists in 2009 showed twofold or greater differences in specific IgE measurements for a panel of common allergens using the three assays [2]. In practice, advice to physicians is to consistently select one of the three tests for allergy diagnostic purposes. Further limitations of the “big three” are that separate tests are needed for each allergen, which is time consuming and uses large amounts of serum. The quality of the tests depends on the allergen source material used on the solid phase, the coupling technology, and the IgE antibody standards used for quantification. Finally, these systems are all laboratory based and require substantial infrastructure that is only available in hospitals, medical centers, and commercial diagnostic laboratories. As such, they do not address the needs of allergic patients at the point of care.
Allergy diagnostics manufacturers have been slow to innovate and introduce new technologies. In part, this may reflect a strong market position and a lack of incentive to move the industry forward. Over the past 5 years, the situation has begun to change and there is now strong impetus and excitement about new technologies based on molecular diagnostics. Measurements of IgE antibodies to specific allergen molecules are more discriminating than extract-based tests and can provide valuable diagnostic information. The shift to molecular diagnostics involves radically different assay platforms incorporating multiplex technologies, new chemistries for allergen coupling, and innovative devices that can provide test results in 15 min—about the time it takes to perform a skin test in an allergist’s office. This chapter will highlight promising new technologies for allergy molecular diagnostics. Critical elements that are necessary to successfully develop these technologies into tools that benefit allergic patients will also be discussed.
Molecular Diagnostics
A subset of patients who are allergic to birch pollen develop oral allergy symptoms when eating apples, pears, or other soft fruits. Some birch pollen-allergic patients may also show positive skin tests or in vitro IgE antibody tests to peanut but have few symptoms and are at low risk of adverse reactions upon eating peanuts. The causes of these reactions were revealed following the cloning, sequencing, and expression of recombinant allergens over the past 20 years which has enabled the molecular basis of allergenic cross-reactivity to be deciphered and the individual allergens responsible for these reactions to be identified. Birch pollen-allergic patients make IgE antibody responses to specific allergens (Bet v 1 and Bet v 2) that also occur as structural homologues in fruits and legumes. Birch-allergic patients who make IgE to Bet v 1 and Bet v 2 also react to Ara h 8 and Ara h 5, respectively, but are not truly peanut allergic and are less likely to have symptoms on eating peanuts [3]. Another important aspect of molecular diagnostics is that some allergens, notably Ara h 2 from peanut, have higher predictive value for diagnosis than natural allergen extracts [4]. The molecular biology and clinical significance of allergenic cross-reactivity is covered by other chapters in this series of Current Allergy and Asthma Reports.
The challenge with molecular diagnostics for allergy is that most allergen sources comprise multiple specific allergen molecules [5, 6]. Measurement of IgE to multiple allergens, typically 2–10 purified allergens, is required for optimal diagnostic efficacy. This can be achieved on the ImmunoCAP, either by coupling purified allergens directly to the CAP or by using biotinylated allergens in the streptavidin CAP [7]. An extensive range of purified allergen CAPs is commercially available. Streptavidin CAP can be used with any purified allergen that can be biotinylated and offers more flexibility, especially for research studies. One drawback for use of CAP technology is that each test requires ~50 μl of serum, which can be a problem for multi-allergen testing in pediatric patients.
The ability to measure IgE to a large number of allergen molecules using a small volume of serum is best resolved using multiplex technologies. The first approach, developed by Hiller and colleagues, used allergens that were chemically coupled as a microarray on a glass slide, with four serum samples tested per slide using a chemiluminescent detection system [8]. The technology was initially developed by VBC Genomics and has been commercialized by Thermo Scientific as ImmunoCAP Immuno Solid-phase Allergen Chip (ISAC). The chip has an array of 112 purified natural or recombinant allergens which can be tested using ~30 μl of serum/array [9]. The ISAC platform has been in use for almost 10 years and has been instrumental in opening up the field of molecular diagnostics [9]. The World Allergy Organization produced a consensus document which reviewed the field of molecular-based allergy diagnostics, focusing almost exclusively on use of the ISAC, as a guide for practicing allergists [10•]. A key recommendation was the use of large population studies to assess the practical importance of molecular diagnostics. Since the publication of the WAO report, another microarray chip system manufactured by Microtest (London, UK) has been launched. The MicrotestDx system is fully automated, measures IgE antibodies to 26 allergens using 100 μl of serum, and can process five serum samples simultaneously (see: www.microtestdx.com). Further development of the ISAC has been initiated by the European Union-funded project, Mechanisms for the Development of Allergies (MeDALL) (http://medall-fp7.eu/). The MeDALL program has greatly expanded the number of allergens on the chip, including additional peanut, tree nut, milk, wheat, dust mite, olive pollen, and insect venom allergens, as well as Staphylococcus aureus enterotoxins. This “super-array” is reported to measure IgE and IgG antibodies to 170 allergen molecules [11].
Next-Generation Innovations
Several critical elements are required to assemble fast, reliable, and sensitive molecular diagnostics: highly purified allergens of known diagnostic efficacy, flexible and robust microarray technology, well-defined serum panels from allergic individuals, and diagnostic platforms that offer versatility and ease of use in both laboratory and point-of-care settings. Let us consider these in more detail:
Purified Allergens
Purified natural or recombinant allergens can be used for molecular diagnostics. Typically, natural allergens are purified using ion-exchange, size exclusion, or hydrophobic interaction separation techniques on HPLC and by affinity chromatography using monoclonal antibodies. With natural allergens, it is important to establish the degree of purity (ideally, >95 %) and the extent of trace contamination with other allergens. Obviously, contamination with other allergens is not a problem for recombinant allergens. These are routinely expressed in Escherichia coli or Pichia pastoris (recommended because of its high yield and constitutive or inducible expression of allergen into the culture medium via pGAP or pPICZ vectors, respectively) [12]. Other advantages of recombinant allergens are the ability to engineer the sequence to remove or modify glycosylation or enzyme cleavage sites. The ability to add histidine tags or other residues or sequences that aid in purification or protein expression is also an advantage. Great strides have been made in allergen molecular biology, allergen manufacturing, quality control, and standardization in recent years. The three-dimensional structures and function of most major allergens are known. Assessments of purity and composition of allergens have been strengthened by the increasing use of mass spectrometry (MS) which can identify allergen isoforms in complex mixtures [13–15]. In our group, MS is now routinely used to determine purity (in addition to immunoassay) (Table 1, [16]).
Table 1.
Mass spectrometry assessments of purity of natural and recombinant allergens
| Allergen | Sequence coverage (%) | Relative abundance (NASF) (%) |
|---|---|---|
| nDer p 1 | 50 | 98.3 |
| nDer p 2 | 91 | 99.6 |
| nAra h 2 | 76 | 96.2 |
| nFel d 1 | 73 | 98.1 |
| rBet v 1 | 79 | 100 |
| rAra h 8 | 81 | 95.6 |
Normalized spectral abundance factor (NSAF) provides assessment of relative abundance based on spectral counts (SpC) normalized to protein MW and expressed as a percentage of the total [16]
Engineering of allergen sequences has resulted in a novel approach to multiplex the allergens into a single molecule, rather than expressing multiple individual allergens. Nilsson and colleagues designed a tetrameric dog allergen molecule which comprised Can f 1, Can f 2, Can f 4, and Can f 6 [17••]. Allergens expressed in the E. coli tetramer were correctly folded, independently positioned, and retained their three-dimensional structure. IgE reactivity to the allergen tetramer was comparable to a mix of the four individually expressed allergens. Natural dog allergenic extracts have highly variable allergen composition and are difficult to standardize. This novel tetramer strategy should improve in vitro diagnostics for dog allergy, especially since the individual allergens are specific for dog. The tetramer strategy does not allow IgE to individual dog allergens to be measured but could be a useful diagnostic approach for allergens which show limited cross-reactivity with other allergen sources.
International purified allergen standards have potential to aid manufacturers to produce quality products by providing yardsticks with which company products can be compared for purity and allergenic potency. The European Directorate for Quality of Medicines (EDQM) has produced standards for rBet v 1 and rPhl p 5 [18]. These standards were produced under good manufacturing practices (GMP) and are approved for use for registration of birch pollen and timothy pollen allergenic products in the European Pharmacopoeia. Production of allergens for use in in vitro diagnostics does not require that the allergens are produced under GMP. However, it is important that manufacturers of purified allergens have quality systems and good laboratory practices that are ISO certified to ensure the quality and reproducibility of allergen molecules.
Microarrays
Limitations of the ISAC are that it is a static or planar array performed on a glass slide (which has to be manually processed). The test is time consuming and requires an Affymetrix (or equivalent) array reader. Static arrays have to be redesigned when changes are made to the number of allergens on the chip. The ISAC test is a semiquantitative screen of all 112 allergen specificities with little flexibility for allergen selection, in some cases providing too much information. A recent paper cautioned against overreliance on the ISAC alone for diagnostic purposes. The array unexpectedly detected IgE to hymenoptera venom allergens in patients with no clinical history of reactions to insect stings [19•]. Using in vitro IgE test results without documenting the patient’s clinical history is contrary to current diagnostic guidelines for venom allergy and poses a dilemma for management of patients. The in vitro test results clearly need to be interpreted in the context of clinical history to provide an optimal allergy diagnosis.
Suspension arrays using allergens linked to polystyrene microbeads provide additional flexibility. The beads can be mixed in different combinations to selectively target allergens that are being investigated based on clinical history. Thus, a patient with seasonal allergic rhinitis during the pollen season could be tested for sensitization to various pollen allergens but not indoor allergens. Similarly, a patient with allergic symptoms on eating nuts need only be followed up with a food allergy panel. Small-scale suspension arrays for 12–16 allergens have been developed on the Luminex xMAP® platform and on the Becton Dickinson cytometric bead array system [20, 21]. Indoor Biotechnologies produces an IgE Quantitative Binding Array (QBA) of 11 allergens plus total IgE on the Luminex xMAP system (Fig. 1). The IgE-QBA is a beta version that has been released for research use, prior to development of a larger IgE panel for clinical diagnostic purposes.
Fig. 1.
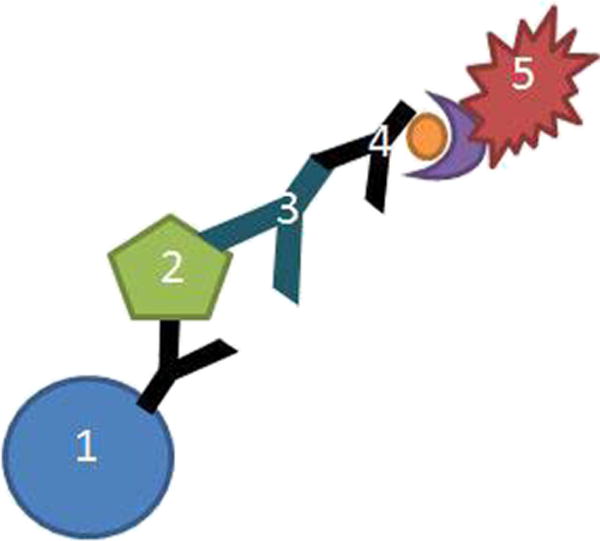
Schematic outline of Luminex xMAP-based microarray for allergen-specific IgE. Allergen (2) binds to antibody which is covalently coupled to magnetic xMAP bead sets (1). Following incubation with patients’ serum (3), bound IgE antibody is detected with biotinylated anti-IgE (4) and streptavidin phycoerythrin (5). Copyright Indoor Biotechnologies Inc., Charlottesville, VA, USA, reproduced with permission
Hydrogel biochips have recently been proposed as carriers for specific IgE measurements for both allergenic extracts and purified allergens [22•]. These three-dimensional gel-based microchips are produced using copolymerization technology which immobilizes allergen in a hydrophilic environment and preserves their native conformation. The hydrogel FEIA can be read on a portable fluorescent biochip analyzer and has been reported to measure IgE to 21 allergens. Finally, a surface plasmon resonance imaging microarray has been developed for peanut allergen, Ara h 2, which measures both peptide and carbohydrate epitopes. This method uses magnetic beads coated with anti-IgE monoclonal antibody to capture IgE, is highly sensitive (0.5 pg/ml), and can be completed in 45 min [23•]. Further studies are needed to investigate whether surface plasmon resonance technology can be used for other allergens.
Biosensors and Point-of-Care Testing
To paraphrase Mungroo and Neethirajan, a biosensor is defined as “instrumentation that comprises two key elements in close proximity: a transducing device and a recognition element with a supporting material. The recognition element consists of two affinity-pairing partners (e.g., antibody/antigen, enzyme/substrate, receptor/ligand, and an analyte that binds specifically to them), one of which is immobilized. The transducer is utilized to detect any contacts between the affinity-pairing partners by converting the biological response into useful electrical signals. A processor then transforms the electrical signal for interpretation” [24•]. Biosensors are being applied to all branches of medicine, including allergy. Biosensors offer capabilities for rapid, sensitive point-of-care diagnostics that can be integrated into “lab-on-a-chip” devices and used with electronic readers or smartphones to provide real-time measurements.
Quantum dot technology has recently been used to develop biosensors for casein-specific IgE. Allergen is immobilized onto a sensor chip using a copolymer, and the reaction with IgE antibodies is detected using dual polarization interferometry with signal enhancement using streptavidin-conjugated quantum dots. The quantum dot assay for casein-specific IgE had comparable sensitivity to ImmunoCAP [25•]. Quantum dots are one example of several types of sensor that have been reported to show promise for IgE detection but have not yet been integrated into functional devices for clinical use.
At the European Association of Allergy and Clinical Immunology (EAACI) annual meeting in 2014, a Swiss company, Abionic, introduced an innovative biosensor for allergy diagnosis—the abioSCOPE. The principle underlying the abioSCOPE is the biomolecular interaction that occurs in nanofluidic biosensors contained in capsules where the allergen IgE interaction takes place (Fig. 2a). The surface of each biosensor contains specific immobilized allergens (either from natural sources or purified allergens). Patient’s serum or blood sample containing allergen-specific immunoglobulin (IgE) is diluted with fluorescently labeled biomolecules and deposited into a capsule where it fills the biosensors inside through capillary action. Within the biosensors, allergen-specific IgE interactions form fluorescent molecular complexes that are immobilized and optically measured by the abioSCOPE reading unit, which contains a miniaturized fluorescent microscope (Fig. 2b). The fluorescent signal quantifies specific IgE antibody levels.
Fig. 2.
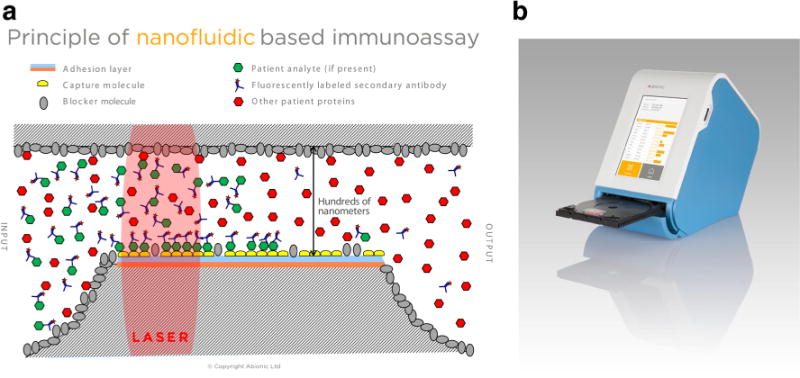
a Principle of nanofluidic immunoassay. The surfaces of the biosensor are chemically prepared and contain specific immobilized allergens. Within the biosensor, the IgE antibodies from the patient’s serum diffuse and interact with the allergens, forming fluorescent molecular complexes which are then measured optically. Due to the nanofluidic configuration, the biomolecular interaction is highly accelerated and reduces assay times to a few minutes. Copyright Abionic SA, Lausanne, Switzerland, reproduced with permission. b Operating the abioSCOPE. The patient’s blood sample is mixed with a proprietary reagent and placed into a test capsule that contains multiple biosensors capable of detecting allergen-specific IgE. The capsule is placed into a disc mounting plate that is then inserted into the abioSCOPE, as a DVD is inserted into a DVD player. The results are presented on a wireless touch screen device (e.g., iPAD) and saved onto a standard SD card. The abioSCOPE conforms to ISO 13485 and provides an intuitive and easy-handling diagnostic system for allergists. Copyright Abionic SA, Lausanne, Switzerland, reproduced with permission
Devices such as the abioSCOPE are designed to deliver specific, quantitative, and personalized information about patients’ allergic sensitization at the point of care and are intuitive in that users’ can easily read the results which are presented on a touch screen. Other approaches to patient-centered testing include lateral flow devices that can be read visually or on mobile devices. The ImmunoCAP Rapid® is designed for use in clinics or pharmacies and measures the presence of IgE to allergen extracts from whole blood within 15 min. The assay principle is the same as a pregnancy test. Allergen-specific IgE is detected using colloidal gold-labeled anti-IgE on a lateral flow device striped with 10 different allergens [26].
Challenges Faced by the Allergy Diagnostics Industry
As noted above, coupling chemistries, purified allergens, and well-defined sera from panels of allergic patients are key elements for developing molecular diagnostics in allergy. Coupling chemistries and array platforms, whether laboratory based or point of care, need to be stable, robust, and flexible enough to measure over 100 allergen specificities with accuracy, sensitivity, and precision. Offering allergists selectivity over panels of specific allergens that they need for diagnostic purposes is a plus, especially if it reduces time and cost.
Purified allergens form the core of any molecular diagnostic platform or device. Allergens should be validated for purity and for binding to IgE antibodies following the principles established in the EU CREATE project [27, 28]. Purity is primarily assessed using SDS-PAGE, amino acid composition, and mass spectrometry (Table 1). The degree of trace contamination in natural allergens should ideally be less than 1 % and can be determined either by MS and/or by immunoassay. As an in-house example, natural Der p 2 purified from spent mite culture using multi-step affinity chromatography is analyzed by MS to determine sequence coverage, abundance, and presence of non-allergen sequences. Purified nDer p 2 is analyzed by monoclonal ELISA for Der p 2 and for Der p 1. The ELISA data also confirm dose-dependent antibody binding for murine IgG antibodies. Similar approaches are used for other allergens. For example, natural peanut allergens are assessed for trace contamination using ELISA for Ara h 1, Ara h 2, and Ara h 6.
With recombinant allergens, the challenges lie in achieving high levels of expression and in establishing that the recombinant molecule represents the predominant isoform and has comparable structural, biologic, and allergenic properties to its natural counterpart. Recombinant allergens have particular advantages where the source material for natural allergen processing is in short supply (e.g., dog hair) or where the natural allergen is present at low concentration, such as Japanese cedar pollen. Cry j 1 and Cry j 2 occur at relatively low concentrations in Japanese cedar pollen and several hundred grams of pollen need to be extracted to yield a few milligrams of purified natural Cry j 1. However, production of recombinant allergens from Japanese cedar also has problems. Cry j 1 is a pectate lyase and this family of proteins, which also includes Jun a 1 from Western Mountain cedar and ragweed Amb a 1, has proved especially intransigent for recombinant allergen production [29••].
The biggest challenge faced by allergy diagnostic companies is finding panels of sera from allergic patients that can be used to verify allergenic activity. With molecular diagnostics, selection of sera requires not only patient reactivity to the allergenic extract but also reactivity to individual purified allergens, covering a broad range of IgE levels. Validation of molecular diagnostics typically requires 20–50 sera from patients with ImmunoCAP values ranging from classes 1–6 (i.e., 0.35 to >100 kU/l) for each allergen source. This means having access to hundreds of sera to adequately validate multiple allergen sources. Commercial serum banks can help supply human sera for some of the most common allergens (dust mite, cat, pollen). Otherwise, allergy diagnostic companies may need to partner with academic, government, or non-profit foundations to access allergic sera. These partnerships are increasingly constrained by concerns about privacy and about making patient samples available to for-profit companies. However, the introduction of innovative diagnostics leads to improvements in public health and patient care which benefit society as a whole. Over the past 15 years, there have been many large, government-funded population studies of the prevalence of allergy in the USA and in Europe. Many sera have been collected and tested in the course of these studies. Once the study is completed, the sera often remain unused, leaving a potentially valuable resource of well-characterized sera underutilized. Providing greater commercial access to these serum sources, under appropriate ethical guidelines, could help alleviate problems with serum resources. Another approach to this dilemma is for allergy diagnostic manufacturers to work with academic partners to commission their own studies and serum collections. However, this is time consuming and expensive and slows the pace of innovation.
Finally, another important point to consider is the development of appropriate thresholds for IgE levels in the next-generation technologies and how these compare with current IgE thresholds and measurements. With the increasing sensitivity of microarray and nanotechnologies, it will be necessary to cross-validate new technologies with gold standards for IgE detection to avoid false-positive results. The good news is that a new WHO International Standard for IgE was released in 2014 which will facilitate direct method comparisons. The new standard, WHO 11/234, was established by the WHO Expert Panel on Biological Standardization, as the 3rd International Standard for IgE, with an assigned value of 13,500 IU/ml [30]. The IgE standard is expected to last 20 years, and by then, one can anticipate that it will be replaced by recombinant human IgE antibodies!
Conclusions
The technology of in vitro IgE testing is undergoing a renaissance. New technological innovations and devices are being developed involving microarrays, biosensors, and other point-of-care tests that will provide clinicians with more sophisticated tools for allergy diagnosis. The flexibility of these testing systems will enable clinicians to take the patient clinical history and then order the specific allergen panel of choice to substantiate the diagnosis. This is personalized medicine applied to the allergy field. Although allergy diagnostic manufactures face technical, clinical, and commercial challenges in developing these new technologies, the field is advancing a pace and strong competition between these innovations can be expected as they are approved by US and EU regulatory authorities and brought to market. Technological innovations will strengthen allergy testing at the point of care and also broaden the diagnostic arena to include additional panels, e.g., biomarkers for asthma and mediators of anaphylaxis. The intuitive nature of the technology means that it can be used within a clinical practice, providing immediate feedback to doctors and their patients about allergy diagnosis.
Acknowledgments
This work is supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI077653. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to Drs. Nicolas Durand and Iwan Merki (Abionic) for the informative discussions about nanotechnology for IgE detection and for providing the images used in Fig. 2.
Footnotes
Conflict of Interest Conflict of Interest Martin D. Chapman declares that he is a founder and co-owner of Indoor Biotechnologies. Sabina Wuenschmann, Eva King, and Anna Pomés declare that they are employees of Indoor Biotechnologies.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010;125:S284–96. doi: 10.1016/j.jaci.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton RG, Williams PB. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol. 2010;126:33–8. doi: 10.1016/j.jaci.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Asarnoj A, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130:468–72. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Beyer K, et al. Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy. 2015;70:90–8. doi: 10.1111/all.12530. [DOI] [PubMed] [Google Scholar]
- 5.Chapman MD, Pomés A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119:414–20. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Thomas WR. The advent of recombinant allergens and allergen cloning. J Allergy Clin Immunol. 2011;127:855–9. doi: 10.1016/j.jaci.2010.12.1084. [DOI] [PubMed] [Google Scholar]
- 7.Erwin EA, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 8.Hiller R, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 9.Wohrl S, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006;61:633–9. doi: 10.1111/j.1398-9995.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 10•.Canonica GW, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. This paper is a thorough review by leaders of the allergy field on molecular-based allergy diagnostics, focusing on ISAC for specific IgE testing in vitro. The review describes many clinical studies that have used this technology and provides recommendations for future studies on molecular diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupinek C, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokoj S, et al. Pichia pastoris is superior to E. coli for the production of recombinant allergenic non-specific lipid-transfer proteins. Protein Expr Purif. 2010;69:68–75. doi: 10.1016/j.pep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Fenaille F, et al. Mass spectrometric investigation of molecular variability of grass pollen group 1 allergens. J Proteome Res. 2009;8:4014–27. doi: 10.1021/pr900359p. [DOI] [PubMed] [Google Scholar]
- 14.Seppala U, et al. Absolute quantification of allergens from complex mixtures: a new sensitive tool for standardization of allergen extracts for specific immunotherapy. J Proteome Res. 2011;10:2113–22. doi: 10.1021/pr101150z. [DOI] [PubMed] [Google Scholar]
- 15.Chapman MD, Briza P. Molecular approaches to allergen standardization. Curr Allergy Asthma Rep. 2012;12:478–84. doi: 10.1007/s11882-012-0282-3. [DOI] [PubMed] [Google Scholar]
- 16.Zybailov B, et al. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5:2339–47. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 17••.Nilsson OB, et al. Designing a multimer allergen for diagnosis and immunotherapy of dog allergic patients. PLoS ONE. 2014;9:e111041. doi: 10.1371/journal.pone.0111041. The study is an excellent paper on how to use recombinant DNA technology to fuse multiple allergens into one for diagnostic purposes. This is an innovative strategy which could simplify molecular diagnostics. The paper lays out a comprehensive approach to ensuring correct folding of dog allergens in the multimer and to assessing IgE reactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieths S, et al. Establishment of recombinant major allergens Bet v 1 and Phl p 5a as Ph. Eur. reference standards and validation of ELISA methods for their measurement. Results from feasibility studies. Pharmeur Bio Sci Notes. 2012;2012:118–34. [PubMed] [Google Scholar]
- 19•.Incorvaia C, et al. A pitfall to avoid when using an allergen microarray: the incidental detection of IgE to unexpected allergens. J Allergy Clin Immunol Pract. 2014 doi: 10.1016/j.jaip.2014.09.020. This rostrum article cautions against interpreting molecular diagnostic data out of context of the clinical history and brings up important questions about how false-positive IgE results should be interpreted. [DOI] [PubMed] [Google Scholar]
- 20.King EM, Vailes LD, Tsay A, Satinover SM, Chapman MD. Simultaneous detection of total and allergen-specific IgE by using purified allergens in a fluorescent multiplex array. J Allergy Clin Immunol. 2007;120:1126–31. doi: 10.1016/j.jaci.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 21.Pomponi D, et al. Allergen micro-bead array for IgE detection: a feasibility study using allergenic molecules tested on a flexible multiplex flow cytometric immunoassay. PLoS ONE. 2012;7:e35697. doi: 10.1371/journal.pone.0035697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Feyzkhanova GU, et al. Development of hydrogel biochip for in vitro allergy diagnostics. J Immunol Methods. 2014;406:51–7. doi: 10.1016/j.jim.2014.03.003. This paper presents initial data using three-dimensional hydrogel chips with 1-nl volumes to measure allergen-specific IgE. The chip results correlate with an IgE ELISA for allergen extracts, though the data on purified allergens was somewhat limited. Nonetheless, the paper is a useful application of this promising nanotechnology. [DOI] [PubMed] [Google Scholar]
- 23•.Joshi AA, Peczuh MW, Kumar CV, Rusling JF. Ultrasensitive carbohydrate-peptide SPR imaging microarray for diagnosing IgE mediated peanut allergy. Analyst. 2014;139:5728–33. doi: 10.1039/c4an01544d. This technology may have applications for measuring IgE to carbohydrate epitopes on allergens. The SPR technology is highly sensitive and fast, but it is unclear how SPR can be used in the clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Mungroo NA, Neethirajan S. Biosensors for the detection of antibiotics in poultry industry—a review. Biosensors (Basel) 2014;4:472–93. doi: 10.3390/bios4040472. The paper is a comprehensive review of biosensors which covers different biosensing techniques and applications of SPR technology in industry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Platt GW, et al. Allergen immobilisation and signal amplification by quantum dots for use in a biosensor assay of IgE in serum. Biosens Bioelectron. 2014;52:82–8. doi: 10.1016/j.bios.2013.08.019. This paper provides a detailed discussion of the merits of copolymer immobilized allergen (casein) for specific IgE detection. Streptavidin-conjugated quantum dots provide optimal signal enhancement and allow dry-form storage which is particularly useful for assay automation. [DOI] [PubMed] [Google Scholar]
- 26.Chapman MD. Lateral flow tests for allergy diagnosis: point-of-care or point of contention? Int Arch Allergy Immunol. 2010;152:301–2. doi: 10.1159/000288282. [DOI] [PubMed] [Google Scholar]
- 27.van Ree R, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–26. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 28.Chapman MD, et al. The European Union CREATE Project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008;122:882–9. doi: 10.1016/j.jaci.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 29••.Gadermaier G, Hauser M, Ferreira F. Allergens of weed pollen: an overview on recombinant and natural molecules. Methods. 2014;66:55–66. doi: 10.1016/j.ymeth.2013.06.014. The paper is an excellent and up-to-date review of allergens of weed pollen from around the world, both natural and recombinant, from the leading experts in the molecular biology of weed allergens. The review covers the molecular biology of pectate lyases, defensins, lipid transfer proteins, profilins and polcalcins: important molecules for diagnostics. [DOI] [PubMed] [Google Scholar]
- 30.Thorpe SJ, et al. The 3rd International Standard for serum IgE: international collaborative study to evaluate a candidate preparation. Clin Chem Lab Med. 2014;52:1283–9. doi: 10.1515/cclm-2014-0243. [DOI] [PubMed] [Google Scholar]


