Abstract
During exercise the renin–angiotensin system is stimulated. We hypothesized that the increase in serum angiotensin II (AngII) levels after exercise is dependent on exercise intensity and duration and secondly that people with the ACE-II genotype will show a higher increase in AngII serum levels. We also assumed that perfusion of upper limbs is transiently reduced with maximal cycling exercise and that subjects with the ACE-II compared to the ACE-ID/DD genotype will have a higher capillary perfusion due to lower AngII levels. Ten healthy subjects completed a maximal exercise test, a 12-min exercise test at ventilatory threshold and a 3-min test at the respiratory compensation point. AngII serum levels and capillary recruitment of the skin in the third finger were measured before and after exercise and breath-by-breath gas exchange during exercise was assessed. Baseline levels of AngII levels were lower prior to the 3-min test which took place on average 5 days after the last exercise. A two-fold increase compared to baseline levels was found for AngII only immediately after the 3-min test and not after the maximal exercise test and 12-min of exercise. Subjects without the I allele showed a decrease in AngII values after the maximal test in contrast to subjects with the ACE-II/ID genotype. Subjects with the ACE-II genotype had a 1.8 times significant higher capillary perfusion in the finger after exercise. A trend was observed for a 34.3% decreased capillary recruitment in the ACE-ID/DD genotype after exercise. We conclude that the rise in AngII after exercise is intensity dependent and that variability in serum AngII and capillary perfusion is related to the ACE I/D polymorphism.
1. Introduction
Angiotensin II (AngII) elevates arterial pressure because of its vasoconstrictor action and restricts blood from entering non-active muscles thereby directing metabolic substrates to active tissues with enhanced energy turnover (Andersen and Saltin (1985)). This mechanism helps to redirect blood to active muscles which work depends on the substrate supply (Secher et al. (1977)). The AngII-mediated vasoconstriction is overridden with the onset of muscle contraction through flow-induced dilatation of conduit arteries and arterioles and promotes angiogenesis by activating the endothelial cell population that constitutes the capillary wall (Brothers et al. (2006); Hahn et al. (1995)). Different studies support the notion that a switch in AngII action from arterioles to the endothelium of the perfused vessel lumen facilitates exercise-induced capillary growth in human skeletal muscle (Petersen and Greene (2007); Staessen et al. (1987)). There is evidence that the role of AngII in stimulating angiogenesis relies on capillary perfusion (Petersen and Greene (2009)).
During cycling exercise, the renin–angiotensin system is stimulated (Staessen et al. (1987)). Different studies show an increase in AngII after high intensity exercise, indicating that organs which cannot overcome AngII mediated vasoconstriction are decreasingly perfused (Fagard et al. (1985); Kosunen and Pakarinen (1976); Staessen et al. (1987); Aldigier. et al. (1993)). Limited studies investigated AngII levels related to exercise intensity and duration, but it seems that there is an increase in AngII at an intensity above the anaerobic threshold (Maher et al. (1975), Staessen et al. (1987), Miura et al. (1994)).
Human subjects carrying the D-allele of the ACE I/D gene polymorphisms (i.e., ACE-ID and ACE-DD genotypes) have respectively approximately 30% and 60% higher serum and tissue ACE activity and more serum AngI is converted via ACE to AngII than in subjects with the ACE-II genotype. However, only less than 20% of the variability in circulating ACE activity can be explained by the ACE I/D polymorphism (Danser et al. (2007)). Subjects with homozygous (ACE-II) or heterozygous (ACE-ID) for the ‘I-allele’ show reduced capillarity at rest but amplified mitochondrial biogenesis after bicycle-type endurance exercise and training, suggesting a critical role of ACE-modulated vascular tone in the regulation of the response to exercise (Defoor et al (2006); Vaughan et al. (2013)).
We aimed to detect changes in human AngII levels after exercises at different intensities. We hypothesized that the increase in serum AngII after exercise is dependent on the exercise intensity and the exercise duration. We also hypothesized that people with the ACE-II genotype will show a higher increase in AngII serum levels after cycling exercise due to their lower AngII resting values (Vaughan et al. (2013)). In order to establish the suggested association between serum AngII and capillary perfusion with exercise (Petersen and Greene (2007)), we tested whether serum AngII levels relate to exercise intensity and capillary perfusion in skin, in a body compartment being accessible during exercise, and whether this depends on the ACE I/D genotype. We assumed that perfusion of the finger is reduced with maximal cycling exercise and that subjects with the ACE-II genotype will have a higher capillary perfusion as they have less increased capillary density and a genetically reduced potential for vasoconstriction (Vaughan et al. (2013); Brothers et al. (2006)). Consequently, the relative change in perfusion and exercise-induced AngII levels in serum will be less pronounced than in D-genotypes for which vasoconstriction is overridden to a larger extent.
2. Methods
On three separate occasions, ten healthy young subjects performed three exercise tests on three separate occasions at the same time of the day on an electronically braked cycle ergometer (Excalibur Sport, Corval Lode B.V., Lode Medical Technology, Groningen, The Netherlands) in an air-conditioned room, where the temperature was kept between 15° and 20°. All subjects gave written informed consent before participating in the study and participation was accepted according to the anamnesis forms. Before the first exercise test the subject's height and weight were measured. Mucosal cells were removed from inside of the cheeks by simply twirling/rubbing an ear bud against the inner cheek wall. During all tests the same personalized bicycle settings were used and the subjects were instructed to keep their pedal frequency constant at 80 rotations per minute (rpm), which was displayed on an analog display on the handlebar. Subjects were asked to rate their perceived exertion (RPE) on a 20-point Borg RPE scale every minute. Two milliliters of blood was collected from the upper arm via the median cubital vein before cycling and 3, 6, 9 and 12 min after the cycling tests. Blood collection was done by a professional person via an intravenous cannula from the upper arm, while the subjects were in a sitting position. During the cycling exercise the subjects' VO2 (oxygen uptake) and VCO2(CO2 output) were measured breath by breath via expired air using a gas analyzing system (Cosmed Quark, Cosmed S.R.L., Rome, Italy) and the subjects' heart rate was measured with a Polar heart rate belt. From these data the maximal oxygen uptake (VO2peak) and the respiratory exchange ratio (RER) were established.
At the first occasion the subjects performed a maximal incremental exercise test (maximal test). Subjects started cycling for 3 min at 50 W and the resistance was increased every minute by 25 W until exhaustion. The test ended when the pedal frequency dropped below 65 rpm. After the first test the ventilatory threshold (VT) and respiratory compensation point were identified using the V-slope and ventilatory equivalent methods (Sue et al. (1988)). To investigate the effect of exercise duration and intensity on AngII levels at and above the AT, subjects performed two other exercise tests at different occasions on the same time of the day with at least 1 day of rest in between. At the second occasion subjects cycled after a 3 min warm up of 50 W for 3 min at the intensity where they reached their RCP during the maximal test. At the third occasion subjects cycled for 12 min at an intensity at which they reached their VT the maximal test. When the RPE of the subject during the tests dropped below 11 or was above 13, the external power was adjusted with 25 W.
2.1. Capillary recruitment
To assess perfusion in a non-active body compartment we used the capillary microscope which can non-invasively and directly visualize the perfusion of the skin in the finger. Absolute and relative capillary recruitment was measured before and directly after the maximal test as described previously (Serne et al. (1999)). Briefly, two separate visual fields of 1 mm2 were recorded 1.5 mm proximal to the terminal row of capillaries in the middle of the nail fold of the third finger. A characteristic capillary (i.e., a capillary that was constantly perfused and had an eye-catching morphological feature) was kept on the same spot exactly in the center of the visual field (marked by a dot on the monitor) to ensure that capillary density was measured in the exact same visual field during the entire experiment. Capillary density was counted as the number of continuously erythrocyte-perfused capillaries per square millimeter (n/mm2) during 15 s and after 4 min of arterial occlusion. Capillary recruitment was calculated as the relative increase in capillary density from baseline to capillary density after the 4 min of arterial occlusion. The procedure was repeated using a visual field adjacent to the first visual field and data concerning capillary densities are the mean of two measurements. The number of capillaries was counted off-line by a single experienced study physician (Jorn Woerdeman).
2.2. Assessment of angiotensin II levels
Blood (2 ml) was withdrawn from the venous cannula and put into vacutainers containing 60 μl AngII inhibitor cocktail, comprising 13.35 μl of O-Phenanthroline and Pepstatine A in DMSO mixed with 46.65 μl of EDTA and PHMB in aqueous solution (SPI bio, Bertin pharma, Versailles, France). The samples were immediately centrifuged at 10,000 rpm (3000 g), at 4 °C for 12 min. The supernatants were separated, snap frozen in liquid nitrogen and stored at − 80 °C, transferred with dry ice to MMU Manchester and stored at − 80 °C until analyzed. Plasma was eluted with C18 phenyl cartridge which was conditioned with 2 ml of methanol and then rinsed with 2 ml of water. 0.9 ml of cold plasma was rapidly passed through the cartridge and subsequently washed with 1 ml of water. Absorbed angiotensins were eluted with 1 ml of methanol into conical polypropylene tubes. The eluate was evaporated to dryness by means of a nitrogen gas stream at room temperature and the residue was stored at − 20 °C.
We have characterized the performance of the AngII assay in pilot experiments where we spiked AngII in the sample before the measurements (data not shown). These experiments show that the assay recovers 85% of the AngII peptide in blood plasma. As well we find that the detection of the AngII signal is linear for the range between 2 and 100 pg/ml.
AngII was assessed with a commercially available AngII enzyme immunoassay kit (SpiBio, Montigny Le Bretonneux, France) according to the manufacturer's instructions. The characterization of this kit performance has been published (Volland et al. (1999)). Briefly, the plasma samples were incubated with 100 μl of EIA, 50 μl, 100 μl of borane-trimethylamine and with 100 μl of anti-AngII IgG tracer. Samples were incubated with 200 μl of Ellman's reagent. The plate was red with a single quick read on an Absorbance Microplate Reader (ELx800, BIO-TEK) with wavelength 405 after 30 min, 1 h and 2 h of incubation.
2.2.1. ACE genotyping
DNA was extracted from mucosal samples using a custom designed protocol. The mucosal mouth swabs were extracted with 800 μl of methanol with vortexing and left to evaporate in air under a stream of nitrogen gas. The pellet was resuspended in 100 μl of sterile water and stored at − 20 °C.
ACE genotyping was carried out with polymerase chain reaction (PCR) as described by Evans et al. (1994). PCR reactions were run with a mix of the three primers using Sybr Green master mix (Applied Biosystems) on an Applied Biosystems Real Time PCR system (SepOnePlus, Life Technologies). This involved 45 standard cycles of denaturing at 95 °C for 15 s followed by annealing and extension at 55 °C for 1 min. Amplicon identification followed using a melting curve analysis between a temperature range of 70 °C and 80 °C. The identity of the amplified sequence for the ACE-I and ACE-D genotype was validated by sequencing of the PCR products with the specific primers (Microsynth, Balgach, Switzerland). The presence of the short amplicon for the I-allele was identified by a lower melting temperature (73.5 °C; 72.5–74 °C) compared to the longer D-allele (75.5 °C; 74.5–76.5 °C) respectively.
2.3. Statistics
Results are mean ± SE. For AngII the data was logarithmic transformed and an analysis of variance (ANOVA) with repeated measures was used to compare time and exercise intensity overall and between the ACE genotypes. To compare the effect of exercise among ACE genotypes, AngII levels were background-corrected and related to the mean values of the respective genotype before exercise. For capillary parameters, capillary recruitment and percentage capillary recruitment were compared by a Student's paired t-test.
3. Results
Subject (n = 10) characteristics at baseline and during the three tests are reported in Table 1a, Table 1b. Four subjects carried the ACE-II allele, and six the ACE-ID/DD allele.
Table 1a.
Baseline characteristics of the studied subjects.
| Age (year) | 29.6 ± 2.1 |
| Length (cm) | 180.9 ± 7.6 |
| Mass (kg) | 76.1 ± 5.6 |
| Resting heart rate (beats/min) | 65.8 ± 9.0 |
Table 1b.
Physiological characteristics of the studied subjects during the three exercise tests.
| Power (W) | Heart rate (beats/min) | VO2 (ml O2/min) | VO2/kg (ml O2/kg/min) | RER | RPE (6–20) | Heart rate before exercise (beats/min) | Days after last exercise test | |
|---|---|---|---|---|---|---|---|---|
| Peak exercise (last 30 s of maximal test) | 353 ± 49 | 187.4 ± 7.4 | 3885.2 ± 508.9 | 51.0 ± 4.8 | 1.32 ± 0.07 | 70.8 ± 3.0 | ||
| Anaerobic threshold | 215 ± 54 | 143.9 ± 12.6 | 2701.8 ± 604.9 | 35.4 ± 6.5 | ||||
| Respiratory compensation point | 301 ± 51 | 161.3 ± 8.3 | 3387 ± 482.7 | 44.24 ± 4.6 | ||||
| 12 min test (last 30 sec) | 230 ± 48 | 157.4 ± 5.2 | 3173.1 ± 527.4 | 41.6 ± 5.3 | 1.04 ± 0.03 | 14.3 ± 0.9 | 73.8 ± 3.5 | 5.0 ± 0.9 (range: 2–9) |
| 3 min test (last 30 s) | 310 ± 42 | 171.7 ± 9.0 | 3589.6 ± 458.3 | 47.14 ± 4.5 | 1.22 ± 0.07 | 16.6 ± 0.8 | 69.9 ± 4.0 | 8.0 ± 1.5 (range: 2–15) |
Blood samples data are shown in Fig. 1, Fig. 2. No correlation was found between the blood lactate levels and the serum AngII levels. All indices of metabolic strain, i.e., VO2peak, RER, heart rate and lactate levels after exercise were elevated after all the three exercise tests and remained high over the study period (Fig. 1, Table 1a). The highest increases in indices of metabolic strain were observed after the maximal test.
Fig. 1.
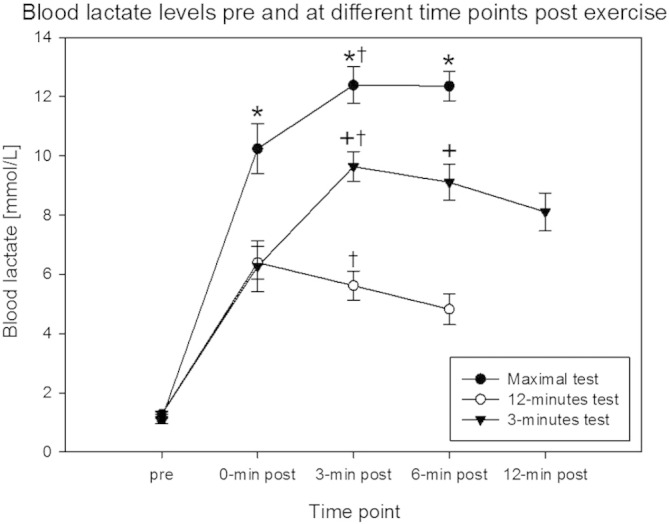
Metabolic strain of the three exercise conditions.
Mean ± SE of serum lactate levels at the measured time points post exercise. * Significantly different from 12-min test and 3-min test (p < 0.01), + significantly different from maximal test and 3-min test (p < 0.01), † significantly different from pre, 0-min post and (if measured) 9-min post (p < 0.05) (repeated measures ANOVA, n = 10).
Fig. 2.
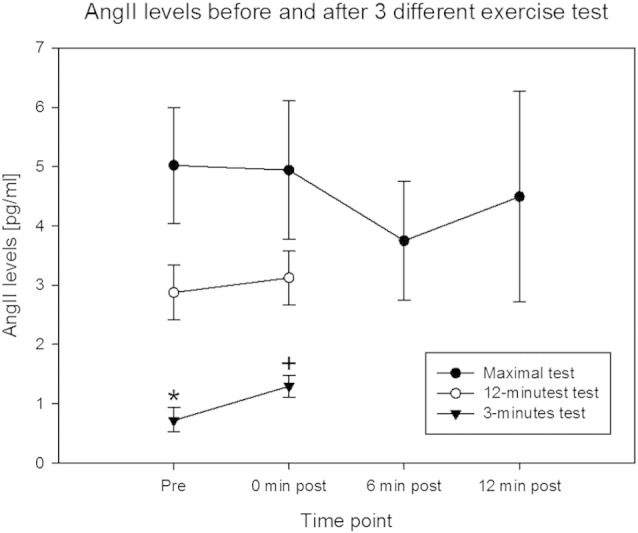
Serum AngII levels after exercise.
Mean ± SE of AngII concentration in capillary blood (serum) at the measured time points post exercise. * Significantly different from 12-min test and maximal test (p < 0.05) and + indicates significantly different from 12-min test, maximal test and 3-min post (p < 0.05) (repeated measures ANOVA, n = 10).
There was a difference in AngII levels before the exercise. AngII levels before the first tests at maximal intensity were higher than after the tests done in the course of the next days. AngII levels were significantly increased after the 3-min exercise test (Fig. 2) but not after the 12-min and maximal test. A main effect of the presence of the I-allele was identified for the course of changes in AngII levels relative to baseline after exercise in the maximal test (p = 0.041), but not the 3-min (p = 0.357) and 12-min test condition (p = 0.395). Subjects with the II genotype showed a maintained level of AngII whereas AngII levels decreased in subjects lacking the I allele after the maximal test (Fig. 3).
Fig. 3.
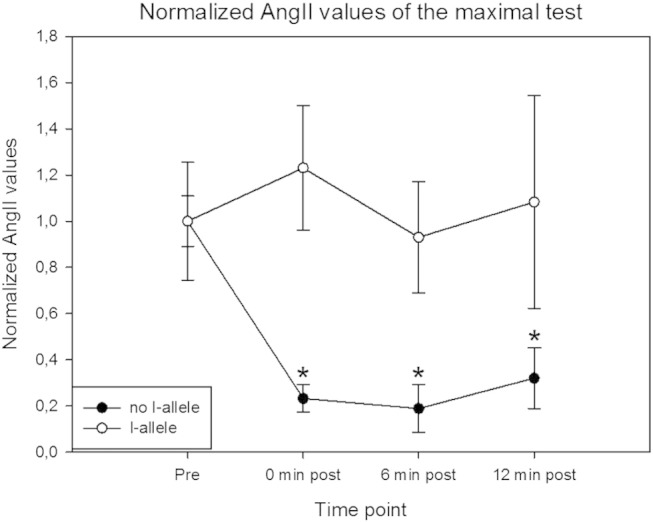
ACE I/D dependence of AngII levels after the maximal exercise test.
Mean ± SE of AngII concentration in capillary blood serum for carriers and non-carriers of the ACE I-allele. * indicates significant different from the I-allele and from no I-allele (pre) p < 0.05 (repeated measures ANOVA, n = 10).
Table 2 shows a non-significant increase in capillary recruitment in subjects carrying the ACE II-allele while a trend for a decreased capillary recruitment after exercise was observed in subjects carrying the ACE-ID/DD allele. When we compared the genotypes we observed that capillary recruitment after exercise was significantly less in the ACE-ID/DD group compared to the ACE II-allele. No indication for an effect for capillary recruitment post exercise was seen in function of the I-allele (data not shown).
Table 2.
Capillary recruitment in numbers and percentages before and after the maximal test.
| Genotype | Test | Pre | Post | p |
|---|---|---|---|---|
| II | Capillary recruitment (%) | 35.5 ± 5.5 | 43.3 ± 4.6 | 0.73 |
| Capillary recruitment | 18.3 ± 2.6 | 26.8 ± 2.2 | 0.11 | |
| ID/DD | Capillary recruitment (%) | 36.2 ± 6.3 | 23.8 ± 4.1 | 0.08 |
| Capillary recruitment | 15.9 ± 2.7 | 12.9 ± 2.4a | 0.19 |
Values are means ± SE.
Significantly different from capillary recruitment II at 0 min post (p < 0.05)
4. Discussion
We aimed to detect changes in human AngII levels after exercise at different intensities.
Our measurements are in line with the values of 7–10 pg/ml for AngII levels in serum which were identified to represent the true values in health subjects (Nussberger et al, 1986). Overall it was observed that highest increases in indices of metabolic strain were observed after the maximal test. We hypothesized that the rise in AngII is dependent on exercise intensity and duration. A two fold increase in serum AngII concentration after intense exercise was found after the 3-min exercise test. This is comparable to the increase found in the study of Fagard et al. (1985). No significant increases in AngII were found after the 12-min test and maximal test with also no significant differences in AngII levels between the different time points after exercise (Fig. 2).
As hypothesized the AngII values after the maximal exercise test were higher in the subjects carrying the ACE-II genotype. Subjects without the I allele showed a decrease in AngII values after the maximal test where people with the ACE-II genotype did not (Fig. 3). A main effect of the presence of the I-allele was identified for the course of changes in AngII levels relative to baseline after exercise under the maximal test, but not the 3-min and 12-min test condition. This might support a role of the duration of exercise for affecting serum AngII. We therefore conclude that the rise in AngII post exercise might be duration dependent and seems to be intensity and ACE genotype dependent.
It was further observed that as hypothesized subjects with the ACE-II genotype had a significant higher capillary perfusion in the finger after exercise compared to the ACE-ID/DD genotype. With a trend for a lower capillary perfusion in the ACE-ID/DD genotype post exercise, these data indicate that this body compartment is less perfused due to AngII dependent vasoconstriction during exercise. This mechanism is comparable with the study of Santana et al. (2011) in which they found a main effect in systolic blood pressure between the ACE genotypes after a maximal test and a cycling test at 90% AT. They also showed a protective effect on post exercise diastolic blood pressure and MAP. The initial vasoconstriction may be overridden by maximal cycling exercise due to the systemic action of released nitric oxide (NO) and adenosine (Santana et al. (2011)). We therefore conclude that there seems to be a relationship between AngII levels and ACE genotype and between ACE genotype and blood flow.
It was astonishing that a difference was noted for AngII levels at rest before each of the three tests (Fig. 2). We note that the values measured for AngII (see Fig. 2) are in the range of reported values (i.e., 2.3–18.6 pmol l− 1; Ueda et al. (1998)). It has been reported that exercise lowers hypertension and that this effect lasts certainly 13 h (Kenney and Seals (1993)). Santana et al. (2011) showed that only people with the I allele of the ACE genotype had post exercise hypotension and increased NO-release. As AngII levels in serum relate to blood pressure (Kaplan and Silah (1964) and the 3-min tests were by some subjects conducted 2 days after the maximal test, including subjects bearing the I allele the hypothesis is raised that the lowered AngII may be related to post exercise hypotension that lasts at least 15 h (Kenney and Seals (1993)). The heart rate before the 3 min test was not significantly higher, compared to the maximal test (Table 1b).
Remarkable is also the variation in AngII levels at the different time points post exercise between the subjects, that can be revealed from the standard errors (Fig. 2). A reason for this variation is that we identified an effect of the ACE genotype on AngII values after exercise. Subjects with an I allele in their ACE-genotype had significant higher AngII values at all the time points after the maximal test than people without the I allele, who demonstrate decreased AngII values after the maximal test (Fig. 3). These results indicate that the D-allele restricts production of AngII after exercise, while people without a D-allele maintain AngII levels after exercise.
A limitation of our study was that capillary recruitment measurements were only performed at the maximal test. Due to the short duration, we expect no exercise induced vasodilatation based on literature after the 3 min cycling test (Tschakovsky (2002)). Therefore we cannot rule out that for instance the increase in serum AngII immediately after the 3-min test is an acute phenomenon of vasoconstriction in non-exercised body parts that also took place in subjects exercising for longer duration and which did not halt exercise to allow sampling of this time point.
The increase in AngII levels after exercise causes the blood pressure to decrease but on the other hand it is expected to promote angiogenesis in the activated muscles by activating the endothelial cell population that constitutes the capillary wall (Brothers et al. (2006)). A trend was observed that subjects with the ACE-DD/ID polymorphism showed a decrease in capillary recruitment after exercise. Subjects with the ACE-II polymorphism had a significant larger recruitment of the relative capillaries after exercise compared to subjects with the ACE-ID/DD polymorphism.
The rise in serum AngII with the 3-min exercise protocol at the RCP highlights a role of high intensity exercise for a short duration. Taking into account that the maximal test also included a similar level of intensity we analyzed the role of exercise time of exercise-induced systemic vasodilatation. The identified decrease in capillary recruitment in the finger of ACE-ID-DD genotypes after the maximal test supports the contention of an elevated potential for vasoconstriction in non-exercised body parts in subjects which do carry the D-allele. In this regard the documented increase in ACE transcript expression in vastus lateralis muscle being recruited during bicycle exercise in ACE-DD genotypes (Vaughan et al. (2013)) suggests a role of the ACE I/D polymorphism in controlling capillary recruitment with exercise via effects on ACE gene expression.
The data indicate that variability in the response of the major vasoconstrictor, AngII, in serum after exercise is ACE genotype-dependent and not related to blood lactate levels. In some contradiction to Danser et al. (2003 or 2007) we see that in the controlled situation of maximal exercise, there is evidence for the role of the ACE I/D polymorphism in explaining variability in serum AngII and capillary perfusion. Thus, physical activity must be considered as an important confounder of previous conclusions. Awareness of the differences in AngII generation after exercise could have clinical repercussions on current health care practice. It may be one of the reasons why people with the ACE-II genotype and/or people taking ACE inhibitors show lowered gains as a result of exercise rehabilitation (Defoor et al. (2006)).
Acknowledgments
We thank David Vaughan for characterizing the performance of the AngII assay. This study was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate program (2011–2015).
References
- Aldigier J.C., Huang H. Angiotensin-converting enzyme inhibition does not suppress plasma angiotensin II increase during exercise in humans. J. Cardiovasc. Pharmacol. 1993;21(2):289–295. doi: 10.1097/00005344-199302000-00015. [DOI] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers R.M., Haslund M.L. Exercise-induced inhibition of angiotensin II vasoconstriction in human thigh muscle. J. Physiol. 2006;577(Pt 2):727–737. doi: 10.1113/jphysiol.2006.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser A.H., Batenburg W.W. ACE phenotyping as a first step toward personalized medicine for ACE inhibitors. Why does ACE genotyping not predict the therapeutic efficacy of ACE inhibition? Pharmacol. Ther. 2007;113(3):607–618. doi: 10.1016/j.pharmthera.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Defoor J., Vanhees L. The CAREGENE study: ACE gene I/D polymorphism and effect of physical training on aerobic power in coronary artery disease. Heart. 2006;92(4):527–528. doi: 10.1136/hrt.2004.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A.E., Poirier O. Polymorphisms of the angiotensin-converting-enzyme gene in subjects who die from coronary heart disease. Q. J. Med. 1994;87(4):211–214. [PubMed] [Google Scholar]
- Fagard R., Lijnen P. Effects of angiotensin II on arterial pressure, renin and aldosterone during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1985;54(3):254–261. doi: 10.1007/BF00426142. [DOI] [PubMed] [Google Scholar]
- Hahn A.W., Schmidt R. Endothelium-modulated proliferation of medial smooth muscle cells: influence of angiotensin II and converting enzyme inhibition. Eur. Heart J. 1995;16(Suppl. C):29–32. doi: 10.1093/eurheartj/16.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- Kaplan N.M., Silah J.G. The Effect of Angiotensin II on the Blood Pressure in Humans with Hypertensive Disease. J. Clin. Invest. 1964;43(4):659–669. doi: 10.1172/JCI104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney M.J., Seals D.R. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension. 1993 Nov;22(5):653–664. doi: 10.1161/01.hyp.22.5.653. [DOI] [PubMed] [Google Scholar]
- Kosunen K.J., Pakarinen A.J. Plasma renin, angiotensin II, and plasma and urinary aldosterone in running exercise. J. Appl. Physiol. 1976;41(1):26–29. doi: 10.1152/jappl.1976.41.1.26. [DOI] [PubMed] [Google Scholar]
- Maher J.T., Jones L.G. Aldosterone dynamics during graded exercise at sea level and high altitude. J. Appl. Physiol. 1975;39(1):18–22. doi: 10.1152/jappl.1975.39.1.18. [DOI] [PubMed] [Google Scholar]
- Miura S., Ideishi M. Angiotensin II formation by an alternative pathway during exercise in humans. J. Hypertens. 1994;12(10):1177–1181. [PubMed] [Google Scholar]
- Nussberger J., Brunner N.B. Specific measurement of angiotensin metabolites and in vitro generated angiotensin II in plasma”. Hypertension. 1986;8:476–482. doi: 10.1161/01.hyp.8.6.476. [DOI] [PubMed] [Google Scholar]
- Petersen M.C., Greene A.S. Angiotensin II is a critical mediator of prazosin-induced angiogenesis in skeletal muscle. Microcirculation. 2007;14(6):583–591. doi: 10.1080/10739680701404697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.C., Greene A.S. Inhibition of Angiogenesis by High Salt Diet Is Associated with Impaired Muscle Performance Following Chronic Muscle Stimulation. Microcirculation. 2009;15(5):405–416. doi: 10.1080/10739680701809093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana H.A1., Moreira S.R. The higher exercise intensity and the presence of allele I of ACE gene elicit a higher post-exercise blood pressure reduction and nitric oxide release in elderly women: an experimental study. BMC Cardiovasc. Disord. 2011;2(11):71. doi: 10.1186/1471-2261-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher N.H., Clausen J.P. Central and redional circulatory effects of adding arm exercise to leg exercise. Acta Physiol. Scand. 1977;100:288–297. doi: 10.1111/j.1748-1716.1977.tb05952.x. [DOI] [PubMed] [Google Scholar]
- Serne E.H., Stehouwer C.D. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99(7):896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- Staessen J., Fagard R. Plasma renin system during exercise in normal men. J. Appl. Physiol. 1987;63(1):188–194. doi: 10.1152/jappl.1987.63.1.188. [DOI] [PubMed] [Google Scholar]
- Sue D.Y., Wasserman K. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Use of the V-slope method for anaerobic threshold determination. Chest. 1988;94(5):931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- Tschakovsky M.E., Sujirattanawimol K., Ruble S.B., Valic Z., Joyner M.J. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J. Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Meredith P.A., Morton J.J., Connell J.M.C., Elliott H.L. ACE (I/D) Genotype as a predictor of the magnitude and duration of the response to an ACE inhibitor drug (enalaprilat) in humans. Circulation. 1998;98:2148–2153. doi: 10.1161/01.cir.98.20.2148. [DOI] [PubMed] [Google Scholar]
- Vaughan D., Huber-Abel F.A. The angiotensin converting enzyme insertion/deletion polymorphism alters the response of muscle energy supply lines to exercise. Eur. J. Appl. Physiol. 2013;113(7):1719–1729. doi: 10.1007/s00421-012-2583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volland H., Pradelles P. A solid-phase immobilized epitope immunoassay (SPIE-IA) permitting very sensitive and specific measurement of angiotensin II in plasma”. J. Immunol. Methods. 1999;228(1–2):37–47. doi: 10.1016/s0022-1759(99)00097-6. [DOI] [PubMed] [Google Scholar]


