Abstract
In the last decade, it has been proposed that the sun's IR-A wavelengths might be deleterious to human skin and that sunscreens, in addition to their desired effect to protect against UV-B and UV-A, should also protect against IR-A (and perhaps even visible light). Several studies showed that NIR may damage skin collagen content via an increase in MMP-1 activity in the same manner as is known for UVR. Unfortunately, the artificial NIR light sources used in such studies were not representative of the solar irradiance.
Yet, little has been said about the other side of the coin. This article will focus on key information suggesting that IR-A may be more beneficial than deleterious when the skin is exposed to the appropriate irradiance/dose of IR-A radiation similar to daily sun exposure received by people in real life.
IR-A might even precondition the skin – a process called photoprevention - from an evolutionary standpoint since exposure to early morning IR-A wavelengths in sunlight may ready the skin for the coming mid-day deleterious UVR.
Consequently IR-A appears to be the solution, not the problem. It does more good than bad for the skin. It is essentially a question of intensity and how we can learn from the sun.
Keywords: Infrared (IR), IR-A, photobiomodulation, MMP-1, photoprevention, irradiance
1 Introduction
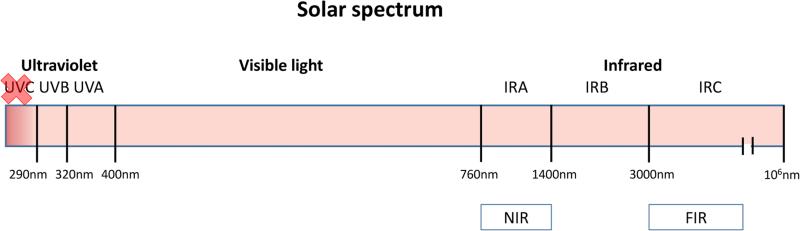
The spectrum of solar radiation reaching the Earth ranges from 290 to more than 1,000,000 nm and is divided as follows: 6.8% UV, 38.9% visible, and 54.3% near infrared radiation (NIR) [1]. Infrared constitutes the waveband longer than 760 nm and up to 1 mm. It accounts for approximately 40% of the solar radiation reaching the ground at sea level. It has been divided into three bands: IR-A (760–1400 nm), IR-B (1400–3000 nm), and IR-C (3000 nm – 1 mm)(Figure 1). IR radiation can penetrate the epidermis, dermis, and subcutaneous tissue to differing extents depending on the exact wavelength range being studied. Exposure to IR is perceived as heat [2].
Figure 1.
Solar spectrum composition. Red X over UVC means they are blocked by the ozone layer. (NIR: Near Infrared, FIR: Far infrared)
The strength of electromagnetic radiation depends on the energy of the individual particles or waves as well as the number of particles or waves present.
Electromagnetic radiation covers a spectrum with a wide range of photon energies that can also be expressed as a range of wavelengths. The spectrum has two major divisions:
non-ionizing radiation
ionizing radiation
Radiation that has insufficient energy to completely remove electrons from atoms and molecules is referred to as non-ionizing radiation. Examples of this kind of radiation are visible light, infrared, microwaves and radio waves. Radiation that falls within the ionizing radiation range has enough energy to remove tightly bound electrons from atoms, thus creating charged ions. This type of radiation includes X-rays and gamma rays.
Ultraviolet (UV) radiation is intermediate between these two broad ranges, and short-wavelength UV has enough energy to break chemical bonds and carry out photochemical reactions.
Although the consequences of sun exposure on the skin have been extensively studied over the years, the impact of IR radiation has received far less attention than its UV counterpart that is well known to cause skin cancer, photoaging, and immune suppression.
Moreover, the solar IR-A (also called NIR) irradiance level is critical to trigger beneficial effects in the skin beyond which it becomes deleterious. Most studies reporting the detrimental effects of IR-A (upregulation of matrix metalloproteinase 1 or MMP-1) used artificial light sources way above the solar IR-A irradiance threshold. This review article highlights the discrepancies in published data in order to bring a new perspective on this controversial topic.
2 NIR & Skin
2.1 NIR detrimental effects: heat
It has been known for a long time in dermatology that chronic IR exposure can be deleterious to the skin. It was classically seen on the legs of those sitting too close to hearth fires named erythema ab igne. Such reticulated, erythematous or hyperpigmented dermatoses resulted from chronic and repeated exposure to relatively low levels of infrared radiation, and generally had a good prognosis. However, this was not necessarily a self-limiting diagnosis as patients were at long-term risk of developing subsequent cutaneous malignancies such as squamous cell and Merkel-cell carcinomas [3]. This diagnosis recently made a comeback with laptop-computer induced erythema ab igne [4] being described. Furthermore, severe skin aging may develop occasionally on bakers' arms because of exposure to hot ovens and on the faces of glass blowers [5]. In the above examples, the skin was exposed to massive heat via convection (hot air flow), conduction (direct contact) and/or radiation (IR). Although the proportion of heat transmitted by radiation is unknown, it can be estimated as far from negligible, at least for people sitting by fires or for bakers. Most importantly, the distinct effect of NIR was not measured independently from the heating effects by convection and/or conduction.
The thermal nature of erythema ab igne means that the irradiance of exposure was elevated and that the cumulative dose (fluence) was very high.
Is heat really an issue in causing the deleterious effects of NIR? Some studies have shown that there is an increase in collagen degradation and ROS generation with a relatively small increase in temperature. Piazena et al. studied the effects of water-filtered infrared-A (wIRA) with convective cooling or heating on viability, inflammation, inducible free radicals and antioxidant enzyme content in natural and viable skin [6]. The water-filtered IR-A, applied over 30 minutes to the skin at an irradiance of 190 mW/cm2, with the skin temperature maintained at 37°C by convective cooling from air ventilation, did not significantly affect the cell viability, the inflammatory status, the free radical content, or the antioxidant defense systems of the skin. This is of clinical relevance since the irradiance exceeded the maximum solar IR-A irradiance at the Earth's surface more than 5 times. Conversely, after convective heating to about 45°C, free radical formation was almost doubled and antioxidant power was reduced to about 50%. This may be also linked to temperature-dependent polymer photodegradation showing a linear increase with radiation dose.
Even a relatively low irradiance of IR may lead to an intradermal temperature rise (inside-out heating). Other studies by Tanaka et al. reported that NIR can non-thermally induce cytocidal effects in cancer cells as a result of activation of the DNA damage response pathway [8, 9]. They used a broadband NIR source (Titan; Cutera, Brisbane, CA, USA) emitting 1100 to 1800 nm, with water filtering to simulate solar NIR radiation. Even though no irradiance is mentioned, they irradiated cells with one to ten rounds of NIR at 20 J/cm2 in vitro and up to 40 J/cm2 in vivo without temperature monitoring in tissues. The use of a broadband NIR source (intense pulsed light (IPL) with a contact cooling tip at 20°C to protect epidermal damage) is totally irrelevant since it is essentially a thermal technology built to destroy chromophores by raising dermal temperature with very high peak power pulses. Consequently, it does not simulate NIR rays from the sun and explains the cytocidal effects of this artificial light source that occur as a consequence of the heat generated.
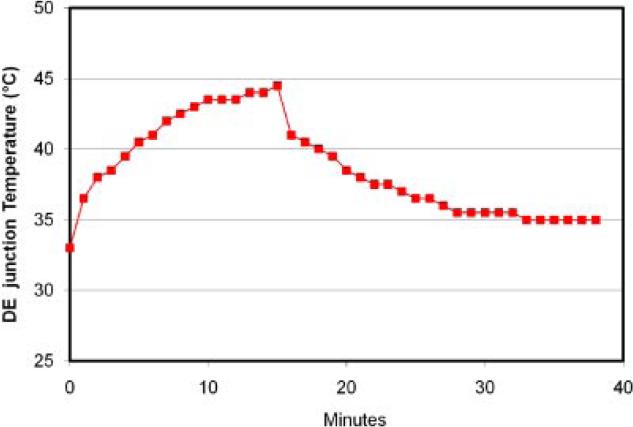
We reported this finding via intra-dermal thermocouple type-T temperature measurements. We observed temperatures up to 44°C with as little as 80 mW/cm2 delivered in 15 minutes (72 J/cm2), using a NIR LED light source at 970 nm (figure 2) [10].
Fig 2.
Temperature increase with 970 nm light emitting diode at 80mW/cm2 was measured at the derma-epidermal (DE) junction as a function of time (minutes) for a patient. Data monitoring demonstrated that the temperature peaked at 45°C after 15 minutes of irradiation and decreased slowly thereafter[10].
Even a simple non-IR heating pad may lead to collagen degradation at 43°C for 15 minutes [11]. In this experiment, dorsal skin of hairless mice was exposed to heat three times per week for a period of 6 weeks. They showed that chronic exposure of the skin to heat can cause skin wrinkling by increasing matrix metalloproteinase 13 (MMP-13)expression and decreasing antioxidant enzyme activity with consequent oxidative damage. MMP-13 promotes closure of skin wounds[12]. Another study by Halper et al. using chicken embryonic gastrocnemius tendon explants at different temperatures (37°C vs. 43°C) reported increases in mRNAs representing several collagen regulators, transforming growth factor beta (TGF-β), heat shock protein 47 (Hsp47) and connective tissue growth factor (CTGF) at 43°C [13].
2.2 NIR induced photoaging & oxidative stress
Other studies have been carried out to determine the effects on human skin cells of IR-A radiation from another type of artificial light source: Hydrosun 500 emitting extraordinarily high irradiance (average: 360 mW/cm2) (at 760-1400 nm), far from what the sun is capable even at zenithal conditions (at noon in the tropics). In a 2002 article by Schieke et al., human dermal fibroblasts were irradiated with this source. The cells were exposed to IR-A radiation in the range of 760-1400 nm, from 10 and 60 minutes, with a corresponding fluence range of 200-1200 J/cm2. This translates to an irradiance range of 333-2,000 mW/cm2. Matrix metalloproteinase 1 (MMP-1), the collagenase involved in the normal turnover of skin collagen was found to be upregulated in the irradiated cells [14].
In order to assess the in vivo relevance of these observations, Schroeder et al. [15] studied normal buttock skin of 23 healthy human volunteers. They were all irradiated with the same artificial source (Hydrosun 500), a single dose of 360 or 720 J/cm2 IR-A radiation and subsequently assessed for MMP-1 expression (mRNA by RT-PCR or protein by Western blotting or by immunohistochemistry). The measured irradiance to which the skin was exposed was 105 mW/cm2. As in the Schieke et al. [14] study, the expression of MMP-1 was found to be upregulated following IR-A irradiation. The authors mention that doses were chosen because they correspond to doses of IR-A radiation which can be achieved in a few hours on a summer day in central Europe. Nevertheless, to get such a total dose under the sun, one would have to be exposed from 6 am to 6 pm in the summer further south in the tropics. In reality, solar IR-A average irradiance is around 20 mW/cm2 during the day with a peak irradiance reaching 40 mW/cm2 [16].
An in vivo mouse model was used to study the long term effects of IR-A exposure followed by UVB exposure over several months [17]. Using an artificial source emitting IR-A covering the wavelength range of 780-1400 nm, the skin of shorn mice was irradiated at a fluence of 135 J/cm2 (no irradiance nor treatment time was mentioned). After two hours, the skin was exposed to UVB light. This process was repeated three times per week for many weeks, with a gradually increasing UVB fluence. It was observed that IR-A radiation did not enhance the frequency of UVR radiation-induced skin tumors but, tumors that had developed became more aggressive. So, tumors in IR-pretreated mice grew faster.
All of these studies used artificial radiation with properties unlike natural sunlight which brings into question the applicability in real life situations of their conclusions regarding the short and long term deleterious effects of IR-A. Considering all wavelengths, the maximum solar irradiance is 130 mW/cm2. Due to absorption by the atmosphere, this value would be reduced (to approximately 100mW/cm2). Of the total irradiance emitted by the sun, infrared A, B, and C make up about 40% of it and about 40% of that represents IR-A. So, natural sunlight, produces an IR-A irradiance of 20% of 100 mW/cm2 or 20 mW/cm2. In Schieke et al., 2002, cells were exposed to a minimum IR-A irradiance of 333 mW/cm2 and, in Schroeder et al., 2008, the IR-A irradiance was 105 mW/cm2, both intensities much greater than that found in natural sunlight. In Jantschitsch et al., 2011, although an irradiance value was not available, the IR-A fluence value of 135 J/cm2 was unnaturally high.
Kim et al. showed that chronic repetitive exposure to heat via IR also leads to skin wrinkling [7]. Unfortunately, the IR artificial radiation source used (Infrared-300, Daekyoung Co., Kyungki, Korea) was set to deliver IR at an unnaturally high irradiance of 2020 mW/cm2 (peak 1100-1200 nm), for a dose (fluence) of 1212 J/cm2, 5 days a week for 15 weeks [7].
In 2010, Piazena et al. overviewed studies in which the effects of IR-A (or IR) have been assessed in an experimental in vitro or in vivo setting. They highlighted the importance of careful choice and characterization of both the incident irradiance and the optical properties of the exposed medium containing the target cells in the in vitro experiment as well as of temperature control in the medium during the exposure if a realistic extrapolation to in vivo conditions is to be made feasible [16]. They point out that results from available studies are contradictory and that it is difficult to draw coherent conclusions on the deleterious effects of IR-A.
2.3 NIR induced photorejuvenation
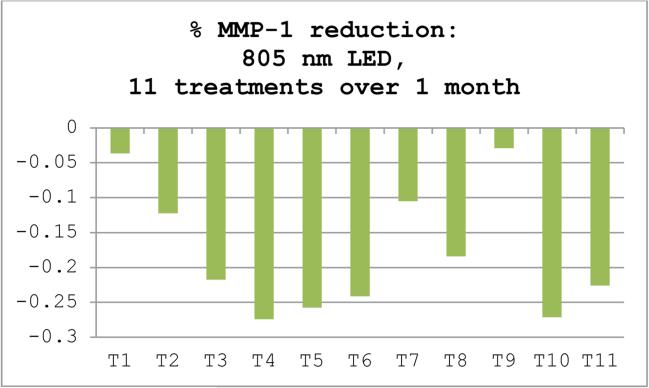
Using a tissue-engineered Human Reconstructed Skin (HRS) model, it has been demonstrated that exposure to 660 nm using an irradiance of no more than 50 mW/cm2 resulted in the downregulation in MMP-1 and the upregulation of type I procollagen [18]. To correlate these results, a split-face single-blinded study was conducted which showed a significant improvement in wrinkles on 660 nm treated skin. As part of the same in vitro experiment, 805 nm (NIR) was also tested with a comparable MMP-1 decrease over 11 treatments (unpublished data) figure 3.
Figure 3.
Reduction of MMP-1 levels by ELISA (enzyme-linked immunosorbent assay) in HRS (Human Reconstructed Skin) after LED treatments. A cyclic pattern of alternating highs and lows was observed in response to 11 consecutive treatments (T1–T11) for MMP levels. Values are percent differences ±SEM (n=9) between treated and untreated control HRS samples in mean levels of MMP-1 assessed in the supernatants after each treatment.
Lee et al. reported the effects of IR on photoaged skin [19]. Twenty patients with mild to moderate facial wrinkles received daily treatments of far infrared radiation (9-10 X 106nm ) for six months. Most patients (51-75%) reported positive improvements in skin texture and roughness. Additionally, 25-50% of patients noted fair skin tone improvement. Furthermore a prospective study showed comparable clinical results using 830 nm LEDs [20]. Histologically, a marked increase in the amount of collagen and elastic fibers in all treatment groups was observed. Ultrastructural examination demonstrated highly activated fibroblasts, surrounded by abundant elastic and collagen fibers. Immunohistochemistry showed an increase of TIMP-1 and 2. RT-PCR results showed the mRNA levels of IL-1ss, TNF-alpha, ICAM-1, and Cx43 increased after LED phototherapy whereas that of IL-6 decreased.
3 Law of reciprocity: irradiance is key
The law of reciprocity (Bunsen-Roscoe law) states that the biological effect is directly proportional to the total energy dose irrespective of the administered regime [21]. Therefore, the same exposure should result from reducing duration and increasing irradiance, and vice versa. However, scientific evidence supporting reciprocity does not apply all the time when considering tissue response in photobiology. Van Breughel irradiated fibroblast cultures with a helium-neon (HeNe) laser (wavelength 632.8 nm) at a constant dose, varying irradiance and exposure duration. Results show that the proliferation and collagen production could be only stimulated with medium irradiances and exposure durations (1.2 mW/cm2 for 145 s) [22]. Also, the immediate pigment darkening threshold of human skin to monochromatic UVA radiation has been shown to depend on irradiance [23]. Dose reciprocity effects were examined in a wound healing model and showed that varying irradiance and exposure duration to achieve a constant specified energy density affects photobiomodulation (PBM) treatment outcomes [24]. In practice, if irradiance is lower than the physiological threshold value for a given target, it does not produce beneficial effects even when irradiation duration is extended. Moreover, photoinhibitory deleterious effects may occur at higher irradiances. Such a biphasic pattern may explain the reported increase in MMP-1 when the artificial IR-A irradiances are too high (> 100 mW/cm2), inducing skin hyperthermia. This provides further evidence of the Arndt-Schulz law which states that there is only a narrow window of opportunity where you can actually activate a beneficial cellular response using precise sets of parameters [25]. This biphasic effect is described thoroughly by Huang et al., 2011 [26]. Lanzafame et al. 2007 completed a study varying irradiance and interval on laser-induced healing of pressure ulcers in mice. Energy density (5 J/cm2) was kept constant but four different irradiance parameters (0.7 – 40 mW/cm2) were compared with a significant improvement only occurring for 8 mW/cm2 [27].
Lower irradiance (<50mW/cm2) is less likely to induce skin hyperthermia leading to potential deleterious effects.
4 The healing power of IR
4.1 NIR (Near infrared)
It has been known for almost 50 years that low energy exposure to visible and NIR wavelengths is beneficial to humans via the promotion of healing processes. This low level light therapy (so called LLLT or PBM) has been reported in thousands of peer reviewed articles since 1968 [28, 29]. Using specific low energy (non-thermal) light parameters within a window of wavelengths from visible to NIR, PBM provides an alternative therapy for patients needing faster healing of wounds and/or for anti-inflammatory purposes. It has been compared to plant photosynthesis with a known photoacceptor molecule (cytochrome c oxidase) located in the mitochondria of eucaryotic cells.
PBM parameters have been improving in the last decade so that it is now part of our therapeutic armamentarium in dermatology as a complimentary treatment modality to treat skin inflammation, promote faster wound healing after ablative procedures or even prevent sunburn [30]. It is also used as a photodynamic therapy light source to photoactivate a photosynthetizer (Protoporphyrin IX or PpIX) when treating actinic keratosis, basal cell carcinoma and acne [31]. Furthermore, low intensity infrared has been shown to induce beta-endorphin hypoalgesic (analgesic) effects [32].
NIR photobiomodulation of tissue pathologies is associated with increased proliferation of specific cells, gene expression of anti-inflammatory cytokines and suppression of the synthesis of pro-inflammatory mediators [33].
4.2 FIR (Far infrared)
Another sub-division of IR radiation (far infrared, FIR, 3-25 μm), has also been observed to stimulate cells and tissue in both in vitro and in vivo studies [34]. Moreover FIR therapy is considered a promising treatment modality for certain medical conditions [35]. Technological advances have provided new techniques for delivering FIR radiation to the human body. Specialty lamps [36] and saunas [37], delivering pure FIR radiation (eliminating completely the near and mid infrared bands), have became safe, effective, and widely used sources to generate therapeutic effects. Fibers impregnated with FIR emitting ceramic nanoparticles and woven into fabrics, are being used as garments and wraps to generate FIR radiation powered by the body-heat of the wearer, and these garments provide diverse benefits to health [38, 39].
5 PBM mechanism of action
Although skin is naturally exposed to light more than any other organ, it still responds well to red and near-infrared radiation [29]. A better understanding of the mechanism of action will direct clinicians in their treatment approach.
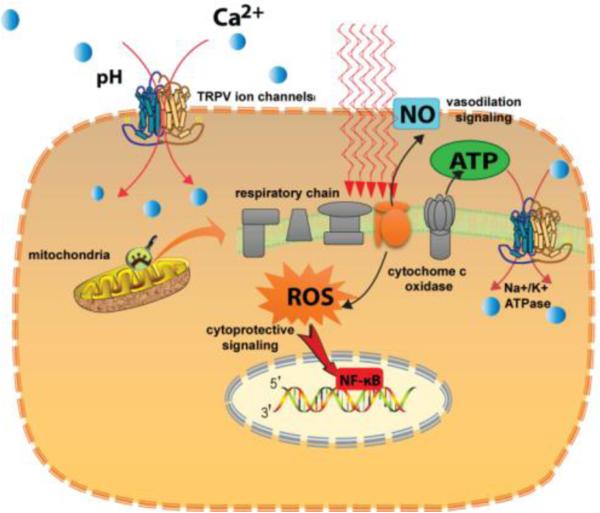
The cellular and molecular mechanisms of action of PBM have become reasonably well-understood in recent years and are summarized in Figure 4.
Figure 4.
Mechanisms of action of PBM. The two principal chromophores are cytochrome c oxidase (CCO) which is unit IV in the mitochondrial respiratory chain, and TRPV ion channels. Photon absorption leads to dissociation of inhibitory nitric oxide from CCO leading to increased enzyme activity and raised ATP production and a burst of reactive oxygen species. The extra ATP produced can activate the Na+/K+ ATPase pump. Another type of calcium ion channel called “transient receptor potential vanilloid” (TRPV) is activated by both visible and infrared light. Calcium signaling is a very important pathway in multiple cell types.
An important finding demonstrates that the NF-kB (nuclear factor-kappa B) cell signaling pathway plays an essential role thought to be activated by mitochondrial cytochrome c oxidase (COX) serving as a generator of ROS (reactive oxygen species) [40]. Changing the redox state of the mitochondrial membrane activates the formation of the transcription factor NF-kB. In the cell cytoplasm, NF-kB is inactive because it is in a complex with its specific inhibitory protein, IkB (I kappa B). ROS stimulates IkB-kinase (IkK), which triggers the phosphorylation of IkB, resulting in IkB complex decay with release of NF-kB. NF-kB is transported into the nucleus, which causes the expression of more than 150 genes many of which are involved in defense mechanisms against cell stress. The correlation between the stimulation of NF-kB and the accumulation of ROS was found in embryonic fibroblasts in vitro subsequent to their IR irradiation (810 nm). The maximal activation of NF-kB and ROS accumulation were observed at a dose of 0.3 J/cm2, while high doses caused less pronounced effects [26].
Mitochondrial ROS show a triphasic dose-response with two distinct peaks. The Janus nature of ROS is such that it may act as a beneficial signaling molecule at low concentrations and a harmful cytotoxic agent at high concentrations. This may partly explain the observed responses in vivo [26].
6 Poly vs mono chromatic light effects on aging skin
Although monochromatic wavelengths are usually used in PBM, combination wavelengths have also been successful.
Some authors emphasize the importance of distinct wavelengths for optimal skin rejuvenation results [20]. In a study, the differences between poly- (570-850nm, 42.8-54.8 mW/cm2, 49.3-51.4 J/cm2) vs mono- (630 nm, 5.9-23.4 mW/cm2, 15.5-16.6 J/cm2) chromatic light treatments in clinical outcome and patient satisfaction were not significant, indicating that despite spectral differences, both light sources were commensurably effective in the study objectives to reduce aging skin [41].
7 Emulating the sun: IR photoprevention
Preconditioning has been described by Decreane et al. in 2005 using UV. A low UVB dose triggered a protective p53-dependent gene mechanism increasing the resilience of keratinocytes against future UVB insults [42]. However, the use of UVB ionizing radiation to prevent further skin damage remains too hazardous, particularly in the long term [43].
Recently, it has become apparent that PBM (visible & NIR) can also be effective if delivered to normal cells or tissue before the actual insult or trauma, in a pre-conditioning mode [44].
Such application of cutaneous PBM called photoprevention employs visible and, for the most part, IR-A radiation to better prepare the skin for upcoming insults like UV induced sunburn. The process of exposing the skin to such radiation before its exposure to deleterious UVR wavelengths, closely emulates processes found in nature. This is understandable from an evolutionary standpoint since exposure to these early morning red and IR-A wavelengths in sunlight may ready the skin for the coming mid-day deleterious UVR.
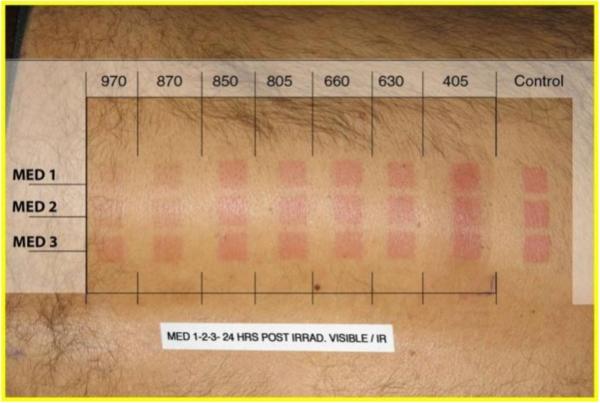
Several in vitro studies have shown that preconditioning fibroblasts with NIR could protect against upcoming UVB damage via p53 cell signaling-induced anti-apoptotic effects [45, 46]. In the first in vivo study, prior to exposure to UVB, test subjects were pre-treated with 660 nm light as compared to controls who were not [30]. The progression in the development of erythema following UVB exposure was used as a measure of the deleterious reaction to UVB. The results of the study showed a reduction in the UVB-induced erythema reaction in a significant number of the pre-treated subjects. Also a SPF-15-like sun protection factor effect and a reduction in post-inflammatory hyperpigmentation were observed. Subsequent experiments (unpublished data) using multiple wavelengths showed superior reduction in erythema in favor of the NIR end of the spectrum (figure 5).
Figure 5.
Visible to NIR wavelengths were applied 24h prior to MED 1, MED 2, and MED 3 (Oriel solar simulator) showing reduced erythema in favor of NIR wavelengths.
In a seminal in vivo study by Sayre et al. (1978) showing that you can duplicate sunlight with a sun simulator for sunscreen Sun Protection Factor (SPF) determination, an IR heat lamp was used to determine the effect of skin temperature on the SPF. The sunscreen was applied followed by heat to raise skin temperature to 33-35°C prior to solar simulator exposure. The control MED was almost unchanged by this treatment (original 2.11; heated 2.05), but the mean MED of the sunscreen protected area was more than 25% lower after heat treatment (original 7.93; heated 5.83), closer to the SPF measured under the sun [47]. In 1988, Chardon et al. exposed the back of 16 caucasian volunteers for 15 and 30 minutes to a total realistic irradiance of 70 mW/cm2 provided by four Philips-IR250S lamps and the effects were followed over six hours. The UVB and IR reactions and the IR*UV interaction were quantified 5 and 24 hours after irradiation. The IR*UVB interaction was found to be significantly positive with 1.5 and 2 MED at 5 hours and strongly reduced at 24 hours [48].
It has been recently shown in a pig model that pulsed 940 nm applied 4h or 24h prior to UVB exposure provided significant protection against UVB-induced acute actinic damage to pig skin compared to control [49]. Furthermore, the same study showed quantitative PCR (polymerase chain reaction) upregulation of procollagen type I and anti-apoptotic Bax gene expression and downregulation of MMP-1 (collagenase) and SOD2 (superoxide dismutase 2 an oxydative stress marker) when a pulsed 940 nm LED was applied 24 h prior to the UV insult [49].
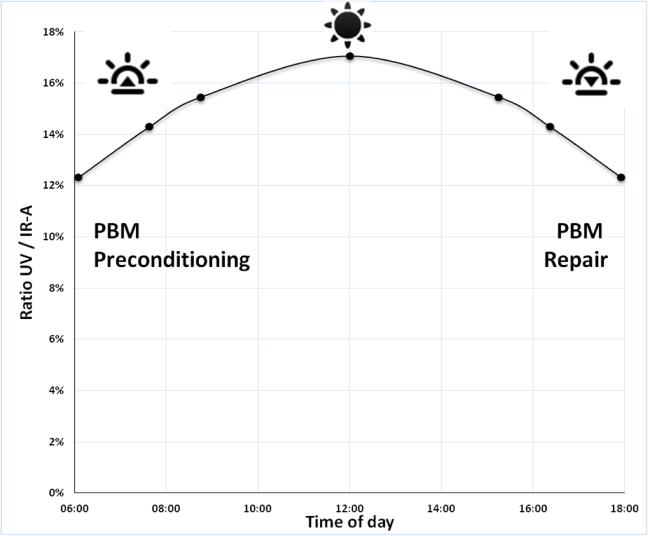
Some indication of the irradiance needed to optimize photoprevention is provided by a measurement and quantification of morning sunlight. As a precursor to the day's coming UV insults to the skin, the ratio of UV to IR-A, as measured in the tropics, is lower in the morning and at the end of the day (figure 6). Cooler morning temperatures combined with the proportionally lower UV/IR-A ratio provide the ideal conditions to trigger IR-A beneficial effects without skin hyperthermia before potential UV insults (higher UV/IR-A ratio at noon). The same applies late in the afternoon with PBM tissue repair, if UV damage occurs. Consequently, IR-A prevents and restores the possible mid-day UVR damage to the skin within beneficial physiological irradiance boundaries (figure 7).
Figure 6.
Ratio of UVR / IR-A solar irradiances, at sea level, with no clouds overhead, as available in the inter-tropics zone (where zenithal sun happens). Calculations were made using the Simple Model of the Atmospheric Radiative Transfer of Sunshine (SMARTS), 2.9.5 model, available from NREL and obtained in July 2015 at http://www.nrel.gov/rredc/smarts/about.html.
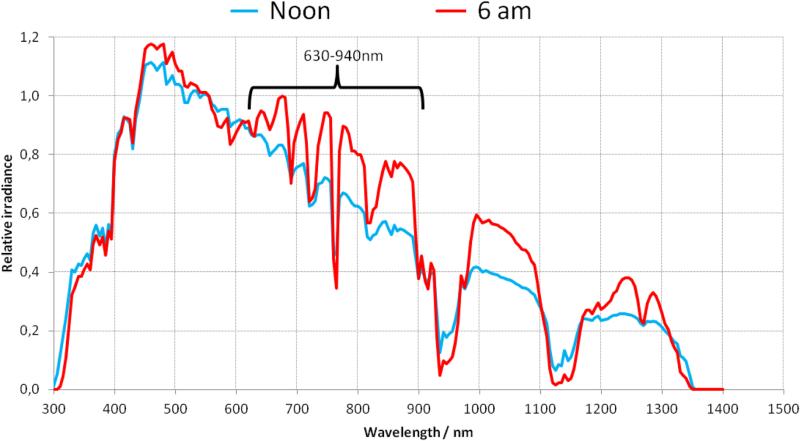
Figure 7.
Early morning (6am) relative irradiance of the sun is higher in the visible and NIR spectrum compared to midday exposure (noon). Calculations were made using the Simple Model of the Atmospheric Radiative Transfer of Sunshine (SMARTS), 2.9.5 model, available from NREL (National Renewable Energy Laboratory) and obtained in July 2015 at http://www.nrel.gov/rredc/smarts/about.html.
Based on the Sun's emission spectra, effective photoprevention wavelengths would fall between 630 and 940nm (figure 8). Additionally, the atmosphere blocks the passage of selective IR wavelengths with only some of the IR radiation above 950 nm making it to the Earth's surface. The water vapor in our atmosphere absorbs the rest.
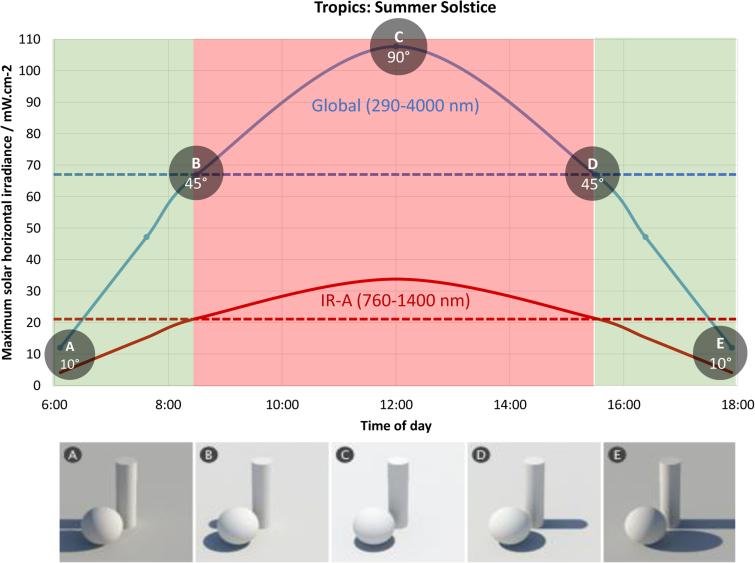
Figure 8.
The solar angle determines the irradiance at different times throughout the day, with a peak at noon (no shadow). The curves represent global solar irradiance for UV-Visible-IR (290-4000 nm) and its IR-A segment (760-1400 nm), as collected on a horizontal surface with no shadow and no clouds. The parameters input into the SMARTS 2.9.5 software are water vapour = 2, ozone = 3, aerosol optical depth at 500 nm = 0.2, and albedo = 0.2. From approximately 8:15 am to 3:45 pm, global sun irradiance climbs above 65 mW.cm−2. However, IRA irradiance peaks at midday at 35mW.cm-2 and remains within a safe therapeutic (PBM) range for the length of the day (mean: 20mW.cm−2). The dotted lines represent mean irradiances.
Emulating the sun with LED (light emitting diodes) devices at specific parameters, photoprevention represents an exciting treatment alternative for polymorphous light eruption patients or photosensitive individuals before they depart for a sunny destination in the winter.
8 DISCUSSION
8.1 Dose-dependent effects
The effects of visible & NIR wavelengths are dose-dependent. This is analogous to the beneficial long-term health effect of drinking a glass of red wine a day as opposed to the detrimental effect of drinking a whole bottle a day once a week. Visible & NIR comprise approximately 45-50% of the sun's emission spectrum respectively. However, when compared to UVR (2-3% of the sun's spectrum) their relative potency (ionizing radiation) is relatively low. Hence, we did not pay attention to the effect of NIR on the skin until recently. Several studies showed that NIR may damage skin collagen content via an increase in MMP-1 activity in the same manner as is known for UVR. Unfortunately, the artificial NIR light sources used in such studies were not representative of the solar irradiance [50]. Schauberger et al. (2004) published a method to evaluate how spectral irradiances from artificial sources compare to solar irradiance. The method, although proposed for UVR, can logically be extended and applied to other wavebands [51]. According to the Grotthus–Draper law only 10–12% of incident irradiance is absorbed in the dermis in vivo. This causes additional difficulties in the correlation of the in vitro experimental situation to in vivo conditions [52]. In this respect, the irradiance used in vitro is equivalent to an incident irradiance at the skin surface (in vivo) of between 210 and 300 mW/cm2 [50], which would cause a marked warming of the tissue resulting in heat pain if a “large” area of skin were to be exposed. Another discrepancy between the in vitro & in vivo situation becomes even greater when considering the effective dissipation of absorbed energy through blood flow in vivo and the fact that the solar IR-A irradiance is generally much less, due to smaller solar elevation angles before and after noon and during seasons other than summer [52]. Thus, the irradiance used in the in vitro experiments showing IR deleterious effects must be considered to be unnaturally high. Actually, the effects observed are most likely thermal effects (due to the increased temperature of the cells), which are not related to specific properties of IR-A radiation.
Chronic sun exposure is chiefly responsible for long term clinical skin changes such as photoaging and skin cancers [53]. These effects have been mostly attributed to the detrimental impact of ultra-violet (UV) radiation involving a combination of UVB (280–320 nm) and UVA (320–400 nm) wavelengths. In order to experimentally assess the effects of solar UV, standard UV spectra have been defined, particularly in the sunscreen industry. These emission spectra represent extreme solar UV exposure conditions with a quasi zenithal sun irradiance, representative of a high UVB level [54]. However, the solar spectrum reaching Earth depends on many parameters including latitude, season, meteorological conditions, ozone layer thickness, and particularly on time of day. Therefore, zenithal sun exposure conditions, corresponding to summer sunlight at noon in the tropics, are rarely found [53]. Thus, studies using extreme conditions with artificial NIR light sources reporting deleterious effects on the skin, would not appropriately reflect real life conditions of daily sun exposure.
To assess more realistic full solar exposure conditions, including visible and IR-A wavelengths, non-zenithal sun spectral irradiance should be used. Such spectra have been calculated and published by the CIE (Commission Internationale de l'Eclairage) [55]. Later, the CIE withdrew the publication because more recent and accurate solar spectral irradiance data became accessible. These are now freely available from the National Renewable Energy Laboratory website [56].
Exposure to visible and IR-A light can be beneficial to the skin depending on the right combination of wavelength, fluence, and irradiance. Produced by natural sunlight at certain times of the day, these favorable conditions may prepare the skin for the deleterious effects of the mid-day UVR.
We are comparing apples and oranges in terms of the irradiance from water-filtered artificial IRA light sources versus natural IR-A emitted by the sun. In most studies, the Hydrosun 500 used was set for high irradiances [14, 17, 57]. Additional studies utilized a pulsed high peak power broadband (IPL) from Cutera [8, 9] and an Infrared-300 by Daekyoung [7] with an unnaturally high power density (irradiance).
These irradiances were incredibly high since beyond 100 mW/cm2, tissue hyperthermia occurs with possible induction of matrix metalloproteinase (MMP) expression in the dermis [11]. Such data needs to be reconciled with the in vivo experimental data that supports the salutary effects of NIR photobiomodulation in murine wound healing [58]. Of course, irradiation using lasers focused at specific wavelengths, typically at the shorter end of the IRA spectrum, could be expected to result in different genes being expressed as compared to those genes expressed as a result of exposure to light from IR-A lamps emitting light spanning the entire IR-A spectrum. The transcriptome differences at different specific IR-A wavelengths would be an interesting subject for further study. However on balance, it is most likely that the reported conflicting effects of IR-A on the skin are principally connected with the well-known biphasic dose-response curves that have been observed in practically every aspect of photobiomodulation [26, 59]. Fluences in the range of tens of J/cm2 are likely to be protective and overall beneficial to the skin, while fluences in the range of hundreds of J/cm2 are likely to be damaging and overall deleterious to the skin. The same would apply for irradiance parameters.
9 CONCLUSION
Photobiomodulation exposure to visible and IR-A light which emulates the conditions of natural sunlight in wavelength, intensity, and dosage can be beneficial to the skin. Such light exposure might even pre-condition the skin, preparing it for upcoming (mid-day zenithal) UVR insults. On the other hand, exposure to artificial IR-A radiation of too broad of a range and intensity/dose can contribute to existing detrimental effects or cause negative effects of its own (increased MMP-1). Several studies (2, 13, 14, 43, 49, 51-55) demonstrate the damaging effects of IR-A radiation in the skin both in vitro and in vivo. However, they use high-intensity artificial IR-A light sources that do not reproduce real life daily sun exposure. The IR-A emitted by the sun and reaching the skin is not of such high intensity. Some investigators even suggest the development of sunscreens protecting against IR-A. Such claims are irrelevant for consumers and medical professionals because the assessment of IR-A-induced damage evidenced at a physiologically realistic intensity is a pre-requisite. Yet, it has been shown that at realistic irradiances/doses, IR-A radiation has beneficial effects on collagen metabolism and upcoming UVR damage. Similar to PBM parameters, daily IR-A sun exposure delivers a much lower irradiance and fluence (dose) than powerful artificial sources (760-1450 nm).
One could therefore assume that early morning “sun salutation” (surya namaskar) and late afternoon procrastination on the beach are actually natural PBM treatments to prevent and repair, respectively. Consequently, if your shadow is taller than you are (in the early morning and late afternoon) you're taking advantage of the beneficial effects of IR-A while avoiding peak (Zenithal) harmful UVR [60, 61]. Ultimately, it is another way of being sun smart.
Highlights.
Challenge to the present status quo with regards to the effects of IR in the skin.
Confronting the current belief that the sun's IR-A is deleterious to the skin.
Solar IR-A emits less irradiance than artificial sources used in most studies.
At realistic irradiances IR-A has beneficial effects on collagen and UVR damage.
IR-A does more good than bad it depends on intensity and how we learn from the sun.
Acknowledgements
We are grateful to Dr Ying-Ying Huang for drawing Figures 1 and 3 and Greg Cormack for careful proofreading and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
DB & FC have no conflicts of interest. MRH was supported by US NIH grant R01AI050875.
REFERENCES
- 1.Kochevar IE, Pathak MA, PJ A. Photophysics, photochemistry, and phobiol-ogy. In: Fitzpatrick, editor. Dermatology in General Medicine. McGraw-Hill; NewYork: 1999. Photophysics, photochemistry, and phobiology. In: Fitzpatrick (Ed.), Dermatology in General Medicine. NewYork: McGraw-Hill. [Google Scholar]
- 2.Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19(5):228–34. doi: 10.1034/j.1600-0781.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller K, et al. Erythema ab igne. Dermatol Online J. 2011;17(10):28. [PubMed] [Google Scholar]
- 4.Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126(5):e1227–30. doi: 10.1542/peds.2010-1390. [DOI] [PubMed] [Google Scholar]
- 5.Cho S, et al. Effects of infrared radiation and heat on human skin aging in vivo. J Investig Dermatol Symp Proc. 2009;14(1):15–9. doi: 10.1038/jidsymp.2009.7. [DOI] [PubMed] [Google Scholar]
- 6.Piazena H, et al. Effects of water-filtered infrared-A and of heat on cell death, inflammation, antioxidative potential and of free radical formation in viable skin--first results. J Photochem Photobiol B. 2014;138:347–54. doi: 10.1016/j.jphotobiol.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim HH, et al. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech Ageing Dev. 2005;126(11):1170–7. doi: 10.1016/j.mad.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, et al. Non-thermal DNA damage of cancer cells using near-infrared irradiation. Cancer Sci. 2012;103(8):1467–73. doi: 10.1111/j.1349-7006.2012.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y, et al. Non-thermal cytocidal effect of infrared irradiation on cultured cancer cells using specialized device. Cancer Sci. 2010;101(6):1396–402. doi: 10.1111/j.1349-7006.2010.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barolet D, Boucher A. Radiant near infrared light emitting Diode exposure as skin preparation to enhance photodynamic therapy inflammatory type acne treatment outcome. Lasers Surg Med. 2010;42(2):171–8. doi: 10.1002/lsm.20886. [DOI] [PubMed] [Google Scholar]
- 11.Shin MH, et al. Chronic heat treatment causes skin wrinkle formation and oxidative damage in hairless mice. Mech Ageing Dev. 2012;133(2-3):92–8. doi: 10.1016/j.mad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Quan T, et al. Matrix-Degrading Metalloproteinases in Photoaging. J Invest Derm Symp P. 14(1):20–24. doi: 10.1038/jidsymp.2009.8. 0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halper J, et al. In vitro culture decreases the expression of TGF(beta), Hsp47 and type I procollagen and increases the expression of CTGF in avian tendon explants. J Musculoskelet Neuronal Interact. 2005;5(1):53–63. [PubMed] [Google Scholar]
- 14.Schieke S, et al. Infrared-A radiation-induced matrix metalloproteinase 1 expression is mediated through extracellular signal-regulated kinase 1/2 activation in human dermal fibroblasts. J Invest Dermatol. 2002;119(6):1323–9. doi: 10.1046/j.1523-1747.2002.19630.x. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder P, et al. Infrared radiation-induced matrix metalloproteinase in human skin: implications for protection. J Invest Dermatol. 2008;128(10):2491–7. doi: 10.1038/jid.2008.116. [DOI] [PubMed] [Google Scholar]
- 16.Piazena H, Kelleher DK. Effects of infrared-A irradiation on skin: discrepancies in published data highlight the need for an exact consideration of physical and photobiological laws and appropriate experimental settings. Photochem Photobiol. 2010;86(3):687–705. doi: 10.1111/j.1751-1097.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 17.Jantschitsch C, et al. Infrared radiation does not enhance the frequency of ultraviolet radiation-induced skin tumors, but their growth behaviour in mice. Exp Dermatol. 2011;20(4):346–50. doi: 10.1111/j.1600-0625.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 18.Barolet D, et al. Regulation of skin collagen metabolism in vitro using a pulsed 660 nm LED light source: clinical correlation with a single-blinded study. J Invest Dermatol. 2009;129(12):2751–9. doi: 10.1038/jid.2009.186. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Roh MR, Lee KH. Effects of infrared radiation on skin photo-aging and pigmentation. Yonsei Med J. 2006;47(4):485–90. doi: 10.3349/ymj.2006.47.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SY, et al. A prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J Photochem Photobiol B. 2007;88(1):51–67. doi: 10.1016/j.jphotobiol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Bunsen R, R.H.E. Photochemische Untersuchungen. Poggendorff's Annalen 1855, 1857, 1857, 1857, 1859. 96100101108:373–394. 43–88, 481–516, 235–263, 193–273. [Google Scholar]
- 22.van Breugel HH, Bar PR. Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med. 1992;12(5):528–37. doi: 10.1002/lsm.1900120512. [DOI] [PubMed] [Google Scholar]
- 23.Kollias N, Bykowski JL. Immediate pigment darkening thresholds of human skin to monochromatic (362 nm) ultraviolet A radiation are fluence rate dependent. Photodermatol Photoimmunol Photomed. 1999;15(5):175–8. doi: 10.1111/j.1600-0781.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 24.Sommer AP, et al. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg. 2001;19(1):29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 25.Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg. 2008;27(4):227–38. doi: 10.1016/j.sder.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Huang YY, et al. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9(4):602–18. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanzafame RJ, et al. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg Med. 2007;39(6):534–42. doi: 10.1002/lsm.20519. [DOI] [PubMed] [Google Scholar]
- 28.Mester E, Szende B, Gartner P. [The effect of laser beams on the growth of hair in mice]. Radiobiol Radiother (Berl) 1968;9(5):621–6. [PubMed] [Google Scholar]
- 29.Avci P, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 30.Barolet D, Boucher A. LED photoprevention: reduced MED response following multiple LED exposures. Lasers Surg Med. 2008;40(2):106–12. doi: 10.1002/lsm.20615. [DOI] [PubMed] [Google Scholar]
- 31.Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32(3):415–25. doi: 10.1016/j.det.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Tam G. Laser Florence 2003 : A Window on the Laser Medicine World. Proc. SPIE; Florence, Italy: 2003. The hypoalgesic effects of low-intensity infrared laser therapy: a study on 555 cases. p. 5610. [Google Scholar]
- 33.Akhalaya MY, et al. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res Rev. 2014;16:1–11. doi: 10.1016/j.arr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Vatansever F, Hamblin MR. Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med. 2012;4:255–266. doi: 10.1515/plm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue S, Kabaya M. Biological activities caused by far-infrared radiation. Int J Biometeorol. 1989;33(3):145–50. doi: 10.1007/BF01084598. [DOI] [PubMed] [Google Scholar]
- 36.Yu SY, et al. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006;22(2):78–86. doi: 10.1111/j.1600-0781.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 37.Mero A, et al. Effects of far-infrared sauna bathing on recovery from strength and endurance training sessions in men. Springerplus. 2015;4:321. doi: 10.1186/s40064-015-1093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrado LA, Munin E. Reduction in body measurements after use of a garment made with synthetic fibers embedded with ceramic nanoparticles. J Cosmet Dermatol. 2011;10(1):30–5. doi: 10.1111/j.1473-2165.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 39.Ko GD, Berbrayer D. Effect of ceramic-impregnated “thermoflow” gloves on patients with Raynaud's syndrome: randomized, placebo-controlled study. Altern Med Rev. 2002;7(4):328–35. [PubMed] [Google Scholar]
- 40.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and nonmitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140C:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 41.Wunsch A, Matuschka K. A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed Laser Surg. 2014;32(2):93–100. doi: 10.1089/pho.2013.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decraene D, et al. A low UVB dose, with the potential to trigger a protective p53-dependent gene program, increases the resilience of keratinocytes against future UVB insults. J Invest Dermatol. 2005;125(5):1026–31. doi: 10.1111/j.0022-202X.2005.23909.x. [DOI] [PubMed] [Google Scholar]
- 43.Halliday GM. Activation of molecular adaptation to sunlight--a new approach to photoprotection. J Invest Dermatol. 2005;125(5):xviii, xix. doi: 10.1111/j.0022-202X.2005.23940.x. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal T, et al. Pre-conditioning with low-level laser (light) therapy: light before the storm. Dose Response. 2014;12(4):619–49. doi: 10.2203/dose-response.14-032.Agrawal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menezes S, et al. Non-coherent near infrared radiation protects normal human dermal fibroblasts from solar ultraviolet toxicity. J Invest Dermatol. 1998;111(4):629–33. doi: 10.1046/j.1523-1747.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 46.Frank S, et al. Infrared radiation induces the p53 signaling pathway: role in infrared prevention of ultraviolet B toxicity. Exp Dermatol. 2006;15(2):130–7. doi: 10.1111/j.1600-0625.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 47.Sayre RM, et al. The correlation of indoor solar simulator and natural sunlight: Testing of a sunscreen preparation. Archives of Dermatology. 1978;114(11):1649–1651. [PubMed] [Google Scholar]
- 48.Chardon A, Moyal D, Hourseau C. Kinetics of the infra-red erythema and IR*UVB interaction. IFSCC Congress. 1988 [Google Scholar]
- 49.Barolet D. In vivo mechanisms of photoprevention in a pig model. Lasers in Surgery and Medicine. 2015;47(S26):42. [Google Scholar]
- 50.Schroeder P, et al. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic Biol Med. 2007;43(1):128–35. doi: 10.1016/j.freeradbiomed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Schauberger G, et al. Evaluation of the goodness of fit of solar simulated radiation to a reference solar spectrum for photobiological experiments. Med Phys. 2004;31(9):2509–19. doi: 10.1118/1.1772572. [DOI] [PubMed] [Google Scholar]
- 52.Piazena H, Kelleher D. Comments on “Cellular response to infrared radiation involves retrograde mitochondrial signaling”. Free Radic Biol Med. 2008;44(10):1869. doi: 10.1016/j.freeradbiomed.2008.01.019. author reply 1870-1. [DOI] [PubMed] [Google Scholar]
- 53.Marionnet C, et al. Different Oxidative Stress Response in Keratinocytes and Fibroblasts of Reconstructed Skin Exposed to Non Extreme Daily-Ultraviolet Radiation. PLoS ONE. 2010;5(8):e12059. doi: 10.1371/journal.pone.0012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marionnet C, Tricaud C, Bernerd F. Exposure to non-extreme solar UV daylight: spectral characterization, effects on skin and photoprotection. Int J Mol Sci. 2015;16(1):68–90. doi: 10.3390/ijms16010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CIE Solar spectral irradiance. 085:1989. ISBN 978 3 900734 22 0. http://www.cie.co.at/index.php/Publications/index.php?i_ca_id=360. [Google Scholar]
- 56.NREL National Renewable Energy Laboratory. http://www.nrel.gov/rredc/smarts/about.html.
- 57.Schroeder P, Haendeler J, Krutmann J. The role of near infrared radiation in photoaging of the skin. Exp Gerontol. 2008;43(7):629–32. doi: 10.1016/j.exger.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomed Laser Surg. 2010;28(3):291–325. doi: 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 59.Huang YY, et al. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–83. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Downham TF., 2nd The shadow rule: a simple method for sun protection. South Med J. 1998;91(7):619–23. [PubMed] [Google Scholar]
- 61.Holloway L. Atmospheric sun protection factor on clear days: its observed dependence on solar zenith angle and its relevance to the shadow rule for sun protection. Photochem Photobiol. 1992;56(2):229–34. doi: 10.1111/j.1751-1097.1992.tb02151.x. [DOI] [PubMed] [Google Scholar]