Abstract
Several important paradigm shifts have occurred in the field of schizophrenia treatment, including an increased focus on early detection, the development of preemptive interventions, and the view of schizophrenia as a neurodevelopmental disease characterized by decreased efficiency and abnormal connectivity in cortical and subcortical neural networks. In this review article, we will briefly describe some of the neural impairments that contribute to the development of schizophrenia, with an emphasis on the impact of stress and trauma on cognitively vulnerable neural systems. We will then present current data on two behavioral interventions that target these critical risk factors and that aim to preempt the onset of schizophrenia in vulnerable individuals or improve the clinical course in recent onset schizophrenia: cognitive therapy and computerized cognitive training.
Keywords: clinical high risk, ultra high risk, recent onset schizophrenia, cognitive therapy, cognitive training, neuroplasticity
Early Detection and Intervention in Schizophrenia: the Concept of the Clinical High Risk (CHR) Population
Psychologists have been interested in intervening early in the course of schizophrenia for decades (Chapman et al. 1982), but they have only recently established early detection and intervention as a priority treatment target and as the basis for delivering highly specialized services across the globe. The aim of intervening early in the course of illness is to decrease the duration of untreated psychosis (DUP)– an approach which is associated with better outcomes (Norman et al. 2005)– and also to provide treatments during a time of maximum receptivity, a period Birchwood et al (Birchwood et al. 1998) call the ‘critical period’. Early intervention represents a major paradigm shift in the diagnosis and treatment of schizophrenia: from a stance of therapeutic nihilism with a focus on long-term disability, to an expectation that providing interventions at the earliest possible point will result in improved outcomes, including amelioration of symptoms and functional recovery.
A recent extension of this approach involves efforts to identify individuals prior to the onset of psychosis. The “prodromal period” preceding psychosis is a well-established concept (Yung & McGorry 1996a) consisting of non-specific symptoms, including depression, anxiety, and sleep disturbance, as well as attenuated positive psychotic symptoms and brief intermittent fully psychotic symptoms (Yung & McGorry 1996b). Assessments and clinical symptom criteria have been developed to identify individuals during this period, but they do not predict development of full psychotic disorders with 100% accuracy. Therefore, rather than using the term “prodromal,” which assumes definite transition to full psychosis, the term “clinical high risk (CHR)” is used to describe this syndrome (French & Morrison 2004). The term Ultra High Risk (UHR) is also often used to refer to this population. However, in order to clearly differentiate between those with clinically significant symptoms and those who may be at a genetic high risk for psychosis but who are not presenting with symptoms, we will use the term Clinical High Risk or CHR in this review.
The drive to identify those at high risk of developing psychosis emanates from attempts to provide pre-emptive interventions to this population. Mrazek and Haggerty (1994) stated that ‘the best hope now for schizophrenia lies with indicated preventive interventions targeted at individuals manifesting precursor signs and symptoms who have not yet met full criteria for diagnosis’ (p154). These interventions aim to prevent or delay the transition to psychosis while treating concurrent problems such as depression, anxiety, and interpersonal difficulties. In addition, should conversion to psychosis occur, a secondary benefit of having provided intervention during the prodrome is that the individual is already engaged in treatment, thus reducing the DUP and providing a less traumatic, and by extension less disruptive, development of full-blown clinical symptoms (Yung & Nelson 2011).
In this review article, we will briefly describe some of the neural impairments that contribute to the development of schizophrenia, with an emphasis on the impact of stress and trauma on cognitively vulnerable neural systems. We will then present current data on two behavioral interventions that aim to preempt the onset of schizophrenia in vulnerable individuals or improve the clinical course in recent onset schizophrenia: cognitive therapy and cognitive training. We will also describe the neuroscience-informed approach to cognitive training used in our laboratory, and discuss other emerging interventions in the field.
Neural System Impairments that Contribute to the Development of Schizophrenia
Schizophrenia is a highly heritable disorder, with various genes accounting for 80–85% of liability, yet concordance rates of monozygotic twins are around 50%, with the remaining variance attributed to environmental insults such as maternal infection during gestation, perinatal hypoxia, fetal malnutrition, and the like (Brown 2011). Genetic factors interact with environmental insults, many of them occurring during the pre- and peri-natal period, with the result that at-risk individuals are vulnerable to a range of environmental stressors. This interaction of genes and environment leads to aberrations in brain development and neural network functioning, which are not typically evident until adolescence or very early adulthood, when brain maturation is nearing completion (Andreasen 2010; Hoffman & McGlashan 1997). At that point, usually as a response to environmental stressors, the individual (who may until then have had no observable symptoms or only mild non-specific symptoms), experiences a break with reality and experiences psychosis—often initially in mild or attenuated form, which if left untreated then progress into a full-blown psychotic episode. Understanding schizophrenia as a neurodevelopmental disorder characterized by decreased efficiency and abnormal connectivity in cortical and subcortical neural networks—rendering them particularly vulnerable to the deleterious effects of stress– sheds light on why interventions that help an individual to respond more adaptively to stressful stimuli, or that target abnormal neural system processing, may help to ameliorate the early course of illness.
In healthy neural development, the maximal proliferation of synapses and the peak of synaptic density occur at approximately two years of age, which is then followed by a steady drop in synaptic density during childhood followed by a steep decline in adolescence (Spear 2003). This process is referred to as synaptic pruning, which especially in prefrontal brain regions is essential for increased efficiency of neural processing (Spear 2003). The behavioral concomitants of synaptic pruning in the prefrontal cortex are an increased ability to solve abstract and complex problems and enhanced reasoning and planning capabilities (Spear 2003). Several studies suggest that an overly aggressive or abnormal synaptic pruning process may be a contributing factor to the onset of psychosis in adolescence (Andreasen et al. 2011; McGlashan & Hoffman 2000); for example, individuals with schizophrenia exhibit decreased dendritic spine density (Glantz & Lewis 2000), but intact number of neurons (Selemon et al. 1995). At present it is not known which specific gene products or molecular/cellular processes may be contributing to the development of abnormal synapse pruning and synaptic functioning in schizophrenia, but the net effect is a reduction in gray matter volume in key cortical and subcortical regions. For example, in a recent meta-analysis on clinical high risk patients, those who later converted to psychosis showed baseline decreases in gray matter in frontal and temporal areas (Fusar-Poli et al. 2012). It is entirely plausible that a cascade of aberrant neural system functioning in schizophrenia, including reductions in neuronal integrity and synaptic density, could be the cumulative result of one or more abnormalities in basic plasticity mechanisms, with ongoing maladaptive changes being generated throughout distributed cortical neural networks (Balu & Coyle 2011).
Another critical process in neurodevelopment is increased white matter density. Starting in adolescence and leading into early adulthood, the hippocampus and frontal lobe undergo a substantial process of myelination, which is in large part driven by experience-dependent plasticity mechanisms in the brain (or simply put, adaptive learning). Again, in schizophrenia, accumulating evidence suggests that this process is disrupted; the net result is that decreased white matter density impairs the fast and efficient integration of information processing both within and across cortical zones, and contributes to cognitive impairment (Dwork et al. 2007). For example, diffusion tensor imaging studies show that clinical high risk patients do not show the normal increase in white matter with age, and that this is associated with poor functional outcome (Karlsgodt et al. 2009; Carletti et al. 2012). In the largest longitudinal study to date investigating brain volume changes over time in a cohort of patients with schizophrenia, the results confirmed decreases in multiple gray and white matter regions, which were most pronounced two years after the first episode of psychosis (Andreasen et al. 2011). It is again highly likely that the reduced white matter development which characterizes schizophrenia is a reflection of the distributed effects of abnormal and inefficient information processing mechanisms that arise from very basic impairments in the normal representational and plasticity functions of the cortex. Overall, the picture in early schizophrenia is one of a brain that has undergone aberrant patterns of neurodevelopment, with reduced functional connectivity among key neural systems and reduced efficiency in its cognitive and socio-affective operations. This suggests that, in order to prevent a deteriorating course, interventions must be designed to address and if possible, “correct” the abnormal neural system functioning before the individual has undergone what might be irreversible maladaptive changes in his/her cortical representational systems.
The Impact of Stress and Trauma on the Development of Schizophrenia
The stress-vulnerability hypothesis of schizophrenia posits that environmental stressors interact with the underlying neurocognitive vulnerabilities described above in at-risk individuals to trigger the onset of psychosis (Nuechterlein & Dawson 1984; Walker & Diforio 1997). A number of studies have shown an association between stressors and psychotic relapse in individuals with established illness, and there is growing evidence of a link between stress and attenuated psychosis during the prodromal phase. The primary mechanism proposed to underlie this relationship is dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, the main neural stress response system in mammals. The HPA axis functions to regulate the response to stress through a cascade of hormones that proceeds through the HPA pathway. Cells in the periventricular nucleus of the hypothalamus secrete corticotropin-releasing hormone, which increase adrenocorticotropic hormone secretion by the pituitary gland, which stimulates the adrenal gland to release glucocorticoids (cortisol in humans), which then feedback via glucocorticoid receptors in the hippocampus to dampen the system once the stressor has passed.
A wealth of basic science evidence demonstrates the neurobiological consequences of stress that can mimic aspects of psychosis. Chronic stress and ongoing elevation of glucocorticoids in response to stress can cause stress “sensitization”, resulting in a reduction in hippocampal neurons, reduction of glucocorticoid receptors in the hippocampus (Sapolsky 1985; Sapolsky et al. 1990; Stein-Behrens et al. 1994) and suppression of long-term potentiation (Pavlides et al. 1993), which impairs declarative memory consolidation. Chronic stress not only impairs hippocampal function but also enhances unlearned and conditioned fear through its effects on the amygdala, which persist even after recovery from stress (Conrad et al. 2004; Vyas et al. 2004). In animal models (Meaney et al. 1989), prolonged stress has been shown to result in a dysregulated negative feedback circuit, whereby damaged hippocampi fail to correctly modulate HPA axis activity (Sapolsky et al. 1990) and has also been shown to increase glutamate in both the prefrontal cortex (PFC) and the hippocampus, which can result in altered dopamine levels (Moghaddam 2002). Although the exact molecular mechanisms linking stress and psychosis are beyond the scope of this review, there is sufficient evidence to suggest that chronic stress and dysregulated stress-responsivity impact the same neural systems implicated in psychosis.
Numerous studies have demonstrated a dysregulated stress response system in schizophrenia, primarily using cortisol assays to assess HPA axis integrity. Walder and colleagues (2000) reported that salivary cortisol levels were related to symptom severity in a sample of patients with schizophrenia, as well as performance on neuropsychological tasks within a combined sample of patients with schizophrenia, affective disorder, or no history of psychiatric illness. Basal cortisol and ACTH levels are higher in recent-onset and chronic schizophrenia patients (medicated and non-medicated) than in healthy controls (for a review, see Bradley & Dinan 2010). Both medicated and unmedicated schizophrenia patients show a heightened cortisol response to laboratory stressors (Jansen et al. 1998) and higher rates of nonsupression in response to dexamethasone, which tests the reduction of natural cortisol secretion in response to negative biological feedback (Mück-Seler et al. 1999), though some inconsistencies have been observed (Jansen et al. 1998; Jansen et al. 2000; Iqbal et al. 1991; Braehler et al. 2005). Although the exact nature of hypo- and hyper-responsiveness of the HPA axis in psychosis is unclear, schizophrenia patients undoubtedly show a dysregulated stress response.
A smaller, but growing area of work also suggests that stress responsivity and HPA-Axis functioning are also dysregulated during the prodromal phase of schizophrenia, relate to symptom levels, and are associated with the transition to full psychosis (for two recent reviews in this area, see Aiello et al. 2012 and Holtzman et al. 2012). In a sample of adolescents with schizotypal personality disorder (SPD), a group with a high rate of transition to schizophrenia, baseline salivary cortisol levels were associated with severity of schizotypal symptoms at 12- and 24-month follow-up (Walker et al. 2001), as well as transition to full psychosis within the group (Walker & Bollini 2002). Corcoran and colleagues found an association between basal cortisol and clinician ratings of anxiety, suspiciousness and impaired stress tolerance (Corcoran et al. 2012). In a third study, CHR youth showed elevated resting baseline cortisol levels relative to healthy controls (Yee et al. 2007). Following a laboratory social stress test, the magnitude of the patients’ cortisol response was less than in controls but they did show a stress response of increased cortisol, with a delayed pattern of cortisol recovery compared to controls (Yee et al. 2007). In two early studies with very small sample sizes, 3 young people who developed full psychosis within the 2-year follow-up period of the study had lower blood cortisol levels at baseline and higher cortisol levels at the latter stages of a dexamethasone/corticotrophin releasing hormone test, compared to 9 nonconverters (Thompson, Berger, et al. 2007; Thompson, Phillips, et al. 2007), but the reliability of these results have not been replicated in larger samples, suggesting that HPA axis overactivation is more common during the prodrome.
Structural magnetic resonance imaging (MRI) studies have revealed some evidence of differences in brain regions associated with the HPA axis in CHR patients that suggests hyperactivity during the prodromal phase. Two reports of increased pituitary volumes in CHR participants who later converted to full psychosis compared to those who did not convert are in line with this theory (Garner et al. 2005; Büschlen et al. 2011). Hippocampal volumes in CHRs are typically larger than those of fully psychotic patients but smaller than those of healthy controls with mixed evidence of size predicting transition to psychosis (Pantelis et al., 2009). Of note, impaired hippocampal function can be both a cause and a consequence of HPA axis overactivity, leaving the “chicken and egg” question of which comes first currently undetermined.
Interestingly, a growing literature has documented higher rates of childhood trauma in schizophrenia patients compared to controls, suggesting an environmental mechanism for increased stress responsivity in schizophrenia, although causality cannot be assumed (see Sideli et al. 2012 for a recent review, and Matheson et al. 2012 for a meta-analysis). The relationship between early adversity and later psychiatric symptoms is not specific to psychosis and is well-documented in depression and other disorders, but it may have some interaction with specific genes in producing different phenotypes in adulthood (Laucht et al. 2012), as well as interacting with other environmental risk factors for schizophrenia, such as early pre- or per-natal insults or later cannabis use (Pelayo-Tern et al. 2012).
More recently, investigators have begun examining the impact of everyday stress on full and attenuated psychosis. Fully psychotic patients who have experienced childhood trauma reported increased negative affect and psychotic symptoms in response to daily stress, compared to psychotic patients without childhood trauma (Lardinois et al. 2011), and a genetic polymorphism has been found to mediate the relationship between daily stressors and momentary increases in psychotic symptoms (Collip et al. 2011). Adolescents with SPD, who also develop full psychosis at an elevated rate of 21% by 2.5 years, have reported a greater number of total, independent and undesirable life events compared to healthy adolescents, and report more distress in response to daily hassles (Tessner et al. 2011). Additionally, frequency of daily stressors predicted an increase in positive symptoms one year later. In another study, CHR youth had lower levels of stressful life events and similar levels of daily hassles as an age-matched healthy control group, but nonetheless reported being more distressed by events and used different coping strategies (Phillips et al. 2012). Most relevant, CHR youth show a dopaminergic hyperresponse via PET imaging after exposure to a laboratory stressor compared to healthy controls and similar to antipsychotic-naïve schizophrenia patients (Mizrahi et al. 2012). Finally, genetic variation on the COMT and MTHFR genes interact to predict reactivity to daily stressors in schizophrenia patients (Peerbooms et al. 2012). Altogether, these studies suggest that stress reactivity is heightened in adolescents with attenuated and full psychosis, triggering fluctuations of dopamine transmission and the severity (and possibly the onset) of psychotic symptoms, and may be influenced by both genetic vulnerabilities and environmental inputs.
Given that CHR patients have been found to report even higher stress levels than first-episode psychosis patients (Pruessner et al. 2011), reducing stress and improving coping in CHR youth is a critical target for the development of preemptive interventions for schizophrenia. New areas of research are starting to focus on the natural plasticity of the HPA axis during adolescent development, with the aim of developing interventions during the prodromal phase that promote resiliency to stress. Some examples include cognitive therapy approaches that help individuals to re-appraise their interpretation of stressful stimuli in a more adaptive manner; behavioral stress-reduction techniques that teach individuals to take an active role in reducing their exposure to stressors; family therapy to reduce stressful familial interactions and increase family support; and cognitive training exercises that aim to improve the accuracy and fidelity of the processing of cognitive and socio-affective stimuli and increase the brain’s “cognitive resilience” to data in its environment.
Shifting the View of Schizophrenia from Neurodegeneration to Neuroplasticity and Recovery
As suggested in the previous section, a shift has occurred in the field of schizophrenia treatment development. The nihilistic conceptualization of the illness as a neurodegenerative disease associated with progressive functional deterioration (a view that dominated clinical thinking for the past 50 years) has changed to a perspective that—with early detection and intervention—intact brain plasticity mechanisms can be harnessed in an adaptive manner to promote healthier neural system functioning, increased resiliency to stressors, symptom reduction, and functional recovery. Indeed, if applied early enough in the course of illness, it is possible to envision harnessing these mechanisms to support true pre-emption of the usual illness trajectory.
Neuroplasticity refers to the brain’s capacity to change the molecular and structural features and systems that dictate its function (Cramer et al. 2011). The basic principles of brain plasticity have been extensively studied in animal experiments and clinically in the context of cortical recovery and remapping after injury or trauma, such as a stroke or amputation of a limb (Nudo et al. 1996; Ramachandran & Rogers-Ramachandran 2000). In the last decade, an increasing understanding of these principles has led to the development of cognitive training interventions aimed at exploiting the malleability of the cortex in order to drive behavioral improvements in neuropsychiatric disorders, with some of the most significant advances occurring in the field of schizophrenia (see Vinogradov et al. 2011 for a review). Previously, there was some skepticism about the ultimate possibility of neural system improvement or restoration of functioning in serious neuropsychiatric disorders such schizophrenia, due in part to the fact that these illnesses are characterized by impairments in multiple distributed limbic, prefrontal and frontostriatal neural circuits. Further, recent findings by Balu and Coyle (2011) indicate that schizophrenia risk genes such as those related to DISC-1, dysbindin, brain-derived neurotrophic factor and the N-methyl-D-aspartate receptor, are involved in regulating neuroplasticity; as described earlier, it is possible that alterations in their expression and/or functioning may contribute to the abnormal patterns of synapse formation and cortical connectivity observed in schizophrenia. This would suggest that there may be some inherent limitations in the “brain plasticity” mechanisms of individuals with schizophrenia.
Despite these areas of skepticism, emerging data suggests that it is indeed possible to develop intensive computerized cognitive training methods that successfully harness the brain learning machinery in schizophrenia to generate widespread adaptive behavioral and neural responses (Dale et al. 2010; Eack et al., 2009; Eack et al., 2010a; Fisher et al. 2009; Subramaniam et al. 2012) These adaptive improvements are accompanied by evidence of plastic changes in distributed cortical representational systems that have been driven by the cognitive training intervention and increases in grey matter volume (Adcock et al., 2009; Eack et al., 2010c; Subramaniam et al., 2012). Although this research has focused on computerized cognitive training methods, we wish to emphasize that a large body of work examining psychological treatment methods in young early psychosis individuals (such as cognitive therapy and multi-family groups), while not explicitly focused on understanding how these methods make use of cortical neuroplasticity, demonstrate successful and enduring behavioral effects. These methods are also likely optimized when they make use of the greater malleability of cortical representational systems early in the course of the illness.
Cognitive Therapy as a Pre-Emptive Intervention in Clinical High Risk Schizophrenia
One of the most frequently studied individual behavioral interventions for schizophrenia, particularly in the UK is cognitive therapy (CT). CT for psychosis addresses the relationship between thoughts, feelings and behaviors with a particular focus on assisting the client to examine, and ultimately alter, their interpretation of a situation. The main goal of this cognitive restructuring is distress reduction as a result of the client learning skills to interpret and assess stressful situations in a more adaptive, resilient, and helpful manner. Within this approach, skills are also taught to assist the client in recognizing and managing personal stressors, thus targeting an individual’s stress responsivity.
Wykes et al. (2008) concluded from a meta-analysis of adults with schizophrenia that CT for psychosis has a beneficial effect on positive symptoms, negative symptoms, mood and functioning. Moreover, recent evidence suggests that CT interventions in schizophrenia and in other disorders, such as depression and anxiety, result in functional neural network changes. For instance, Goldapple et al. (2004) examined regional changes in glucose metabolism and modulation of the limbic-corticol network with positron emission tomography (PET) in 17 unmedicated unipolar depressed outpatients who completed a 15–20 session course of CT relative to 13 paroxetine-treated responders. A full course of CT resulted in increased glucose metabolic changes in the hippocampus and dorsal cingluate and decreases in dorsal, ventral and medial frontal cortex. In contrast, paroxetine-responders demonstrated prefrontal increases and hippocampal and subgenual cingluate decreases. While both groups experienced ‘clinical recovery,’ as indicated by Hamilton Depression Ratings, the CT group’s recovery appeared to be facilitated through top-down frontal neural modulation of limbic and cortical regions. Goldapple and colleagues speculated that the directionality of the findings in the CT group suggests CT-induced increases in attention to appropriate emotional and environmental stimuli and a decrease in over-processing of irrelevant information and ruminations. These findings suggest that successful CT can modulate maladaptive responses to anxiety-provoking stimuli, making it an ideal intervention for high-risk clinical populations.
Furmark and colleagues (2002) also conducted a PET study to examine region specific functional changes in social phobia patients treated with 8 sessions of CT or citalopram, relative to a wait-list comparison group. Similar to Goldapple et al., (2004), both treatments resulted in symptomatic remission as well as attenuated neural activity during a public speaking challenge in the amygdala, hippocampus, and rhinal, parahippocampal, and periamygdaloid cortices. Only in the CT group, however, was decreased activation in the amygdaloid-hippocampal region predictive of one-year clinical outcome—indicating that enduring plastic changes in cortical representational systems may require behavioral interventions and may not occur from pharmacotherapy alone. Again, these data underscore the potential suitability of CT as a pre-emptive intervention in early schizophrenia.
Van der Gaag (2006) proposed that CT for psychosis would have the potential to modify or ‘rewire’ neural circuitry in a similar manner as shown in these anxiety and depression treatment trials. Van der Gaag suggested that CT trains an individual to re-appraise and reinterpret potentially threatening stimuli, resulting in reduced emotional responsivity to the stimuli. And indeed, very recently, Kumari and colleagues (2011) demonstrated that CT for psychosis, delivered over an average of sixteen sessions, led to significant clinical improvement and attenuation of activation in neural systems associated with threat perception. Specific functional regions included the inferior frontal gyrus, anterior insula, putamen, thalamus, and occipital areas. Interestingly, contrary to the investigators’ hypothesis, CT did not induce a significant change in amygdale activation. This study was the first to show that CT was associated with a neurofunctional change suggesting a modification in the representation and/or appraisal of stressful environmental stimuli, consistent with the behavioral skills learned in CT. The average age of the participants in this study was 35 years old, with an average length of psychosis of 10 years. As such, one would predict that, if CT were administered to those at risk of developing psychosis–who are typically 10–20 years younger with far less exposure to repeated episodes of psychotic symptoms and environmental insults- the neural effects of CT for psychosis would induce even more significant adaptive plasticity in neural representational systems and would prove to be pre-emptive.
To date, there exist only six randomized control trials of CT for the CHR population, and interpretation of results from these trials is hampered by their small sizes, the use of concomitant treatments, and attrition at follow up. Two studies (Morrison et al. 2004; Addington et al. 2011) explored the effectiveness of CT delivered as a stand-alone treatment. McGorry et al. (2002), and Yung et al. (2011) both examined CT combined with an antipsychotic medication, although Yung and colleagues (2011) include a CT plus placebo group. Bechdolf et al. (2012) compared supportive counseling with a package of interventions they termed ‘Integrated Psychological Intervention’ (IPI) which included CT along with multi-family psychoeducation, group social skills and cognitive remediation. More recently Morrison et al. (2012) reported a multi-site randomized control trial of CT compared with monitoring alone, the largest RCT to date exploring CT as an intervention to delay or prevent transition to psychosis. Table 1 briefly summarizes these studies.
Table 1.
Randomized Controlled Trials of Cognitive Therapy in Individuals at Clinical High Risk.
| Study and Sample Size | Intervention and control group | Conversion to psychosis1 | Clinical outcomes (e.g. symptoms, functioning) |
|---|---|---|---|
| McGorry et al (2002); N=59 | Intervention: CT plus low dose atypical antipsychotic (risperidone) for six months Control: Care management |
Lower conversion rate to psychosis at end of treatment (p=.03) No significant difference at six month follow up |
No significant difference between groups on any of the symptom or functioning measures |
| Phillips et al (2007); N=41 | 36 month follow up to McGorry et al (2002) | No significant difference | Active treatment group less negative symptoms (p=0.004) |
| Morrison et al (2004); N=58 | Intervention: CT plus monitoring Control: Monitoring alone |
96% reduction in odds of making transition to psychosis (Odds ratio 0.04, p = 0.028) | Active treatment group less positive symptoms (p=0.001) |
| Morrison et al (2007); N=27 | 36 month follow up to Morrison et al (2004) | No significant difference | 87% reduction in prescription of anti-psychotics (p=0.024) |
| Yung et al (2011); N=130 | Intervention: CT plus risperidone or CT plus placebo, over 12 months Control: Supportive Therapy plus monitoring or monitoring only, over 12 months |
No significant difference | No significant difference between groups on any of the symptom or functioning measures |
| Addington et al., (2011); N=51 | Intervention: CT over six months Control: Supportive Therapy |
No significant difference | Rapid decline in positive symptoms for both groups *CT improved faster with most improvement in first 3 months (p=0.0001) |
| Morrison et al, 2012; N=288 | Intervention: CT + Monitoring + TAU Control: Monitoring + TAU |
No significant difference | Significant difference in severity of psychotic symptoms (p=0.005) |
| Bechdolf et al, 2012; N=127 | Intervention: Computerized cognitive training (CogPack), individual cognitive-behavioral therapy, group skills training, and multifamily psychoeducation Control: Supportive Counseling |
At 12 months lower conversion rate to psychosis (p=0.08) At 24 months lower conversion rate to psychosis (p=0.019) |
No significant difference between groups in anti-depressant use |
conversion to psychosis compared with comparison group.
Despite early optimism that CT would be effective in preventing the onset of psychosis in at-risk individuals (McGorry et al. 2002; Morrison et al. 2004), initial promising results have not been maintained at follow up and/or have not been replicated in later studies (Addington et al. 2011; Yung et al. 2011). As is common in longitudinal research, the follow-up studies suffered from attrition and small sample sizes, which may have compromised power to detect clinically significant differences between groups. Morrison et al. (2012) collaborated across five sites and recruited a total of 288 participants, but despite this larger sample size, the study failed to detect differences between the CT group and the control group (who only received monitoring of symptoms) in conversion to psychosis. The authors attributed this to the low rate of conversion across the two groups, and to the possible benefit to participants in the control condition of receiving regular assessment and monitoring. This study also raised the question of accurate detection of a truly “clinical high risk” population.
In contrast, Bechdolf et al. (2012) demonstrated that their multimodal IPI package, studied in 63 participants, was effective in reducing conversion to psychosis in participants exhibiting Early Initial Prodromal States (EIPS) when compared with supportive therapy (n=65). These individuals were identified as experiencing self-reported changes in thought and perception, which are believed to precede the onset of sub-threshold psychotic symptoms that defines CHR; in earlier studies, 70% of those identified with the EIPS are found to convert to psychosis over 5 years (Klosterkötter et al. 2001). Bechdolf et al. (2012) suggest that individuals with EIPS experience less severe symptoms, lower disability, and fewer neurobiological deficits than the UHR population, and are therefore more amenable to pre-emptive interventions. The IPI package provided participants with CT, multi-family psychoeducation, group social skills, and computerized cognitive remediation. This multi-domain approach to intervention, which targeted both the individual and family, capitalized on multiple “points of entry” into reducing stressors and improving cognitive resilience in vulnerable youth.
A number of factors may account for the unclear findings on the usefulness of CT for pre-emption of psychosis in CHR individuals. First, multiple investigators have noted a global trend towards lower conversion rates in CHR youth, from an average range of 40–50% conversion reported in the earliest published studies to rates of around 10–15% reported in 2007 (Yung et al. 2007). Yung et al. (2011) discuss this in relation to their longitudinal study of CT and pharmacologic treatment and posit that this may be the explanation for a lack of difference between treatment groups. They suggest that the decrease in conversion may be an artifact of improved referral strategies, resulting in more people entering treatment with ‘incidental psychotic experiences’ that are not in fact the manifestation of an incipient psychotic illness. If this is indeed the case, then further research is required in order to establish assessment methods that differentially identify those truly at risk of developing psychotic illness from those experiencing ‘incidental psychotic experiences’.
Second, Yung et al. (2011) also note that individuals in the very early stages of the CHR period may be quite responsive to non-specific psychological therapies, such as supportive therapy. This has led Yung et al. to propose a clinical staging model to aid treatment decision making: individuals in the earliest stages of clinical presentation should receive non-specific, and thus less costly and less intensive treatments, such as supportive therapy, while those later in the course of the CHR period and who present with specific attenuated symptoms should receive specialized CT.
However, Bechdolf et al. (2012) demonstrated that individuals experiencing EIPS benefited from a specialized multi-modal intervention package that was superior to supportive counseling. Again, a clinical staging approach requires advances in our ability to accurately differentiate between individuals exhibiting incidental psychotic experiences (who may benefit from less intensive treatment) and those experiencing true risk for a psychotic illness (such as demonstrated by the EIPS, for example), who may require specialized intervention.
Addington et al. (2011) have also endorsed the clinical staging model, but highlight the difficulties associated with providing specialized CT to the CHR population. In interpreting the non-significant difference they found between supportive therapy vs. CT, they cited the lack of experience of their trial therapists, who were not doctoral level CT specialists. Addington et al. suggest that interventions aimed at treating individuals with specific attenuated psychotic symptoms should be undertaken by specially trained and experienced therapists. However, community treatment settings are predominantly staffed by Master’s level clinicians, and further research must explore the feasibility and effectiveness of training community clinicians to provide high-fidelity specialized CT for the CHR population.
Although conversion to psychosis, or pre-emption, is the key outcome measure for CT studies in CHR individuals, it is also important to consider the impact of CT on symptoms and functioning. Table 1 shows that, in some instances, CT appears to induce a more rapid amelioration of positive symptoms (Morrison et al. 2004; Addington et al. 2011) as well as decreased severity of positive symptoms (Morrison et al. 2012). This may be of benefit, given research that suggests that DUP is associated with long term morbidity (Thompson et al., 2001). Thus a more rapid amelioration of psychotic symptoms results in less exposure to potentially traumatizing and distressing experiences, as well as less induction of maladaptive neuroplastic changes in response to those experiences.
Future Directions for Cognitive Therapy
While early results are inconclusive for CT delivered as a stand-alone pre-emptive treatment, a number of treatment elements require further investigation and development. Studies typically have used either French and Morrison’s (2004) manual or the manual developed as the Personal Assessment and Crisis Evaluation (PACE) described by Phillips and Francey (2004). French and Morrison’s manual is based on Morrison’s cognitive model of psychosis (2001); although there are no formal modules, treatment advances through engagement, assessment, formulation, intervention, and relapse prevention. The PACE model is based upon a stress-vulnerability rationale (Zubin & Spring 1977) and works through specific phases (engagement, assessment, treatment, termination), with modules for each of these. Thus, within CT there are both specific and non-specific treatment elements, such as specific cognitive formulation of attenuated psychotic symptoms (Morrison 2001) vs. more generic therapeutic elements such as engagement and interpersonal effectiveness. Further study is required to identify the ‘active ingredients’ in CT so that the development and implementation of a staged intervention approach can reflect the trajectory from nonspecific supportive interventions to specific CT that targets attenuated psychotic symptoms. Such a staged intervention approach could also guide training of clinicians in early psychosis clinical services, with less experienced staff providing early engagement and supportive therapy to clients early in the CHR period, while more experienced and specialized staff work with clients who have identified target symptoms.
In sum, while some early results show promise, further research is needed to determine whether CT is an effective pre-emptive treatment using sufficiently powered sample sizes, algorithm-derived and operationalized staged interventions, assessment of effectiveness in community settings, and generalizability to non-western contexts. Kim et al. (2011) suggest that this is a transportable intervention beyond traditional western clinical settings in their study of the clinical efficacy of CT in a CHR sample in Korea, but further research into this area is required. The recent work of Bechdolf et al. (2012) suggests that pre-emption may best be achieved when CT is one component of a multi-modal intervention that targets multiple domains of psychological, interpersonal, and cognitive functioning.
The Role of Impaired Cognition in Clinical High Risk and Recent Onset Schizophrenia
Abundant evidence indicates that, in addition to increased stress responsivity, CHR individuals show cognitive impairment well before illness onset. Relative to normative samples and healthy control subjects, high risk individuals show deficits in vigilance, speed of processing, working memory, verbal learning and memory, executive functioning, global cognition and IQ prior to the first psychotic episode (Becker et al., 2010; Brewer et al., 2005; Eastvold et al., 2007; Jahshan et al., 2010; Keefe et al., 2006; Kim et al., 2011; Lencz et al., 2006; Niendam et al., 2006; Seidman et al., 2006; Simon et al., 2007). A recent meta-analysis comparing 1188 high risk subjects and 1029 healthy controls found small to moderate impairments in high risk subjects in general intelligence, executive functioning, verbal and visual memory, verbal fluency, attention and working memory, and social cognition (Fursa-Poli et al., 2012). In the largest study to date on neurocognition in high risk individuals, the NAPLS consortium (North American Prodrome Longitudinal Study) found that processing speed and verbal learning and memory were the most sensitive in discriminating high risk individuals from controls (Seidman et al., 2010).
From high risk status to first psychotic episode, individuals show continued cognitive impairment (Becker et al., 2010; Hawkins et al., 2008), or further cognitive decline (Keefe et al., 2006; Simon et al., 2007; Wood et al., 2007; Jahshan et al., 2010), and CHR patients have been shown to perform intermediately between recent onset patients and control subjects (Keefe et al. 2006; Seidman et al., 2010; Simon et al. 2007). At first psychotic episode, multiple cognitive deficits are evident that approach or match the degree of deficit shown in chronic schizophrenia, with the largest impairments seen in processing speed and immediate verbal memory (Mesholam-Gately et al., 2009, meta-analysis of 43 studies (N=2204) of recent onset schizophrenia).
Most importantly, strong evidence indicates that cognitive functioning in the prodromal phase is a significant predictor of conversion to psychosis. At risk individuals who later develop psychosis show deficits in a range of cognitive abilities such as verbal memory, verbal executive functions, verbal IQ, visuospatial processing, visual memory, composite measures of cognition, and social cognition (Brewer et al., 2005; Eastvold et al., 2007; Lencz et al., 2006; Keefe et al., 2006; Kim et al., 2011; Pukrop et al., 2007). In the meta-analysis of Fursa-Poli et al., (2012) high risk individuals who later transitioned to psychosis showed significantly lower general intelligence and poorer cognitive functioning in verbal fluency, verbal and visual memory, and working memory compared to high risk individuals who did not transition to psychosis.
Verbal memory appears to be a particularly important indicator of vulnerability to psychosis. For example, Pukrop et al., (2007) found that verbal memory was the strongest predictor of conversion to psychosis followed by verbal IQ, verbal learning, and processing speed, while the NAPLS group found that worse verbal memory predicted more rapid conversion to psychosis (Seidman et al., 2010). Simlarly, Lencz et al. (2006) found deficits on a range of measures of verbal memory, executive functioning, and working memory in high risk individuals, while those who later converted to psychosis showed significant impairment in verbal memory. The authors suggest that verbal memory deficits may be an important risk marker for later conversion to psychosis, while generalized cognitive impairment in high risk individuals may be a nonspecific vulnerability marker.
Cognitive deficits not only predict psychosis but are also associated with poorer functional outcome. The relationship between cognition and functional outcomes in individuals with chronic schizophrenia is well documented (e.g. Green, 1996; Green et al., 2000), while emerging evidence suggests a similar relationship in high risk individuals. For example, Niendam et al. (2006) found that poorer verbal learning and memory performance was significantly associated with poorer social functioning in individuals at high risk, and a trend level association was found between poorer performance on reasoning and problem solving and poorer global functioning. Further, verbal memory predicted social functioning more strongly than negative symptoms. Similarly, in recent onset schizophrenia, verbal memory, speed of processing, and attention have been shown to predict psychosocial and vocational functioning 7 years later (Milev et al., 2005).
The stronger impact of cognition on functioning relative to the effect of symptoms has important treatment implications. In both recent onset and chronic schizophrenia, evidence suggests that cognitive functioning and symptom severity are for the most part independent of one another: cognitive deficits are present in the absence of symptoms, and while antipsychotic medications are effective in ameliorating positive symptoms, they have little effect on cognitive impairment or long-term outcome (e.g., Keefe et al., 2007; Goldberg et al., 2007). In high risk studies, evidence suggests a similar dissociation. Simon et al. (2007) found that an increase in symptom severity was not paralleled by an increase in cognitive impairment, while Hawkins et al. (2008) found that neither the onset of frank psychosis nor olanzapine treatment altered the course of cognitive impairment. Given the relative independence of cognitive functioning and symptoms, as well as the deleterious effects of cognitive impairment on social and occupational outcomes and quality of life, it is clear that cognitive enhancement is a critical treatment goal in early psychosis, and may be a means for intervening preemptively to ameliorate the course of illness, or perhaps even to inoculate the at-risk individual against the onset of a first psychotic episode.
While a range of cognitive deficits are evident in individuals at high risk, and additional research is needed in this area, verbal memory appears to be a particularly relevant pre-emptive target given that deficits in this domain have been shown to predict conversion to psychosis, are associated with poorer functional outcome, and show the largest impairment in recent onset schizophrenia. Thus, the field must move towards: 1) early detection of cognitive impairment in individuals at high risk for psychosis, and 2) the application of targeted interventions to enhance cognition, with an emphasis on verbal memory. To date, novel cognitive-enhancing pharmacological agents have proven disappointing (for a review see Keefe et al., 2011) however, cognitive training approaches are showing promise and are a growing area of investigation.
Overview of Cognitive Training in Early Psychosis
The past 15 years have seen explosive growth in research and clinical interest in cognitive remediation for chronic schizophrenia. Meta-analytic work confirms that a wide range of non-computerized and computerized approaches results in moderate increases in global cognition measures, while significantly stronger effects on functioning are evident when cognitive training is provided together with other psychosocial rehabilitation (McGurk et al., 2007; Wykes et al., 2011). Although several randomized controlled trials are currently in progress to determine the effects of cognitive training in early psychosis (e.g., Breitborde et al., 2011; Vesterager et al., 2011; Vinogradov et al., ClinicalTrials.gov Identifier: NCT00655239 and Fisher et al., under review), only two studies to date have reported the effects of cognitive training in individuals at clinical high risk (Bechdolf et al., 2012; Rauchensteiner et al., 2011), and four have reported on the effects in recent onset schizophrenia (Eack et al., 2009, 2010a, 2010b, and 2010c; Hansen et al., 2012; Ueland & Rund, 2004 and 2005; Wykes et al, 2007). A variety of intervention methods have been used, including computerized cognitive training, therapist guided training with paper/pencil tasks, and adaptive training designed to bypass cognitive deficits using compensatory strategies. Below, we review these 6 studies and then describe the work being carried out by our group.
Cognitive Training in Individuals at High Risk for Psychosis
CogPack
To date, two studies have examined the effects of cognitive training in individuals at clinical high risk for psychosis, and both used computerized exercises from CogPack (Marker, 1987–2007). Designed in Germany, CogPack contains multiple exercises in each of the following sub-programs: Visuomotor Skills, Vigilance/Comprehension/Reaction, Language Material, Memory, Numbers/Logic, Knowledge/Orientation/Everyday Skills, and Special Elements (e.g. executive functioning and tone and pitch discrimination). Thus, exercises provide training across a broad range of cognitive functions including attention, psychomotor speed, learning and memory, and executive functions.
In an exploratory pilot study, Rauchensteiner et al. (2011) tested the effect of 10 sessions of CogPack delivered over a 4 week period in 10 individuals at risk for psychosis (mean age of 27.2 years) relative to 16 individuals with fully manifested schizophrenia (mean age of 30.13 years). At the group level, there were no significant differences in age. Eight CogPack tasks of attention, memory, and executive functioning were used. Before and after training, subjects were assessed on verbal learning and memory, attention/working memory (i.e. the Continuous Performance Test Identical Pairs, CPT-IP), and progress on the 8 CogPack training exercises. Between group analyses revealed that the high risk group made greater gains in verbal memory and on 3 of the 8 CogPack exercises relative to the schizophrenia subject group. In a within-group analysis, the high risk group showed significant improvement in verbal memory, hits on the CPT-IP Shapes (but not numbers), and significant improvement on 5 of the 8 CogPack exercises, while the schizophrenia group showed improvement on false alarms of the CPT-IP Shapes only. While results from this pilot study need to be interpreted with caution, these data raise two interesting possibilities. First, individuals at high risk show greater gains in verbal memory and on the exercises relative to individuals with established schizophrenia, which suggests increased capacity for neuroplasticity in the high risk individuals. Second, these individuals showed gains after only 10 sessions of training, while 10 sessions was of limited benefit in individuals with chronic schizophrenia. However, CogPack has shown significant effects in chronic schizophrenia in several prior studies where training was delivered at a higher dose (i.e. 15–36 sessions) (Cavallaro et al., 2009; Lindenmayer et al., 2008; McGurk et al., 2005; McGurk et al., 2009; Sartory et al., 2005). These differential responses to a relatively brief course of training suggest that a meaningful treatment response can be obtained with a more efficient intervention if we capitalize on the neuroplastic capacity in individuals at high risk, before the deleterious effects of medications and illness chronicity set in.
As discussed earlier, Bechdolf et al. (2012), in an unblinded study, tested the effects of an integrated psychological intervention (IPI) relative to supportive counseling in 128 individuals with EIPS. IPI combined 25 sessions of individual cognitive-behavioral therapy, 15 sessions of group skills training, 12 sessions of cognitive remediation with the CogPack software, and 3 multifamily psychoeducation sessions delivered over a 12 month period. Subjects in the control condition completed 30 sessions of supportive counseling over the same period. The supportive counseling was designed to provide a minimal level of support and included basic assessment, psychoeducation, and counseling. Subjects in IPI showed a significantly lower rate of conversion to psychosis: at the end of treatment 2 of the 63 patients in IPI converted to psychosis compared to 11 of the 65 patients in supportive counseling. At a follow-up 12 months after treatment, 2 additional patients in each condition converted to psychosis. While these results are very encouraging, the trial design did not allow assessment of the relative contribution of computerized cognitive training to this outcome, and the effects on cognitive performance were not reported. Additional studies on the effects of cognitive training in high risk samples are needed to determine whether this intervention can prevent conversion to psychosis or avert further cognitive decline.
Cognitive Training in Recent Onset Schizophrenia
Therapist Guided Paper/Pencil Tasks
Wykes et al. (2007) tested the effects of cognitive remediation therapy (CRT) compared to treatment as usual in 40 young, primarily in-patients with adolescent onset schizophrenia (average age of 18) who showed evidence of cognitive and social behavioral difficulties. Participants received 40 hourly sessions of CRT at an average rate of 3 sessions per week over 3 months. CRT targets memory, complex planning, and problem solving using simple paper and pencil tests and small task equipment, with the guidance of a therapist. The therapist provides information processing strategies, and discusses the regulation, organization and monitoring of behavior for each task to minimize errors.
Out of 3 primary outcome measures (cognitive flexibility, working memory, and planning), CRT showed a significant effect on cognitive flexibility (Wisconsin Card Sorting Task), with durability at 3 month follow-up. There was no significant effect of CRT on any secondary outcome measures of symptoms or functioning which, as the authors note, might only be evident when cognitive remediation is integrated with rehabilitation and other treatments where the learned strategies can be implemented. However, a moderating effect was found between planning improvements and a decrease in symptoms, and cognitive change in the total sample was associated with change in social functioning and symptoms.
Ueland & Rund (2004) tested the effects of a therapist-guided pencil/paper cognitive remediation program combined with psychoeducation compared to psychoeducation alone in 26 adolescent inpatients (average age of 15) with adolescent onset schizophrenia. The cognitive remediation program consisted of 30 hours of individual training with a therapist over 12 weeks. Training targeted cognitive differentiation, memory, social perception, and attention, which included a 3-day intensive training on the Span of Apprehension Task (SPAN) with enhanced instructions and monetary reinforcement. The psychoeducation treatment included parent seminars, problem-solving sessions, milieu therapy and network groups.
The results showed no significant difference between the groups, possibly due to the small sample size. Given that the study was underpowered, within-group analyses were conducted and showed that the cognitive remediation group improved on five of the 10 cognitive measures and three of the five functional outcome measures, while the control group improved on three cognitive and one functioning measure. Only the cognitive remediation group showed significant improvement in early visual information processing (SPAN task), visual memory, symptoms, and psychosocial functioning. The improvement in the control group on some measures suggests that the psychoeducation program may have some benefits on cognition, and/or that some “placebo” effects are possible in cognitive performance during a RCT (indeed, recent data show that, in pharmacologic trials, placebo responses have increased in schizophrenia participants in the past decade compared to prior decades (Kemp et al., 2010)).
In contrast to Wykes et al. (2007), the authors found no relationship between change in cognition and change in functioning which, again, might be due to low statistical power. At one-year follow-up (Ueland & Rund, 2005) no significant group-by-time interactions were found on the cognitive, clinical or functioning measures with the exception of the SPAN task, which indicates that durable improvements in visual attention can be achieved through intensive training in these young individuals.
Cognitive Enhancement Therapy (CET)
Eack et al. (2009) examined the effects of Cognitive Enhancement Therapy (CET) in recent onset schizophrenia. CET is a small group approach that combines 60 hours of computerized training in attention, memory, and problem solving using CogRehab software (Bracy, 1995), with 45 1.5-hour sessions of social cognitive group exercises delivered over two years. CogRehab software (Bracy, 1995) was originally designed for patients with traumatic brain injury but has been used in a number of studies in chronic schizophrenia. The exercises emphasize simple attention, executive skills, visuospatial skills, memory, and problem solving. Eight modules are completed sequentially, with two modules of increasing complexity within each cognitive domain. The developers recommend 1–3 hours per day of training, carried out several times a week over the course of one year, with weekly therapy sessions during which compensatory skills are taught, patients’ progress is reviewed, and program parameters, such as difficulty level, are adjusted.
In Eack et al. (2009), 58 individuals with recent onset schizophrenia were randomized to CET or Enriched Supportive Therapy (EST)—an individual approach involving illness management and psychoeducation. After one year of treatment, improvements on cognitive measures were not evident, but showed moderate improvement after two years of treatment. At both one and two years, CET subjects showed significant gains on measures of social cognition, cognitive style, social adjustment, and symptoms, and a significantly greater proportion of CET subjects were engaged in competitive employment at 2 years. However, neither participants nor raters were blind to group assignment and most of the social, occupational, and symptom measures were interview-based. Further, subjects in the CET condition received significantly more hours of clinician contact and therapeutic intervention, and it is not possible to differentiate the effects due to the cognitive training alone vs. those due to increased therapist contact and the social skills groups.
A follow-up study one year after the completion of CET (Eack et al., 2010a), showed that the gains on social and symptom measures were broadly maintained, while the effect on employment was no longer significant (i.e. a similar proportion of EST and CET subjects were employed). Cognitive assessments were not conducted at the follow-up. Consistent with Wykes et al. (2007), the gains in cognition from baseline to two years of treatment were significantly associated with gains in functional outcome, as were increases in social cognition (MSCEIT Managing Emotions) (Eack et al., 2010b).
Eack at al. (2010c) also found greater preservation of gray matter volume over two years in the left hippocampus, parahippocampal gyrus, and fusiform gyrus, and significantly greater gray matter increases in the left amygdala in CET subjects relative to EST subjects. Further, less gray matter loss in the left parahippocampal and fusiform gyrus and greater gray matter increases in the left amygdala were significantly related to improved cognition. The findings of Eack et al. indicate that computerized cognitive remediation combined with intensive group-based social skills therapy has beneficial effects on both cerebral grey matter volume and psychosocial functioning in recent onset schizophrenia. However, a perplexing issue is the fact that it took two years for subjects in CET to show gains in cognition, and it is unclear as to which components of the intervention contributed to these gains. It is also unknown whether this treatment can induce cognitive gains in less time if a more intensive schedule is employed.
Cognitive Adaptation Training (CAT)
CAT is a compensatory treatment for cognitive impairment in schizophrenia designed to bypass cognitive deficits by providing training in solutions to concrete, daily life problems using various tools (e.g. schedules, schemes, signs). Hansen et al., (2012) tested the effects of CAT in combination with assertive community treatment (ACT) versus ACT alone in 62 outpatients with recent onset schizophrenia. ACT included medication management, weekly contact with professionals, psychoeducation, social skill training groups, and psychosocial intervention with relatives. CAT was conducted in patients’ homes every 2 weeks over a period of 6 months. Subjects were assessed at baseline, post-intervention (6 months), and follow-up (9 months) on global functioning, social problems, social needs, symptoms, quality of life, and hospitalizations. The results showed no significant group differences at post-intervention or follow-up. At follow-up, both groups showed a small improvement in social problems, positive symptoms, and quality of life. These results are inconsistent with previous studies where CAT has shown positive effects in chronic schizophrenia (e.g. Velligan et al., 2009) and may be due to the sample size (the estimated number of subjects required was not reached due to a high refusal rate). Further, CAT treatment was provided by staff without practical training in CAT or supervision from experienced CAT providers, and the control condition was more intensive than in previous studies, which may have obscured group differences.
Brain Fitness Program (BFP)
BFP is an auditory processing/verbal learning training program that is designed to restore and enhance auditory perceptual and working memory processes, with the goal of increasing the accuracy and temporal resolution of auditory inputs feeding working memory and long-term verbal memory encoding (developed by Positscience, Inc.). BFP was originally designed to address the verbal memory impairments associated with aging, but has been applied to patients with schizophrenia based on the known impairments in auditory processing and frontally mediated verbal memory operations in the illness (e.g., Foucher et al., 2005; Friston and Frith, 1995; Javitt, Shelley, and Ritter, 2000; Kasai et al., 2002; Light and Braff, 2005; Ragland et al., 2004; Wible et al., 2001). Though many of the exercises have a heavy emphasis on auditory perceptual processing, they also explicitly require sustained attention and working memory and repeatedly engage cognitive control and response-selection mechanisms.
This explicitly “systems neuroscience”-informed approach harnesses principles of training-induced neuroplasticity (Vinogradov et al., 2012) and has the following features: (1) intensive—many thousands of learning trials are performed for each specific exercise; (2) neuroadaptive—the dimensions of each exercise (e.g., speed, working memory load) are parametrically and continuously modified on a trial-by-trial basis for each individual user during the course of each exercise in order to maintain performance at ~80% accuracy; (3) attentionally engaging—each trial is gated by a “ready” signal from the user to indicate and require directed attention; (4) rewarding—correct responses are continuously rewarded by amusing auditory and visual stimuli in order to drive high levels of training compliance and to engage reward and novelty detection systems for successful learning. The training program consists of six exercises of increasing complexity. Exercise 1 requires subjects to make gradually more difficult distinctions between frequency modulation sweeps (i.e., progressively faster sweeps and shorter inter-stimulus intervals) that mimic the features of important formants in speech. Once mastered, subjects progress to exercises that use phonemes, then full words, and finally, full sentences. The first four exercises heavily engage auditory and verbal working memory functions. Exercise 5 engages both working memory and verbal learning as the listener must remember a sequence of verbal instructions in order, then carry them out; the instructions get longer and more complex as the listener improves. In Exercise 6, stimuli consist of real-world scenarios of conversational narrative, and the listener must sharply focus on increasingly more difficult and elusive details of longer narratives.
Our group has previously reported the effects of BFP delivered as a stand-alone treatment in individuals with chronic schizophrenia (Fisher et al., 2009). Twenty-nine schizophrenia outpatients completed 50 hours of the training over a 10 week period and were compared to an active computer games control condition (N=26) designed to control for the effects of computer exposure, contact with research personnel, and monetary payments. Relative to the control group the auditory training showed positive effects on measures of verbal working memory, verbal learning and memory, and global cognition. Effect sizes in verbal learning and memory, and in global cognition, were large (Cohen’s d 0.86 – 0.89). At a 6 month follow-up assessment, improved cognition was significantly associated with improved functional outcome.
Our findings of the same training in individuals with recent-onset schizophrenia show similar results (Fisher et al., under review). In this randomized controlled trial (ClinincalTrials.gov Identifier: NCT00694889), 80 individuals with recent onset schizophrenia (mean age of 21 years) were given laptop computers to take home where they performed either 40 hours of neuroplasticity-based auditory training or 40 hours of commercial computer games over an 8 week period, in conjunction with standard care. We examined MATRICS neurocognitive outcome measures, symptoms, and functioning. We also investigated psychophysical improvement in auditory processing and its association with cognitive gains.
Auditory training subjects demonstrated significant improvements in global cognition, verbal learning and memory, and problem solving compared to computer games control subjects. Both groups showed a slight but significant decrease in symptoms, likely as a result of receiving clinical attention and standard treatment. Subjects in the training group also showed significant psychophysical improvement in early auditory processing that correlated with gains in cognition.
These findings suggest that the cognitive deficits of schizophrenia can be addressed very early in the course of illness, using an intensive schedule of training at home that is delivered via a portable computing device. Further, we posit that the association of improvement in early auditory processing with improvement in higher-order cognition reflects training-induced distributed plastic changes occurring throughout the neural systems subserving verbal learning and memory. Indeed, MEG experiments in our adult subjects indicate that training induces an increase in the amplitude of the M100 response in auditory cortex (Dale et al., 2010), accompanied by changes in gamma-band oscilllatory power in lateral frontal cortex (Brown et al., under review), while Popov et al. (2011) found normalization of early auditory gating impairments in schizophrenia after 20 hours of this training. Future studies will investigate whether this training improves long-term outcome and community functioning.
Future Directions for Computerized Cognitive Training
Taken together, the studies above provide the first indications that computerized cognitive training is a promising treatment for both clinical high risk and recent onset schizophrenia (Table 2). Individuals at high risk show greater cognitive gains relative to adults with chronic schizophrenia (Rauchensteiner et al., 2011), while a combined treatment of cognitive training, cognitive-behavioral therapy, group skills training, and multifamily psychoeducation resulted in lower rates of conversion to psychosis (Bechdolf et al., 2012). In recent onset schizophrenia, computerized cognitive training, when delivered in sufficient doses, appears effective in improving multiple cognitive and social cognitive domains both as part of a multimodal treatment targeting social functioning (Eack et al., 2009) and as a “stand-alone” treatment delivered along with usual care (Fisher et al., under review). Further, there is indication that gains in cognition are associated to gains in functional outcome (Wykes et al., 2007; Eack et al., 2010b).
Table 2.
Cognitive Training Programs to Target Impaired Cognition in Clinical High Risk and Recent Onset Schizophrenia.
| Study and Sample Size | Cognitive Function Target and Type of Training | Cognitive Training Treatment Intensity | Effects1 | |
|---|---|---|---|---|
| Clinical High Risk | Rauchensteiner et al., 2011; N=26 | Target: attention, memory, and executive functioning Training: Computerized cognitive training (CogPack) |
10 hours over a maximum of 4 weeks | Improvement in verbal memory and 3 out of 8 CogPack exercises |
| Bechdolf et al., 2012; N=128 | Target: Concentration, attention, vigilance, and memory Training: Computerized cognitive training (CogPack), individual cognitive-behavioral therapy, group skills training, and multifamily psychoeducation |
12 sessions over 12 months | Lower rate of conversion to psychosis at 12 and 24 month follow-up | |
| Recent Onset Schizophrenia | Wykes et al., 2007; N=40 | Target: Memory, complex planning, and problem solving Training: Paper and pencil tasks with the guidance of a therapist |
40 hours, 3 times per week, over 3 months | Improvement in problem solving |
| Ueland & Rund, 2004 and 2005; N=24 | Target: Cognitive differentiation, memory, social perception, and attention including the Span of Apprehension Task (SPAN) Training: Paper and pencil tasks and practice on cognitive measures (e.g. SPAN) with the guidance of a teacher or therapist |
30 hours, 2–3 times per week over 12 weeks | Improvement in SPAN task at 1-year follow up | |
| Eack et al., 2009; N=58 | Target: Attention, memory, problem solving, and social cognition Training: Computerized cognitive training (CogRehab) and social cognitive group exercises |
60 hours, 1 time per week over 1–2 years | Improvement in neurocognition, social cognition, cognitive style, social adjustment, symptoms, and competitive employment | |
| Hansen et al., 2012; N=62 | Target: Global and social functioning Training: Compensatory training (e.g., schedules, schemes and signs to solve concrete problems related to daily life) along with assertive community treatment (ACT) |
12 sessions, 1 session every 2 weeks, over 6 months | None | |
| Fisher et al., under review; N=80 | Target: Auditory processing and verbal memory functions Training: Computerized cognitive training (BFP) of lower level auditory processes and auditory/verbal working memory |
40 hours, 5 times per week over 8 weeks | Improvement in global cognition, verbal learning and memory, problem solving |
Significant improvement in cognitive training subjects relative to control subjects.
While encouraging, it is not yet possible to make direct comparisons between these cognitive training programs or draw any firm conclusions about their pre-emptive effects, given the small number of studies to date in early psychosis, the variety of training programs, adjunctive treatments, and control groups studied, and the disparity between studies in the assessment measures used. Until we can systematically examine the effects of an intervention under sufficiently powered, carefully controlled, randomized, and double-blind conditions, using well-defined cognitive, neurological, and functional outcome measures—and until we make a concerted effort to investigate the durability or sustainability of both behavioral and neurological effects as well as the additive (or synergistic) effects of psychosocial, vocational, and pharmacological treatments– it will continue to be difficult to assess and compare the various cognitive training methods currently available.
Future research must determine not only the optimal form of training for individuals at high risk and recent onset schizophrenia, but also the ideal number of hours of training and treatment intensity, how best to disseminate and supervise cognitive training in real-world settings, and the subject characteristics of those who can benefit most from this intervention. In studies of cognitive training in chronic schizophrenia, moderator variables explored to date have shown little to no effect (Wykes et al., 2011), but it is highly likely that several unexplored moderators (such as genotype, “learning potential,” motivational state, and baseline neural system functional connectivity) all play a role in influencing treatment outcome. Indeed, our group has reported preliminary data showing an effect of COMT genotype on response to cognitive training (Panizzutti et al., in press), as well as an effect of baseline prefrontal cortical connectivity (Hinckley et al., under review).
Emerging Areas of Investigation
There is now a consensus in the field that behavioral interventions, such as CT or computerized cognitive training, offer a number of significant advantages as we seek to develop meaningful approaches to pre-empt schizophrenia. Bentall and Morrison (2002) note that, as compared to pharmacologic treatments, behavioral interventions have fewer deleterious side effects, are less stigmatizing, and target the presenting non-specific symptoms through a normalizing approach. In addition, they are generally more tolerable and acceptable than medications (Morrison et al. 2004). We posit that, in at least some forms, they are also more scalable, and can be delivered via web-based technology to remote or under-resourced clinical sites.
While the emerging data are promising, much important work remains to be done. Basic methodologic issues such as sample size and sample characteristics, assessment criteria, clinical staging, and rigorous trial design and data analytic approaches, must be implemented in order to build a convincing evidence base for both CT and computerized cognitive training as pre-emptive behavioral interventions. With the current rapid advances in the development of cognitive-enhancing medications, we must also be prepared to explore the most advantageous ways in which these agents can– and probably should– be combined with behavioral treatments in order to optimize patients’ outcomes (Keefe et al. 2010). Indeed, some agents may be of no value when given alone, but may substantially facilitate the effects of CT or of cognitive training in early psychosis individuals.
Medications are not the only possible adjuvants to enhance the brain’s response to behavioral interventions. The use of neuromodulatory techniques such as transcranial magnetic stimulation (TMS) and direct current stimulation (tDCS), in combination with sensory stimulation, have both shown to be inducers of cortical plasticity (Celnik et al. 2009; Khedr et al. 2010). Exercise is another potent, safe, and valuable ‘neurotrophic agent’ that could easily become a significant part of the treatment armamentarium for pre-empting schizophrenia (see Pajonk et al. 2010).
Finally, advances in information technology and in interactive and entertainment software indicate that web-based treatment delivery methods can be developed that will generate the same interest, engagement, perceived value, and social acceptability as web-based games. Indeed, novel collaborative efforts are already underway to develop socially-networked browser-based cognitive therapy for schizophrenia as well as highly engaging game-like cognitive training tools for early psychosis patients. We are only a few steps away from the development of web-deliverable behavioral treatments that will be available on a scale never before imagined for any other therapeutic intervention, and that may finally allow us to reach—and treat—young high-risk individuals at a population level. We are at a conceptual threshold that could not have been imagined a decade ago: facing the very real possibility that we may be able to move beyond pre-emption in schizophrenia, to innoculation.
Figure 1.

Neurodevelopmental model of schizophrenia, highlighting the pre-emptive period and intervention targets discussed in this review (adapted and modified by permission of Tyrone Cannon, PhD). DA = dopamine, HPA + hypothalamic-pituitary-adrenal).
Figure 2.

The impact of stress on neural circuits related to psychosis.
Figure 3.
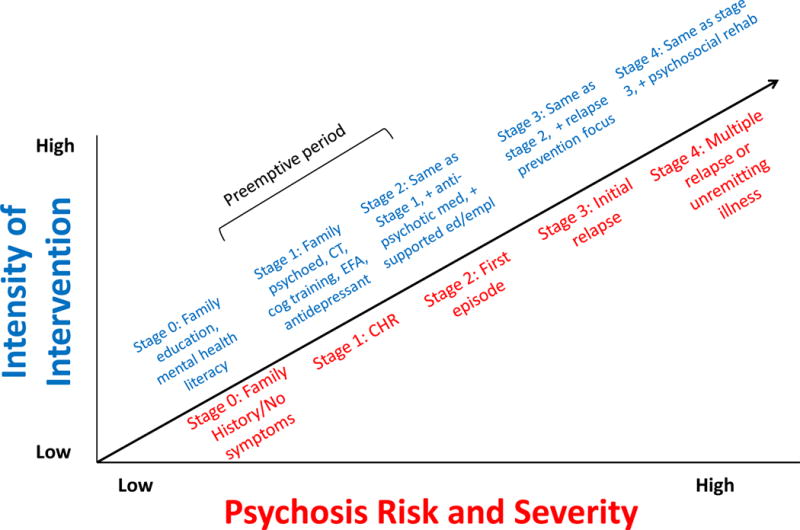
Clinical Staging Model
Footnotes
Disclosure Statement
Dr. Vinogradov is a paid consultant to Brain Plasticity Inc., a company with a commercial interest in cognitive training software. She is also a paid consultant to Amgen, Genentech, and Hoffman-LaRoche. None of the other authors have any financial interest in Brain Plasticity Inc. or Posit Science. Drs. Vinogradov, Fisher, Loewy, Hardy, and Schlosser report no competing interests.
References
- Adcock A, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson G, Nagarajan S, Vinogradov S. When Top-Down Meets Bottom-Up: Auditory Training Enhances Verbal Memory in schizophrenia. Schizophrenia bulletin. 2009;35(6):1132–41. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington Jean, et al. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia research. 2011;125(1):54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Aiello G, et al. Stress abnormalities in individuals at risk for psychosis: A review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.05.003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22663896 [Accessed July 25, 2012] [DOI] [PubMed]
- Andreasen NC, et al. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biological psychiatry. 2011;70(7):672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues in clinical neuroscience. 2010;12(3):409–415. doi: 10.31887/DCNS.2010.12.3/nandreasen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neuroscience and biobehavioral reviews. 2011;35(3):848–70. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechdolf A, et al. Preventing progression to first-episode psychosis in early initial prodromal states. The British journal of psychiatry: the journal of mental science. 2012;200(1):22–29. doi: 10.1192/bjp.bp.109.066357. [DOI] [PubMed] [Google Scholar]
- Becker HE, et al. Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psychological medicine. 2010;40(10):1599–1606. doi: 10.1017/S0033291710000048. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Morrison AP. More harm than good: The case against using antipsychotic drugs to prevent severe mental illness. Journal of Mental Health. 2002;11(4):351–356. [Google Scholar]
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. The British journal of psychiatry Supplement. 1998;172(33):53–59. [PubMed] [Google Scholar]
- Bracy O. Cogrehab software. Indianapolis, Indiana: Psychological Software Services; 1995. [Google Scholar]
- Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24(4 Suppl):91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- Braehler C, et al. Diurnal cortisol in schizophrenia patients with childhood trauma. Schizophrenia research. 2005;79(2–3):353–354. doi: 10.1016/j.schres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Breitborde NJ, et al. Multifamily group psychoeducation and cognitive remediation for first-episode psychosis: a randomized controlled trial. BMC psychiatry. 2011;11:9. doi: 10.1186/1471-244X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162(1):71–8. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Progress in neurobiology. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, et al. Increases in cortical gamma oscillatory activity corelate with improved behavior after computerized cognitive training in schizophrenia under review. [Google Scholar]
- Büschlen J, et al. Pituitary volume increase during emerging psychosis. Schizophrenia research. 2011;125(1):41–48. doi: 10.1016/j.schres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Carletti F, et al. Alterations in White Matter Evident Before the Onset of Psychosis. Schizophrenia bulletin. 2012 doi: 10.1093/schbul/sbs053. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22472474 [Accessed July 23, 2012] [DOI] [PMC free article] [PubMed]
- Cavallaro R, et al. Computer-aided neurocognitive remediation as an enhancing strategy for schizophrenia rehabilitation. Psychiatry research. 2009;169(3):191–196. doi: 10.1016/j.psychres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Celnik P, et al. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40(5):1764–71. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Miller EN. Reliabilities and intercorrelations of eight measures of proneness to psychosis. Journal of consulting and clinical psychology. 1982;50(2):187–195. doi: 10.1037//0022-006x.50.2.187. [DOI] [PubMed] [Google Scholar]
- Collip D, et al. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychological medicine. 2011;41(11):2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Conrad CD, et al. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of learning and memory. 2004;81(3):185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, et al. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophrenia research. 2012;135(1–3):170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011 doi: 10.1093/brain/awr039. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21482550. [DOI] [PMC free article] [PubMed]
- Dale CL, et al. Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol. 2010;75(2):183–93. doi: 10.1016/j.ijpsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2007;10(4):513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Eack SM, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatric Services (Washington, DC) 2009;60(11):1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, et al. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophrenia research. 2010a;120(1–3):210–216. doi: 10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Pogue-Geile MF, et al. Mechanisms of functional improvement in a 2-year trial of cognitive enhancement therapy for early schizophrenia. Psychological medicine. 2010b:1–9. doi: 10.1017/S0033291710001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Archives of general psychiatry. 2010c;67(7):674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophrenia research. 2007;93(1–3):266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, et al. Neuroplasticity-Based Auditory Training Via Laptop Computer Improves Cognition in Young Individuals with Recent Onset Schizophrenia. doi: 10.1093/schbul/sbt232. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–11. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher JR, et al. Functional integration in schizophrenia: too little or too much? Preliminary results on fMRI data. NeuroImage. 2005;26(2):374–388. doi: 10.1016/j.neuroimage.2005.01.042. [DOI] [PubMed] [Google Scholar]
- French P, Morrison AP. Early detection and cognitive therapy for people at high risk of developing psychosis a treatment approach. Chichester, West Sussex, England; Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience (New York, NY) 1995;3(2):89–97. [PubMed] [Google Scholar]
- Furmark T, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of general psychiatry. 2002;59(5):425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, et al. Cognitive Functioning in Prodromal Psychosis: A Meta-analysisCognitive Functioning in Prodromal Psychosis. Archives of general psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Garner B, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biological psychiatry. 2005;58(5):417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of general psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goldapple K, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Archives of general psychiatry. 2004;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives of general psychiatry. 2007;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? The American journal of psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hansen JP, et al. Cognitive adaptation training combined with assertive community treatment: a randomised longitudinal trial. Schizophrenia research. 2012;135(1–3):105–111. doi: 10.1016/j.schres.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, et al. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophrenia Research. 2008;105(1–3):1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Synaptic elimination, neurodevelopment, and the mechanism of hallucinated “voices” in schizophrenia. The American journal of psychiatry. 1997;154(12):1683–1689. doi: 10.1176/ajp.154.12.1683. [DOI] [PubMed] [Google Scholar]
- Holtzman CW, et al. Stress and the prodromal phase of psychosis. Current pharmaceutical design. 2012;18(4):527–533. doi: 10.2174/138161212799316280. [DOI] [PubMed] [Google Scholar]
- Iqbal N, et al. The MCPP challenge test in schizophrenia: hormonal and behavioral responses. Biological psychiatry. 1991;30(8):770–778. doi: 10.1016/0006-3223(91)90233-c. [DOI] [PubMed] [Google Scholar]
- Jahshan C, et al. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24(1):109–120. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LM, et al. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophrenia research. 1998;33(1–2):87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology. 2000;149(3):319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111(10):1733–7. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, et al. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Kiyoto, et al. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. The American Journal of Psychiatry. 2002;159(4):546–553. doi: 10.1176/appi.ajp.159.4.546. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M, et al. Clinical Trials of Potential Cognitive-Enhancing Drugs in Schizophrenia: What Have We Learned So Far? Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr153. advance access published online November 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, et al. Report From the Working Group Conference on Multisite Trial Design for Cognitive Remediation in Schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq010. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20194249. [DOI] [PMC free article] [PubMed]
- Keefe Richard SE, et al. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88(1–3):26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Keefe Richard SE, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. The American journal of psychiatry. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Kemp AS, Schooler NR, Kalali AH, et al. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophrenia bulletin. 2010;36(3):504–9. doi: 10.1093/schbul/sbn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, et al. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121(1):30–7. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, et al. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophrenia research. 2011;130(1–3):170–175. doi: 10.1016/j.schres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Kim KR, et al. Clinical efficacy of individual cognitive therapy in reducing psychiatric symptoms in people at ultra-high risk for psychosis. Early intervention in psychiatry. 2011;5(2):174–178. doi: 10.1111/j.1751-7893.2011.00267.x. [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, et al. Diagnosing schizophrenia in the initial prodromal phase. Archives of general psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kumari V, et al. Neural changes following cognitive behaviour therapy for psychosis: a longitudinal study. Brain: a journal of neurology. 2011;134(Pt 8):2396–2407. doi: 10.1093/brain/awr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardinois M, et al. Childhood trauma and increased stress sensitivity in psychosis. Acta psychiatrica Scandinavica. 2011;123(1):28–35. doi: 10.1111/j.1600-0447.2010.01594.x. [DOI] [PubMed] [Google Scholar]
- Laucht M, et al. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: Findings from a longitudinal study. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.06.002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22748421 [Accessed July 25, 2012] [DOI] [PubMed]
- Lencz T, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff David L. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 2005;62(2):127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, et al. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatric Services. 2008;59(3):241–247. doi: 10.1176/ps.2008.59.3.241. [DOI] [PubMed] [Google Scholar]
- Marker KR. COGPACK. The Cognitive Training Package Manual. Marker Software; Heidelberg & Ladenburg: 1987. Available at: www.markersoftware.com. [Google Scholar]
- Matheson SL, et al. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychological medicine. 2012:1–13. doi: 10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of general psychiatry. 2000;57(7):637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGorry Patrick D, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Archives of general psychiatry. 2002;59(10):921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- McGurk Susan R, et al. A meta-analysis of cognitive remediation in schizophrenia. American Journal of Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk Susan R, et al. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophrenia Bulletin. 2009;35(2):319–335. doi: 10.1093/schbul/sbn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk Susan R, Mueser KT, Pascaris A. Cognitive training and supported employment for persons with severe mental illness: one-year results from a randomized controlled trial. Schizophrenia Bulletin. 2005;31(4):898–909. doi: 10.1093/schbul/sbi037. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, et al. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50(5):597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, et al. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Milev P, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. The American journal of psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, et al. Increased stress-induced dopamine release in psychosis. Biological psychiatry. 2012;71(6):561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biological psychiatry. 2002;51(10):775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Morrison AP, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. The British journal of psychiatry: the journal of mental science. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- Morrison AP, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ (Clinical research ed) 2012;344:e2233. doi: 10.1136/bmj.e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AP. The interpretation of intrustions in psychosis: an integrative cognitive approach to hallucinations and delusions. Behavioural and Cognitive Psychotherapy. 2001;29(03):257–276. [Google Scholar]
- Mrazek PB, Haggerty RJ, editors. Reducing risks for mental disorders: frontiers for preventive intervention research. Washington, D.C.: National Academy Press; 1994. [PubMed] [Google Scholar]
- Mück-Seler D, et al. Platelet serotonin, plasma cortisol, and dexamethasone suppression test in schizophrenic patients. Biological psychiatry. 1999;45(11):1433–1439. doi: 10.1016/s0006-3223(98)00174-7. [DOI] [PubMed] [Google Scholar]
- Niendam TA, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophrenia research. 2006;84(1):100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Norman RMG, Lewis SW, Marshall M. Duration of untreated psychosis and its relationship to clinical outcome. The British journal of psychiatry. 2005;48(Supplement):s19–23. doi: 10.1192/bjp.187.48.s19. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophrenia bulletin. 1984;10(2):300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–43. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Panizzutti R, Hamilton S, Vinogradov S. Genetic correlates of cognitive training response in schizophrenia. doi: 10.1016/j.neuropharm.2012.07.048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Yücel M, Bora E, Fornito A, Testa R, Brewer WJ, Velakoulis D, Wood SJ. Neurobiological markers of illness onset in psychosis and schizophrenia: The search for a moving target. Neuropsychol Rev. 2009;19(3):385–98. doi: 10.1007/s11065-009-9114-1. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3(2):183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Peerbooms O, et al. Evidence that interactive effects of COMT and MTHFR moderate psychotic response to environmental stress. Acta psychiatrica Scandinavica. 2012;125(3):247–256. doi: 10.1111/j.1600-0447.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Pelayo-Tern JM, et al. Gene-Environment Interactions Underlying the Effect of Cannabis in First Episode Psychosis. Current pharmaceutical design. 2012 doi: 10.2174/138161212802884609. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22716151 [Accessed July 25, 2012] [DOI] [PubMed]
- Phillips LJ, Francey SM. Changing PACE: Psychological interventions in the pre-psychotic phase. In: McGorry PD, Gleeson J, editors. Psychological Interventions in Early Psychosis: A Treatment Handbook. Chichester, West Sussex, England; Hoboken, N.J.: J. Wiley; 2004. pp. 23–41. [Google Scholar]
- Phillips Lisa Jane, et al. Comparison of experiences of stress and coping between young people at risk of psychosis and a non-clinical cohort. Behavioural and cognitive psychotherapy. 2012;40(1):69–88. doi: 10.1017/S1352465811000397. [DOI] [PubMed] [Google Scholar]
- Popov T, et al. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biological Psychiatry. 2011;69(5):465–471. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Pruessner M, et al. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophrenia research. 2011;129(1):29–35. doi: 10.1016/j.schres.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Pukrop Ralf, et al. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophrenia research. 2007;92(1–3):116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Ragland J Daniel, et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. The American Journal of Psychiatry. 2004;161(6):1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Archives of neurology. 2000;57(3):317–320. doi: 10.1001/archneur.57.3.317. [DOI] [PubMed] [Google Scholar]
- Rauchensteiner S, et al. Test-performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiatry research. 2011;185(3):334–339. doi: 10.1016/j.psychres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain research. 1985;359(1–2):300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, et al. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartory G, et al. Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia. Schizophr Res. 2005;75(2–3):219–23. doi: 10.1016/j.schres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophrenia bulletin. 2006;32(3):507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of general psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of general psychiatry. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Sideli L, et al. Do child abuse and maltreatment increase risk of schizophrenia? Psychiatry investigation. 2012;9(2):87–99. doi: 10.4306/pi.2012.9.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, et al. Cognitive functioning in the schizophrenia prodrome. Schizophrenia bulletin. 2007;33(3):761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Neurodevelopment during adolescence. In: Cicchetti D, Walker E, editors. Neurodevelopmental Mechanisms in Psychopathology. New York, NY: Cambridge University Press; 2003. pp. 62–83. [Google Scholar]
- Stein-Behrens B, et al. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14(9):5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam Karuna, et al. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Mittal V, Walker Elaine F. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophrenia bulletin. 2011;37(2):432–441. doi: 10.1093/schbul/sbp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KN, Berger G, et al. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. Journal of psychiatric research. 2007;41(5):446–450. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Phillips LJ, et al. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. Journal of psychiatric research. 2007;41(7):561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Thompson PM, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Rund BR. A controlled randomized treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta psychiatrica Scandinavica. 2004;109(1):70–74. doi: 10.1046/j.0001-690x.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- Ueland T, Rund BR. Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta psychiatrica Scandinavica. 2005;111(3):193–201. doi: 10.1111/j.1600-0447.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Van der Gaag M. A neuropsychiatric model of biological and psychological processes in the remission of delusions and hallucinations. Schizophrenia Bulletin. 2006;32(1):s113–s122. doi: 10.1093/schbul/sbl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, et al. The short-term impact of generic versus individualized environmental supports on functional outcomes and target behaviors in schizophrenia. Psychiatry research. 2009;168(2):94–101. doi: 10.1016/j.psychres.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterager L, et al. Cognitive training plus a comprehensive psychosocial programme (OPUS) versus the comprehensive psychosocial programme alone for patients with first-episode schizophrenia (the NEUROCOM trial): a study protocol for a centrally randomised, observer-blinded multi-centre clinical trial. Trials. 2011;12:35. doi: 10.1186/1745-6215-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov Sophia, Fisher Melissa, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Walker EF, Lewine RJ. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biological psychiatry. 2000;48(12):1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological review. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and psychopathology. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Walker Elaine, Bollini AM. Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophrenia research. 2002;54(1–2):17–23. doi: 10.1016/s0920-9964(01)00347-4. [DOI] [PubMed] [Google Scholar]
- Wible CG, et al. A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. The American Journal of Psychiatry. 2001;158(6):938–943. doi: 10.1176/appi.ajp.158.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood Stephen J, et al. Cognitive decline following psychosis onset: data from the PACE clinic. The British journal of psychiatry. 2007;51(Supplement):s52–57. doi: 10.1192/bjp.191.51.s52. [DOI] [PubMed] [Google Scholar]
- Wykes T, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. The American journal of psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes Til, et al. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophrenia bulletin. 2008;34(3):523–537. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes Til, et al. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophrenia research. 2007;94(1–3):221–230. doi: 10.1016/j.schres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Yee CM, et al. Stress reactivity and affective modulation during the prodromal, first-episode and chronic phases of schizophrenia 2007 [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. The Australian and New Zealand journal of psychiatry. 1996a;30(5):587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophrenia bulletin. 1996b;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Yung AR, et al. Declining Transition Rate in Ultra High Risk (Prodromal) Services: Dilution or Reduction of Risk? Schizophrenia Bulletin. 2007;33(3):673–681. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Alison R, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. The Journal of clinical psychiatry. 2011;72(4):430–440. doi: 10.4088/JCP.08m04979ora. [DOI] [PubMed] [Google Scholar]
- Yung Alison R, Nelson Barnaby. Young people at ultra high risk for psychosis: a research update. Early intervention in psychiatry. 2011;5(Suppl 1):52–57. doi: 10.1111/j.1751-7893.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability–a new view of schizophrenia. Journal of abnormal psychology. 1977;86(2):103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]


