Abstract
Burkholderia pseudomallei is the causative agent of melioidosis. The complete genome sequences of this pathogen have been revealed, which explain some pathogenic mechanisms. In various hostile conditions, for example, during nitrogen and amino acid starvation, bacteria can utilize alternative sigma factors such as RpoS and RpoN to modulate genes expression for their adaptation and survival. In this study, we demonstrate that mutagenesis of rpoN2, which lies on chromosome 2 of B. pseudomallei and encodes a homologue of the sigma factor RpoN, did not alter nitrogen and amino acid utilization of the bacterium. However, introduction of B. pseudomallei rpoN2 into E. coli strain deficient for rpoN restored the ability to utilize amino acids. Moreover, comparative partial proteomic analysis of the B. pseudomallei wild type and its rpoN2 isogenic mutant was performed to elucidate its amino acids utilization property which was comparable to its function found in the complementation assay. By contrast, the rpoN2 mutant exhibited decreased katE expression at the transcriptional and translational levels. Our finding indicates that B. pseudomallei RpoN2 is involved in a specific function in the regulation of catalase E expression.
1. Introduction
Melioidosis is an endemic disease in Southeast Asia and northern Australia. The causative agent of melioidosis in humans is Burkholderia pseudomallei which is a facultative intracellular pathogen. This organism is a polar flagellated Gram negative bacterium that can infect both humans and animals [1–3]. The mechanism by which B. pseudomallei causes melioidosis and its virulence is partly understood. The major regulatory control mechanisms for the expression of genes including virulent genes are the sigma factors. There are two major families of sigma factors which are sigma 70 (RpoD) and sigma 54 (RpoN) [4]. Sigma 54 is required only for a specific metabolic pathway such as nitrogen utilization and amino acids synthesis [5]. Moreover, sigma 54 regulates the transcription of virulence associated genes including pili, flagella, and alginate biosynthesis operons in Pseudomonas aeruginosa and Vibrio species [6–8].
The analysis of B. pseudomallei genome has been identified in two copies of rpoN genes in two genomic locations, rpoN1 on chromosome 1 and rpoN2 on chromosome 2. To date, nothing is known at the molecular level regarding the function of sigma 54 (RpoN) in B. pseudomallei. Therefore, it was of interest to determine whether B. pseudomallei RpoN1 or RpoN2 is involved in nitrogen and amino acids utilization. To investigate this, the defined B. pseudomallei strain 844 rpoN1 and rpoN2 knockout mutants were constructed. However, only rpoN2 knockout mutant was successfully constructed. The role of RpoN2 in nitrogen and amino acids utilization was examined and compared to that of E. coli lacking RpoN by complementation assay in rpoN mutant derivative E. coli JKD 814. A comparative proteomic analysis of the B. pseudomallei wild type and its rpoN2 isogenic mutant was performed to elucidate its amino acids utilization property. Moreover, we have identified RpoN2 specific function and found that it is involved in regulation of catalase E expression both at the transcriptional and at the translational levels.
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
The bacterial strains used are listed in Table 1. B. pseudomallei is routinely maintained in Luria-Bertani (LB) medium. Pseudomonas agar base supplemented with SR103E (Cetrimide, Fucidin, and Cephaloridine) from Oxoid was used after conjugation as selective medium to inhibit growth of E. coli. Ashdown agar plate was also used for B. pseudomallei specific selective medium. All cultures were grown at 37°C in an aerobic condition with 250 rpm shaking. Tetracycline (60 μg/mL) and chloramphenicol (40 μg/mL) were added to media when required.
Table 1.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Genotype or relevant characteristic | Source (reference) |
|---|---|---|
| Bacteria strains | ||
| PP844 | Wild type, clinical isolate from blood | This study |
| E. coli SM10 λpir | λpir (thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Km) used for transformation of recombinant plasmid pKNOCK::rpoN2 Bp | [15] |
| E. coli JKD 814 | rpoN::tet | [16] |
| Plasmids | ||
| pKNOCK-Tc | Mobilizable suicide vector carrying TetR gene | [10] |
| pKRpoN2 | pKNOCK-Tc containing 363 bp internal segment of B. pseudomallei rpoN2 gene | This study |
| pBBR1MCS | Broad-host-range cloning vector, Cmr | [17] |
| pBSDRpoN2 | pBR1MCS containing full-length rpoN2 gene | This study |
| pCR 2.1-TOPO | Cloning vector for PCR product, Kanamycin and Ampicillin resistance | Invitrogen, California, USA |
| pTOPO::rpoN2 Bp799 | pCR 2.1-TOPO vector containing 799 bp fragment of truncated rpoN2 gene from B. pseudomallei strain 844, Kanamycin and Ampicillin resistance | This study |
| pTOPO::rpoN Ec | pCR 2.1-TOPO vector containing full-length rpoN gene from E. coli, Kanamycin and Ampicillin resistance | This study |
2.2. Construction of B. pseudomallei rpoN2 Mutant and Its Complemented Strain
rpoN2 knockout mutant (Table 1) was created to be pKRpoN2 according to a previously described procedure [9]. pKRpoN2 was constructed by transferring the 363 bp partial digested PstI fragment from genomic DNA of B. pseudomallei into the mobilizable suicide vector pKNOCK-Tc [10]. The constructed B. pseudomallei rpoN2 mutant was analyzed by Southern blot analysis and PCR as described elsewhere [11]. To confirm that all changes in phenotypes were caused by the disruption of rpoN2 and were not due to polar effects on downstream genes, a plasmid (pBSDRpoN2) containing the complete full-length rpoN2 coding sequence under control of the lacZ and cat promoters was constructed and transferred into the B. pseudomallei mutant strains for complementation analysis. Likewise, pTOPO::rpoN2 Bp and pTOPO::rpoN2 Bp799 were constructed and used to complement into JKD 814 E. coli rpoN negative mutant. All plasmids constructed are listed in Table 1.
2.3. Nitrogen and Amino Acids Utilization Tests
Nitrogen and amino acids utilization tests were performed in MM9 salts minimal agar containing either 20 mM ammonium chloride (NH4Cl) or other alternative nitrogen sources such as arginine, glutamine, glycine, histidine, lysine, methionine, phenylalanine, tryptophan, and valine at 5 mM concentration. 10 μL of overnight growth of the desired bacterial strains (A 600 = 1) was inoculated onto the above agar medium and incubated at 37°C. Growths of the bacterial colony were observed daily for five consecutive days.
Statistical measurements of all assays were carried out in three separate times. The results were expressed as the mean ± standard deviation of days of growth. The significance of differences in nitrogen and amino acids utilization of bacterial strains was analyzed by Student's paired t-test (2-tailed) using SPSS statistical software program.
2.4. Protein Extraction and Two-Dimensional Gel Electrophoresis (2DE)
Bacterial cultures were grown until early stationary phase. Proteins were extracted using 500 μL lysis buffer (8 M urea, 4% w/v CHAPS, 2 mM TBP, 1% v/v IPG buffer, pH 4–7) (Amersham Biosciences, Uppsala, Sweden) and 1% v/v protease inhibitor cocktail set II (Calbiochem, La Jolla, CA). The supernatant after cells lysis was transferred into clean microcentrifuge tubes and stored at −80°C until use. Protein concentrations were determined using RC DC protein assay kit (Bio-Rad, Hercules, CA) as previously described [12]. For 2DE, the first-dimensional isoelectric focusing (IEF) was carried out using 500 μg protein samples with the rehydration buffer (8 M urea, 2% w/v CHAPS, 20 mM DTT, and 1% v/v IPG buffer, pH 4–7) adjusted to 350 μL total volume. Precast 18-cm Immobiline DryStrip with a linear pH 4–7 was used with the IPGphor II system (Amersham Biosciences) to perform IEF. The strips were rehydrated with the protein samples for 12 h at 20°C following three voltage steps as previously described [12]. All profiles were controlled at the current 50 μA/strip. The IPG strips were then equilibrated and transferred to the second-dimensional SDS-PAGE using 12.5% polyacrylamide gel. SDS-PAGE was performed at 4°C with the constant electrical current at 10 mA/gel. Protein spots were visualized by Coomassie Brilliant Blue G-250 (CBBG-250) staining and gels were scanned with an ImageMaster Scanner (Amersham Biosciences). Image analysis was performed using PDQuest software version 7.1 (Bio-Rad). Images from three independent cultures were compared. A master gel used for spot matching process was created from a wild type 2D gel. The master gel was then used for matching of the corresponding protein spots between 2D gels. The relative intensity of each protein spot was determined by normalizing to the total intensity of the gel. Protein expression with intensity representing at least 3-fold difference with P < 0.05 was considered in this analysis. The biosynthesis pathways such as cysteine synthesis, histidine synthesis, purine metabolism, and pentose phosphate pathway were performed by using KEGG database [13].
2.5. Protein Extraction and Activity Staining for Catalase
Bacterial cultures were grown in various growth phase (12, 24, 48, and 72 hours) conditions. The bacterial pellets were lysed on ice by sonication in one-tenth of the original culture volume of phosphate buffer (5 mM potassium phosphate, pH 7.0, 5 mM EDTA, 10% glycerol, and 25 mM phenylmethylsulfonyl fluoride). Proteins were extracted as previously described [14]. The total protein concentration in each sample was determined with Bradford Reagent (Sigma Chemical, St. Louis, MO).
Catalase activities present in crude extracts of B. pseudomallei cells were determined by loading 20 μg of protein in 12% nondenaturing polyacrylamide gel. After polyacrylamide gel electrophoresis, the gels were washed three times with PBS (20 min each) to remove surface attached buffer ions as previously described [14]. Immediately, the gels were incubated with a solution of 2% (w/v) ferric chloride-potassium ferricyanide, until the gel was stained green.
2.6. RNA Isolation, cDNA Synthesis, and Relative Gene Expression Analysis
RNA extraction procedures were performed using TRIzol reagent (Invitrogen, USA). 1 mL of various growth phase cultures (24, 48, and 72 hours) was harvested and collected by centrifugation. RNA extraction was carried out following the manufacturer's instructions. Each RNA sample was treated with RQ1 RNase-free DNase (Promega, USA) and tested for DNA contamination as previously described [11]. Measurement of RNA concentration was performed using Nanodrop 2000 (Thermo Fisher Scientific, USA). cDNA synthesis was performed using Superscript III Reverse Transcriptase First Strand cDNA synthesis kit (Invitrogen, USA) following the manufacturer's instructions.
Relative quantification real-time PCR for the katE gene was performed using Rotor-Gene 3000 (Corbett Research, Australia). Primers (katE-F primer 5′-TCT ACA CCG ACG AGG GCA AC-3′ and katE-R primer 5′-TTC CTC CGG AAT CAG CTT GG-3′) were designed using Primer3 software [19], and the reactions were quantified using SYBR GreenER qPCR SuperMix Universal (Invitrogen, USA). All reactions were programmed with dissociation curve analysis to prevent nonspecific and primer-dimer formation as previously described [20]. Gene encoding 23s rRNA was used as a reference control for normalization. The results were analyzed using the comparative Ct method or ΔΔCt method (Applied Biosystems).
Statistical analysis of this study was performed from at least 3 independent experiments, each carried out in duplicate or triplicate. Values were presented as means ± standard error. Statistical significance of differences between the two means was calculated using SigmaStat 3.5 software and evaluated by Student's t-test and P value <0.01 was considered significant.
3. Results
3.1. Nitrogen and Amino Acids Utilization Tests in Escherichia coli
In order to determine a constructed rpoN2 mutant whether RpoN2 is essential for the B. pseudomallei growth in the absence of amino acid supplementation, we compared the growth of the wild type rpoN2 isogenic mutant and the rpoN2 mutant carrying rpoN2-complementing plasmid. No differences in growth were observed among the strains (data not shown). These results indicate that rpoN2 may be either not necessary or lacking the functions for amino acid utilization phenotypes.
To determine whether the rpoN2 is not necessary or lacks the functions in amino acid utilization in B. pseudomallei, we performed the complementation assay by transforming full-length B. pseudomallei rpoN2 (TOPO::rpoN2 Bp plasmid DNA) and 799-fragment rpoN2 (TOPO::rpoN2 Bp799 plasmid DNA) into E. coli JKD 814 which lacks of rpoN and then compared with E. coli JKD 814 complemented with TOPO::rpoN Ec plasmid DNA using as a positive control. 10 μL (A 600 = 1) of bacterial culture was inoculated on M9 minimal agar containing each amino acid and incubated at 37°C for 5 days. E. coli rpoN mutant JKD 814 and E. coli wild type JM 109 were negative and positive controls, respectively. The experiments were carried out in three biological replicates. The differences in utilization of each amino acid for each construct were compared to that of JKD 814 and analyzed using Student's paired t-test. B. pseudomallei wild type strain 844 showed similar colony growth rate to E. coli wild type in the utilization of histidine as sole nitrogen source. Like E. coli wild type, B. pseudomallei also grew on M9 minimal agar supplemented with NH4Cl, lysine, and tryptophan but the growths were approximately one-day delay (Table 2). Although the growth of B. pseudomallei could be investigated on arginine and valine sole nitrogen source, the colonies were not observed until after day four of incubation. On the other hand, B. pseudomallei wild type utilized glutamine and phenylalanine faster than E. coli wild type. However, unlike E. coli wild type, B. pseudomallei could not utilize glycine as a growth substrate.
Table 2.
Nitrogen and amino acid utilization of B. pseudomallei wild type 844 and E. coli derivatives.
| Nitrogen source |
Day of growth appearance (mean ± SD)a | ||||||
|---|---|---|---|---|---|---|---|
|
E. coli
JM 109 |
JKD 814 rpoN mutant |
JKD 814 harboring pTOPO vector |
JKD 814 harboring TOPO::rpoN Ec |
JKD 814 harboring TOPO::rpoN2 Bp |
JKD 814 harboring TOPO::rpoN2 Bp799 |
B. pseudomallei
wild type 844 |
|
| NH4Cl | 3.7 ± 0.6∗ | ± | ± | 2.7 ± 0.6∗ | 2 ± 1 | NG | 4.7 ± 0.6∗ |
| Arginine | 1.3 ± 0.6∗ | ± | ± | 3.3 ± 0.6∗ | 3.3 ± 0.6∗ | ± | 4.7 ± 0.6∗ |
| Glutamine | 2.7 ± 0.6∗ | NG | NG | 4.6 ± 0.6∗ | 3.7 ± 0.6∗ | NG | 1.3 ± 0.6∗ |
| Glycine | 2.7 ± 0.6∗ | ± | ± | 2.3 ± 0.6∗ | 2.7 ± 0.6∗ | ± | ± |
| Histidine | 1.3 ± 0.6∗ | NG | NG | 4 ± 1∗ | 2.3 ± 0.6∗ | NG | 1.3 ± 0.6∗ |
| Lysine | 3.3 ± 0.6∗ | NG | NG | 3.7 ± 0.6∗ | 4.3 ± 1.2∗ | NG | 4.7 ± 0.6∗ |
| Methionine | 4.7 ± 0.6∗ | NG | NG | NG | 4.7 ± 0.6∗ | NG | ± |
| Phenylalanine | 4.7 ± 0.6∗ | NG | ± | 4.7 ± 0.6∗ | 3.7 ± 0.6∗ | ± | 1.3 ± 0.6∗ |
| Tryptophan | 1.7 ± 0.6∗ | ± | ± | 1.7 ± 0.6∗ | 1.7 ± 0.6∗ | NG | 2.7 ± 0.6∗ |
| Valine | 1.3 ± 0.6∗ | NG | NG | NG | NG | NG | 4.7 ± 0.6∗ |
aData represent geometric mean (±standard error) from three independent experiments. NG indicates the absence of growth. ± indicates the very sparing growth inspected on day 6. ∗Significant differences in amino acid utilization compared to E. coli rpoN mutant JKD 814 (P ≤ 0.05, Student's paired t-test).
The rpoN mutant E. coli JKD 814 and JKD 814 harboring either pCR 2.1-TOPO vector alone or plasmid containing only partial ORF of rpoN2 (TOPO::rpoN2 Bp799 [799 bp]) exhibited no growth on minimal media supplemented with all nitrogen sources tested in this study after 5 days of incubation (Table 2).
Although the delay in growth was demonstrated, when TOPO::rpoN Ec recombinant plasmid which contained entire ORF of E. coli rpoN was complemented into JKD 814 E. coli rpoN mutant, the growth phenotypes were able to restore all tested amino acids except methionine and valine (Table 2). In contrast, the mutant JKD 814 complemented TOPO::rpoN Ec strain exhibited faster growing on the simple nitrogenous compound NH4Cl compared to the E. coli wild type. Similar growth patterns were also observed in the mutant JKD 814 complemented with TOPO::rpoN2 Bp with the exception of methionine. Moreover, in minimal media supplemented with sole lysine, the mutant JKD 814 complemented with TOPO::rpoN2 Bp appeared to grow slower than the mutant complemented with TOPO::rpoN Ec and the E. coli wild type.
Compared to B. pseudomallei wild type strain 844, rpoN mutant JKD 814 complemented with TOPO::rpoN2 Bp grew slightly faster on M9 minimal media supplemented with NH4Cl, arginine, and tryptophan while it grew slower in glutamine, histidine, and phenylalanine. No difference in growth rate was inspected when lysine was used as a sole nitrogen source. Moreover, the valine utilization could not be restored in the rpoN2 Bp complemented strain. Surprisingly, the rpoN mutant JKD 814 complemented with TOPO::rpoN2 Bp was able to grow on media supplemented with glycine and methionine which were the characteristic of E. coli wild type and not of B. pseudomallei wild type strain 844 phenotype (Table 2).
The overall results suggested that any difference in nitrogen utilization observed in this study was mediated by RpoN from either E. coli or B. pseudomallei. Therefore, we have demonstrated that B. pseudomallei rpoN2 has full functions in regulation of nitrogen and amino acids utilization.
3.2. Partial Proteomic Analysis of B. pseudomallei rpoN2 Mutant Compared with Its Wild Type
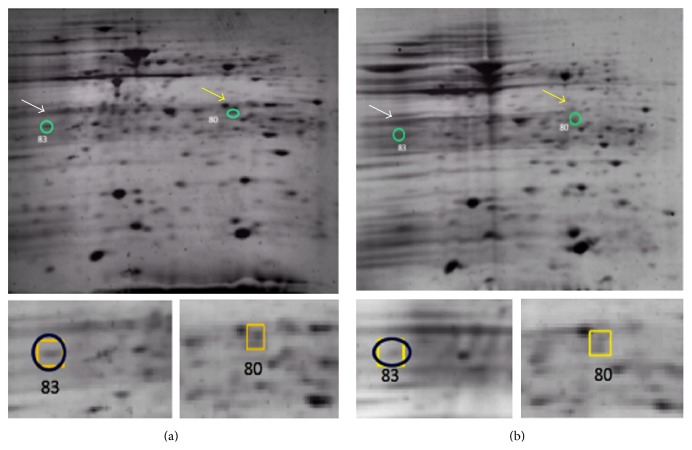
A comparison of proteomic expressions in wild type and rpoN2 mutant strains of B. pseudomallei was set as the cutoff to be a threefold difference between the wild type and rpoN2 mutant. The results for proteins located in pH range 4.5 to 7 and molecular weight 20–75 kDa are shown in Figure 1. The identity of proteins was determined using the reference map from the wild type analysis [18]. A total of 21 spots were identified. The upregulated proteins in the rpoN2 mutant strain included 14 proteins and the downregulated proteins in rpoN2 mutant included 7 proteins as indicated in Figures 1(a) and 1(b). The numbers of each spot are referred to the same numbers as mentioned in the B. pseudomallei wild-type proteome reference map [18] which was used to study the RpoS regulon of B. pseudomallei [21]. The 7 proteins are downregulated in rpoN2 mutant including proteins involved in energy metabolism, lipid metabolism, transcription, and several hypothetical proteins of unknown function. In addition, 14 proteins appeared to be upregulated in rpoN2 mutant, the majority of which are involved in carbohydrate metabolism, posttranslation modification, cell envelope biogenesis, and outer membrane formation as summarized in Table 3. In order to identify the enzymes responsible for nitrogen utilization and amino acid synthesis and uptake, we found phosphoribosyl formimino-5-aminoimidazole carboxamide ribotide isomerase (HisA) (spot number 83) and cysteine synthase (CysM) (spot number 80) that are involved in histidine and cysteine synthesis, respectively (Figures 1(a) and 1(b)). However, both histidine and cysteine biosynthetic pathway can produce these amino acids from several starting points using both de novo synthesis and the intermediates in other biosynthetic pathways (Figures 2 and 3) [13]. Therefore, B. pseudomallei HisA and CysM are not essential for production of histidine and cysteine. Our partial proteomic analysis supported the function of RpoN2 that is not essential for nitrogen and amino acids utilization.
Figure 1.
2D-gel electrophoresis of B. pseudomallei wild type (a) and rpoN2 mutant (b) obtained from stationary phase of growth. PDQuest program was used for analysis of the group's sample of wild type and mutant compared with reference map of B. pseudomallei. The circles indicate spot numbers 80 (CysM) and 83 (HisA) that are downregulated in rpoN2 mutant strain.
Table 3.
Comparative partial proteomic analysis of rpoN2 mutant compared to wild type of B. pseudomallei. The spot numbers assigned in this study are identical to the number of spots in B. pseudomallei 2DE reference map [18].
| Protein orthologues | Expression alteration ratio in B. pseudomallei strains | pI | MW | Spot number | |
|---|---|---|---|---|---|
| Wild type | rpoN2 mutant | ||||
| (1) Cell envelope biogenesis and outer membrane | |||||
| Acetyltransferase (GNAT) family protein | 1 | ↑ 8.30 | 4.9 | 50.17 | 9 |
| UDP-N-acetylmuramyl pentapeptide synthase | 1 | ↑ 5.02 | 5.7 | 41.55 | 2 |
| (2) Energy metabolism | |||||
| Polyphosphate kinase 2 family (Ppk2) | 1 | ↑ 3.17 | 4.89 | 44.26 | 40 |
| ATB synthase subunit B | 1 | ↑ 3.40 | 4.97 | 53.93 | 15 |
| NADH-dehydrogenase delta subunit | 1 | 0.17 ↓ | 5.34 | 36.59 | 26 |
| Aldehyde dehydrogenase (NAD) family protein | 1 | 0.19 ↓ | 6.02 | 55.39 | 24 |
| (3) Carbohydrate metabolism | |||||
| Glyceraldehyde 3-phosphate dehydrogenase (GapA) | 1 | ↑ 3.38 | 4.82 | 20.88 | 48 |
| Nucleoside-diphosphate-sugar epimerases | 1 | ↑ 12.58 | 5.21 | 27.78 | 56 |
| (4) Lipid metabolism | |||||
| 4-Hydroxyl-3-methylbut-2-enyl diphosphate reductase (lspH) | 1 | ↑ 5.07 | 5.94 | 40.81 | 71 |
| Nonribosomally encoded peptide/polyketide synthase (CmaB) | 1 | 0.21 ↓ | 6.19 | 37.19 | 73 |
| (5) Amino acid metabolism | |||||
| Phosphoribosyl formimino-5-aminoimidazole carboxamide ribotide isomerase (HisA) | 1 | 0.20 ↓ | 4.42 | 31 | 83 |
| Cysteine synthase (CysM) | 1 | 0.33 ↓ | 5.75 | 36.11 | 80 |
| (6) Nucleotide metabolism | |||||
| Orotidine 5′-phosphate decarboxylase | 1 | ↑ 6.42 | 4.99 | 32.79 | 88 |
| (7) Translation | |||||
| 50S ribosomal protein L3 | 1 | ↑ 3.49 | 4.14 | 27.8 | 150 |
| (8) Transcription | |||||
| Transcriptional regulator, ArsR family 1 | 1 | 0.20 ↓ | 4.92 | 37.92 | 124 |
| (9) Posttranslational modification | |||||
| Thioredoxin | 1 | ↑ 3.37 | 5.97 | 16.15 | 179 |
| Peptidyl-prolyl cis-trans isomerase B | 1 | ↑ 22.15 | 6.05 | 19.99 | 174 |
| Glutathione S-transferase | 1 | ↑ 3.05 | 6.5 | 22 | 176 |
| (10) Cell motility, intracellular trafficking, and secretion | |||||
| Type III effector protein BipD | 1 | ↑ 5.04 | 4.38 | 34.37 | 194 |
| Type III secretion chaperone LcrH/SycD | 1 | ↑ 6.23 | 4.92 | 18.52 | 190 |
| (11) Stress responses | |||||
| Carbohydrate porin, OprB family | 1 | ↑ 3.16 | 5.01 | 38.71 | 233 |
| (12) Hypothetical protein | |||||
| Hypothetical protein BPSL0349 | 1 | ↑ 3.15 | 5.28 | 16.53 | 253 |
| Hypothetical protein BPSS0931 | 1 | 0.03 ↓ | 4.64 | 23.63 | 274 |
Figure 2.
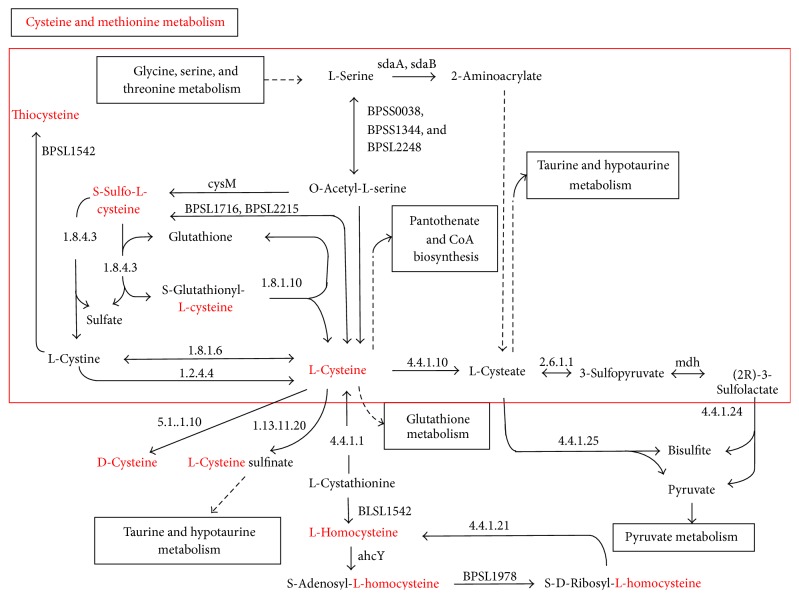
Cysteine and methionine metabolisms. The square is a pathway synthesis from glycine, serine, and threonine metabolisms to L-cysteine. http://www.genome.jp/kegg.
Figure 3.
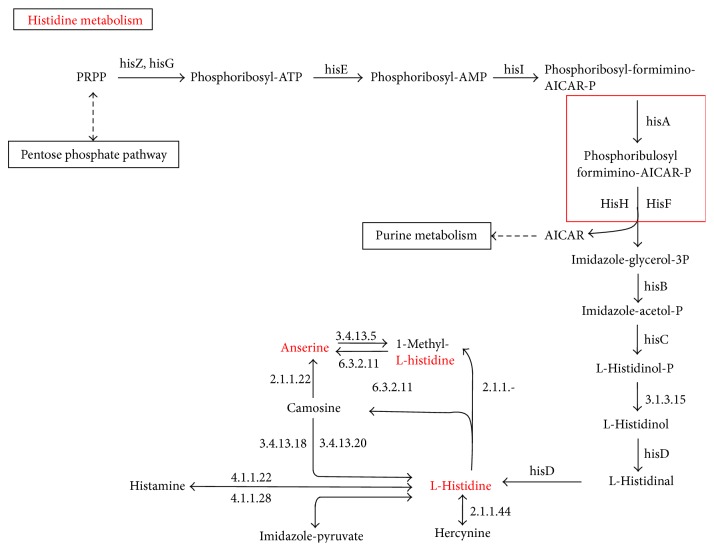
Histidine metabolism. The square is the phosphoribosyl formimino-5-aminoimidazole carboxamide ribotide isomerase (HisA). http://www.genome.jp/kegg.
3.3. Positive Regulation of RpoN2 on katE in B. pseudomallei
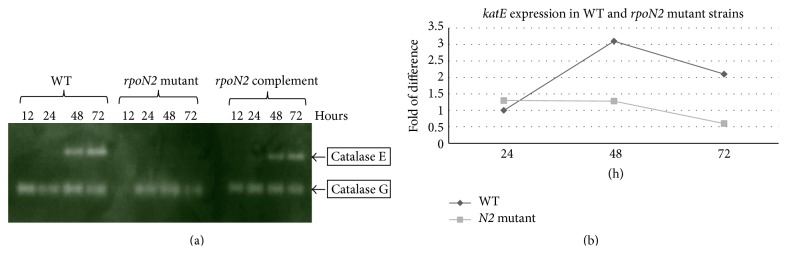
Using bioinformatics prediction for chromosome 2, a potential RpoN box [4] was identified in front of the katE gene. An activity staining assay for catalase [14] was performed for the wild type rpoN2 isogenic mutant and the rpoN2 mutant carrying the rpoN2-complementing plasmid. The results suggest that catalase E or KatE activity is regulated by RpoN2 as shown in Figure 4(a). In particular, the rpoN2 complemented strain fully restores the KatE activity as shown in Figure 4(a). To demonstrate that catalase E activity is regulated at the transcriptional level, qRT-PCR was performed as shown in Figure 4(b). katE expression in the wild type was detected from 48 hours to 72 hours of bacterial growth, whereas no katE expression was detected in the rpoN2 mutant.
Figure 4.
(a) Zymography of catalase activities during various stages. B. pseudomallei PP844 wild type (WT), the rpoN2 isogenic mutant (rpoN2 mutant), and the rpoN2 mutant carrying the rpoN2-complementing plasmid (rpoN2 complement) were grown aerobically in LB medium for 12, 24, 48, and 72 hours. The extracted cells (15 μg of protein) were prepared for electrophoresis in 10% nondenaturing polyacrylamide gel and stained for catalase activity in a solution of 2% (w/v) ferric chloride-potassium ferric cyanide. (b) Relative quantification real-time RT-PCR (qRT-PCR) for B. pseudomallei katE expression was compared between the rpoN2 isogenic mutant (N2 mutant) and the wild type PP844 (WT) at various stages (24, 48, and 72 hours) of growth. The katE gene primers were designed using Primer3 software. All fold difference values were normalized with 23s rRNA expression and the values are the mean standard deviations; analysis is performed in triplicate.
4. Discussion
In several bacterial pathogens, RpoN (δ 54) is known to be involved in pathogenesis and virulence such as nitrogen utilization and amino acid assimilation; capsular polysaccharide and lipopolysaccharide synthesis; flagella and pili biosynthesis; type III secretion; and biofilm formation [5–8, 22–24]. Rhizobium etli has two copies of the rpoN gene and experiments with the mutant strains revealed that rpoN1 and rpoN2 genes are both active but under different physiological conditions [25]. The rpoN1 gene is essential during the growth phase while rpoN2 is required for metabolism [25]. In B. pseudomallei, each chromosome contains a member of the sigma 54 family (RpoN1 and RpoN2) which are known to be involved in amino acid utilization and some virulence factors but the function of each RpoN is still unclear. In this study, construction of both rpoN1 and rpoN2 knockout mutants was performed but, unfortunately, the rpoN1 mutant could not be obtained. We therefore focused on the role of B. pseudomallei δ 54 (RpoN2) in comparison with the wild type strain PP844, rpoN2 mutant, and complemented rpoN2. MM9 culture medium containing only inorganic salts, a carbon source, and water was used for testing whether RpoN2 was necessary for growth in the absence of amino acid supplementation. The wild type and the complemented rpoN2 contain all the genes needed for survival and as predicted both of these strains can grow in the absence of the addition of exogenous amino acids (MM9). However, the rpoN2 mutant is missing RpoN2 activity that has been proposed to regulate the transcription of some proteins involved in amino acid biosynthesis. If RpoN2 has a major role in amino acid utilization, the rpoN2 negative mutant would be unable to grow in the absence of amino acid supplementation. Our results show that the B. pseudomallei rpoN2 mutant strain was able to grow in MM9 medium indicating that RpoN2 is not essential in regulating amino acid utilization. However, the possibility that these phenotypes might be compensated by another rpoN gene product on chromosome 1 of B. pseudomallei could not be excluded.
In order to demonstrate the ability of B. pseudomallei rpoN2 gene product to have a function in regulation of amino acid utilization, a complementation assay by restoration growth of E. coli rpoN mutant in minimal media supplemented with various amino acids (Table 2) was performed. Indeed, the ability to grow in the presence of glycine and methionine was the characteristic of E. coli wild type. Moreover, the ability to utilize glycine and methionine as sole nitrogen source in E. coli (Table 2) was previously shown to be regulated by RpoN [16, 23–27]. Due to the fact that all of the RpoNBp functional domains are almost identical to that of RpoNEc, therefore, it is possible that RpoNBp is able to induce the transcription of the heterologous glycine and methionine utilization genes in E. coli JKD 814 in similar fashion to the E. coli RpoN.
In phenylalanine and glutamine utilization test, slower growth rate of JKD harboring TOPO::rpoN2 Bp compared to B. pseudomallei wild type was demonstrated (Table 2). It has been previously reported that the decrement in gene transcription by RpoN regulon is likely to be the consequence of the differences in the nucleotide sequence around the RpoN-conserved recognition sites 12 and 24 located upstream of the target genes [28]. Thus, it is possible that RpoNBp may prefer to recognize and induce the promoter sequence upstream of the genes required for phenylalanine and glutamine utilization in B. pseudomallei wild type more than that of E. coli JKD 814. However, it is also possible that phenylalanine and glutamine utilization system in B. pseudomallei wild type and E. coli may differ or operate differently. This hypothesis is also underscored by the data presented in Table 2 that E. coli wild type usually utilized phenylalanine and glutamine at least one day slower than B. pseudomallei wild type. Currently, the phenylalanine and glutamine utilization mechanisms in B. pseudomallei have not yet been elucidated. In addition, due to the difference in their native habitats, the discrepancy in the range of RpoN-controlled nitrogen utilization genes between E. coli and B. pseudomallei is also probably due to the specific control circuits to achieve the optimal adaptation to the different environments. The transport of nitrate across the membrane or the production of nitrate reductase and lysine decarboxylase in B. pseudomallei is not under the control of RpoN2Bp as there was no different result observed among E. coli wild type, E. coli JKD 814, and JKD 814 derivatives. In E. coli and other Gram negative bacteria, lysine is metabolized by two major pathways, the lysine oxygenase and the pipecolate route [29, 30]. In the former route, L-lysine is oxidatively decarboxylated by lysine oxygenase to form 5-aminovaleramide, whereas in the latter, L-lysine is converted to D-lysine, which is then oxidatively deaminated, cyclized, and finally reduced to L-pipecolate [29, 30]. Moreover, E. coli can also utilize lysine as a sole carbon source and this ability is also under the control of σ 54 (RpoN) [29]. In contrast, Pseudomonas putida, the B. pseudomallei related species, was able to utilize lysine only as a nitrogen source and this ability was also under the control of σ 54 (RpoN) [15]. However, our results are not able to draw conclusion that the growth on minimal medium supplemented with lysine of B. pseudomallei and JKD 814 harboring TOPO::rpoN2 Bp is due to either the utilization of carbon or nitrogen source.
For better understanding the role of RpoN2 in amino acid utilization, we used 2-dimension polyacrylamide gels to identify proteins with altered levels in the rpoN2 mutant since RpoN2 may regulate these proteins. Cells have many ways to obtain amino acids such as transport from environment, de novo synthesis, or synthesis from other amino acids and we speculated that RpoN2 might be involved in the regulation of some proteins involved in these processes. By analysis of soluble extracts using 2D gels and PDQuest software package to identify the proteins with altered abundance, we found that cysteine synthase (CysM) and phosphoribosyl formimino-5-aminoimidazole carboxamide ribotide isomerase (HisA) were both downregulated in the rpoN2 mutant. While the levels of CysM and HisA were decreased in the rpoN2 mutant, it was able to synthesize/take up sufficient cysteine and histidine to grow on MM9 medium. It is possible that both cysteine and histidine could be produced using intermediates from other pathways or other amino acids as precursors. Investigating the connections between cysteine synthesis and other biosynthetic pathways using http://www.genome.jp/kegg [13] revealed that in addition to the de novo pathway for cysteine synthesis it is possible to produce cysteine from glycine, serine, and threonine (Figure 2). The connection between cysteine synthesis and the production of other amino acids may explain why the rpoN2 mutant, which should not be able to produce cysteine from the de novo pathway utilizing CysM, can grow on MM9 medium. As was seen with cysteine synthesis, histidine can also be synthesized from several precursors. HisA is a protein in amino acid metabolism specific to histidine biosynthesis and function in the synthesis of L-histidinol-P and purine metabolism from the pentose phosphate pathway as shown in Figure 3 (http://www.genome.jp/kegg) [13]. This protein was decreased in rpoN2 mutant indicating that it is regulated by RpoN2; however alternative pathways are present that can compensate for loss of HisA dependent histidine production. Our study is the first to identify and demonstrate a role of RpoN2 in B. pseudomallei.
In contrast to the similarity of amino acid utilization between the rpoN2 mutant and the wild type, the rpoN2 mutant exhibited decreases in katE expression, both at the transcriptional and at the translational levels. Catalase is ubiquitous, well-studied enzyme that catalyzes the decomposition of hydrogen peroxide. It is an important enzyme for the survival of facultative aerobic organisms exposed to oxidative stress conditions. Using bioinformatics prediction for chromosome 2, a potential RpoN box [4] was identified in front of the katE gene. An activity staining assay for catalase [14] was performed. The results suggest that catalase E or KatE activity is regulated by RpoN2. In particular, the rpoN2 complemented strain fully restores the KatE activity indicating that it is not due to polar effects on downstream genes. To demonstrate that the catalase E activity is regulated at the transcriptional level, qRT-PCR [11, 19, 20] was performed and the result is comparable to the KatE activity detecting from 48 hours to 72 hours of bacterial wild type growth. Interestingly, it has been reported that B. pseudomallei KatE activity [14] may be controlled by RpoS. In that study, the authors demonstrated the catalase activities at the translational level in which the catalase E activity was assessed only by activity staining assay. The contradictory results found in this study are more reliable because we determined that an RpoN box is in front of a katE gene and also because the katE gene expression was detected by qRT-PCR. We therefore hypothesized a possibility of cross communication between these two sigma families, RpoS and RpoN2, via KatE regulation, and that is currently under our investigation.
In conclusion, we report a novel finding that B. pseudomallei has 2 copies of RpoN and that RpoN2 is located on chromosome 2. rpoN2 does not directly function in amino acid utilization in B. pseudomallei but it can restore this function in E. coli. The constructed B. pseudomallei rpoN2 mutant lacking KatE activity is demonstrated by activity staining assay and qRT-PCR. In contrast to RpoN1, RpoN2 is therefore involved in a specific function for the regulation of catalase E expression both at the transcriptional and at the translational levels.
Acknowledgments
The research project was supported by the National Science and Technology Development Agency, Pathum Thani, Thailand, and the Thailand Research Fund. Duong Thi Hong Diep was supported by the TRIG project from the University of Medicine and Pharmacy at Ho Chi Minh City.
Abbreviations
- B. pseudomallei:
Burkholderia pseudomallei
- 2DE:
Two-dimensional gel electrophoresis
- KatE:
Catalase E
- qRT-PCR:
Quantitative Reverse Transcriptase-Polymerase Chain Reaction.
Conflict of Interests
The authors declare that their financial supports do not have any conflict of interests regarding the publication of this paper and the grants were only for academic study.
References
- 1.Howe C., Sampath A., Spotnitz M. The Burkholderia pseudomallei group: a review. Journal of Infectious Diseases. 1997;124:598–606. doi: 10.1093/infdis/124.6.598. [DOI] [PubMed] [Google Scholar]
- 2.Sanford J., Douglas J. R. Principles and Practice of Infectious Diseases. New York, NY, USA: Churchill Livingstone Press; 1990. [Google Scholar]
- 3.Leelarasamee A., Bovornkiti S. Melioidosis: review and update. Reviews of Infectious Diseases. 1989;11(3):413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Wösten M. M. S. M. Eubacterial sigma-factors. FEMS Microbiology Reviews. 1998;22(3):127–150. doi: 10.1016/S0168-6445(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson E. L., Plotnikova J., Mahajan-Miklos S., Rahme L. G., Ausubel F. M. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. Journal of Bacteriology. 2001;183(24):7126–7134. doi: 10.1128/jb.183.24.7126-7134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Totten P. A., Cano Lara J., Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. Journal of Bacteriology. 1990;172(1):389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative σ factor (RpoN) of RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramphal R., Koo L., Ishimoto K. S., Totten P. A., Lara J. C., Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infection and Immunity. 1991;59(4):1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low K. B. Conjugational methods for mapping with Hfr and F-Prime strains. Methods in Enzymology. 1991;204:43–62. doi: 10.1016/0076-6879(91)04005-9. [DOI] [PubMed] [Google Scholar]
- 10.Alexeyev M. F. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques. 1999;26(5):824–828. doi: 10.2144/99265bm05. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook J., Russell W. Molecular Cloning: A Laboratory Manual. New York, NY, USA: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 2001. [Google Scholar]
- 12.Wongtrakoongate P., Mongkoldhumrongkul N., Chaijan S., Kamchonwongpaisan S., Tungpradabkul S. Comparative proteomic profiles and the potential markers between Burkholderia pseudomallei and Burkholderia thailandensis . Molecular and Cellular Probes. 2007;21(2):81–91. doi: 10.1016/j.mcp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways mapping for cysteine and histidine. 2015, http://www.genome.jp/kegg/pathway.html.
- 14.Jangiama W., Lopraserf S., Tungpradabkula S. Role of Burkholderia pseudomallei rpoS in regulation of catalase activities under hydrogen peroxide induction. ScienceAsia. 2008;34(1):23–29. doi: 10.2306/scienceasia1513-1874.2008.34.023. [DOI] [Google Scholar]
- 15.Donnenberg M. S., Kaper J. B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infection and Immunity. 1991;59(12):4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reitzer L., Schneider B. L. Metabolic context and possible physiological themes of sigma 54-dependent genes in Escherichia coli . Microbiology and Molecular Biology Reviews. 2001;65(3):422–444. doi: 10.1128/mmbr.65.3.422-444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovach M. E., Phillips R. W., Elzer P. H., Roop R. M., II, Peterson K. M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16(5):800–802. [PubMed] [Google Scholar]
- 18.Wongtrakoongate P., Roytrakul S., Yasothornsrikul S., Tungpradabkul S. A proteome reference map of the causative agent of melioidosis Burkholderia pseudomallei . Journal of Biomedicine and Biotechnology. 2011;2011:5. doi: 10.1155/2011/530926.530926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 20.Wongtrakoongate P., Tumapa S., Tungpradabkul S. Regulation of a quorum sensing system by stationary phase sigma factor RpoS and their co-regulation of target genes in Burkholderia pseudomallei . Microbiology and Immunology. 2012;56(5):281–294. doi: 10.1111/j.1348-0421.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- 21.Osiriphun Y., Wongtrakoongate P., Sanongkiet S., Suriyaphol P., Thongboonkerd V., Tungpradabkul S. Identification and characterization of RpoS regulon and RpoS-dependent promoters in Burkholderia pseudomallei . Journal of Proteome Research. 2009;8(6):3118–3131. doi: 10.1021/pr900066h. [DOI] [PubMed] [Google Scholar]
- 22.Magasanik B. The regulation of nitrogen utilization in enteric bacteria. Journal of Cellular Biochemistry. 1993;51(1):34–40. doi: 10.1002/jcb.240510108. [DOI] [PubMed] [Google Scholar]
- 23.Merrick M. J., Edwards R. A. Nitrogen control in bacteria. Microbiological Reviews. 1995;59(4):604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiological Reviews. 1989;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michiels J., Moris M., Dombrecht B., Verreth C., Vanderleyden J. Differential regulation of Rhizobium etli rpoN2 gene expression during symbiosis and free-living growth. Journal of Bacteriology. 1998;180(14):3620–3628. doi: 10.1128/jb.180.14.3620-3628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmer D. P., Soupene E., Lee H. L., et al. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14674–14679. doi: 10.1073/pnas.97.26.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen B. P. Basic amino acid transport in Escherichia coli . The Journal of Biological Chemistry. 1971;246(11):3653–3662. [PubMed] [Google Scholar]
- 28.Arai H., Hayashi M., Kuroi A., Ishii M., Igarashi Y. Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa . Journal of Bacteriology. 2005;187(12):3960–3968. doi: 10.1128/jb.187.12.3960-3968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler T., Harayama S., Ramos J., Timmis K. Involvement of Pseudomonas putida RpoN σ factor in regulation of various metabolic functions. Journal of Bacteriology. 1989;171(8):4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y. F., Adams E. D-Lysine catabolic pathway in Pseudomonas putida: interrelations with L-lysine catabolism. Journal of Bacteriology. 1974;117(2):753–764. doi: 10.1128/jb.117.2.753-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]