Abstract
Stinging nettle (Urtica dioica L.) has a long history of usage and is currently receiving attention as a source of fiber and alternative medicine. In many cultures, nettle is also eaten as a leafy vegetable. In this study, we focused on nettle yield (edible portion) and processing effects on nutritive and dietary properties. Actively growing shoots were harvested from field plots and leaves separated from stems. Leaf portions (200 g) were washed and processed by blanching (1 min at 96–98°C) or cooking (7 min at 98-99°C) with or without salt (5 g·L−1). Samples were cooled immediately after cooking and kept in frozen storage before analysis. Proximate composition, mineral, amino acid, and vitamin contents were determined, and nutritive value was estimated based on 100 g serving portions in a 2000 calorie diet. Results show that processed nettle can supply 90%–100% of vitamin A (including vitamin A as β-carotene) and is a good source of dietary calcium, iron, and protein. We recommend fresh or processed nettle as a high-protein, low-calorie source of essential nutrients, minerals, and vitamins particularly in vegetarian, diabetic, or other specialized diets.
1. Introduction
Stinging nettle (Urtica dioica L.) has a long history as one among plants foraged from the wild and eaten as a vegetable [1, 2]. Although not fully domesticated, the species remains popular even in the current era for food and medicine as reported, for example, in Nepal [2] and Poland [3].
Despite U. dioica being recognized as an edible and highly nutritious vegetable, research attention has focused more on its value as a source of alternative medicine and fiber. Clinical trials have confirmed the effectiveness of nettle root and saw palmetto (Serenoa repens (Bart.) Small) fruit extracts in the treatment of benign prostatic hyperplasia [4]. Dried nettle leaf preparations are also known to alleviate symptoms associated with allergic rhinitis [5], and a technology for granulating lipophilic leaf extracts for medicine has been developed [6]. A recent report from ongoing work in Italy confirms the potential of U. dioica as a sustainable source of textile fiber [7].
There are a number of reports that address the role of U. dioica in human nutrition. Fatty acid and carotenoid content in leaf, stem, root, and seed samples have been measured [8], and the properties of phenolic compounds in leaves, stalks, and fibers have been reported [9]. Furthermore, the quality and safety [10] and microbiological properties [11] of sucuk, a Turkish dry-fermented sausage, incorporating dried U. dioica leaf have been studied, and the capacity of nettle extracts to improve oxidative stability in brined anchovies has been reported [12]. In the Basque region of Spain, young shoots are reportedly eaten raw or included in omelets [13]. In terms of postharvest processing for long-term storage, microwave drying at 850 W was found to be the best method for preservation of leaf color, energy consumption, and processing time [14]. Mineral content [15] and trace metal concentrations [16] in nettle leaf tea made by infusion or decoction have also been determined.
However, nettle is consumed primarily as a fresh vegetable whereby it is added to soups, cooked as a pot herb, or used as a vegetable complement in dishes. In this sense, more work needs to be done on nutritive value of fresh nettle, and the fate of minerals and bioactive compounds in processed products. This information is essential because the capacity of fresh nettle to irritate bare skin may discourage potential consumers and postharvest processing methods that make it safe to handle, while maintaining nutritive value will benefit the development of U. dioica as a specialty vegetable.
In this study, we report dietary values, mineral properties, and other quality attributes of raw, blanched, and cooked stinging nettle.
2. Materials and Methods
2.1. Plant Materials
Plant samples were obtained from field plots planted as a part of an ongoing agronomic study on U. dioica at Randolph Farm (37.1°N; 77.3°W), Virginia State University (VSU). Samples from fall and spring growth were collected in October 2011 and May 2012, respectively, by harvesting actively growing shoots (20 ± 2 cm) before the onset of flowering. Individual shoots were clipped with a pair of shears and consolidated in vented plastic bags before transfer to a demonstration kitchen located at the VSU Farm Pavilion for further processing.
2.2. Sample Processing
In the kitchen, the shoots were washed, and twelve 200 ± 5 g units were weighed before separating leaves and tender shoot tips from the woody stem. The edible portion (leaves and tender shoot tips) was weighed, and mean yield was determined by presenting the weight of edible portion as a percentage of total unit mass. Treatments, each replicated three times, were applied as follows: raw samples were packaged and frozen without further processing, blanched samples were immersed in boiling water (98-99°C) for 1 min, and cooked samples were boiled (98–100°C) with or without salt (5 g·L−1 H2O) for 7 min. Both blanched and cooked samples were cooled to 0°C with shaved ice immediately after treatment. All samples were kept in frozen storage (−4°C) before analysis. Samples for proximate composition analysis were submitted frozen, while those for fatty and amino acid analysis were freeze-dried and ground to a fine powder before analysis.
2.3. Proximate Analysis
All analysis was done according to the Association of Analytical Chemists (AOAC) methods (AOAC, 2000). Moisture content was determined by drying samples to constant weight using a convection oven. Nitrogen (N) content was measured using a CN analyzer (LECO 528, LECO Corp., St. Joseph, MI), and protein content was derived by multiplying N values with 6.25. Total fat was determined by gas chromatography (Agilent 5890, Agilent Technologies, Santa Clara, CA, USA) after extraction of saponifiable and unsaponifiable fractions, and ash content was measured by ignition at 550°C to constant weight. Carbohydrate content and calorie values were calculated by difference. Total dietary fiber was determined following methods described by the American Association of Cereal Chemists (AACCI method 32-07.01).
2.4. Vitamin and Mineral Analysis
Total vitamin A and vitamin A as β-carotene were determined by colorimetry after alkaline digestion followed by extraction with hexane. Vitamin C was extracted in acid and sample content determined by titration. For mineral analysis, samples were subjected to wet digestion before calcium, iron, and sodium content was determined using an ICP spectrometer (AOAC, 2000).
2.5. Amino Acid Analysis
For amino acid analysis, a ground subsample of nettle tissue was hydrolyzed with 6 M HCl at 100°C for 24 hr as previously described [17]. Acid hydrolyzed amino acids were derivatized with phenyl isothiocyanate (Acros Organics, Geel, Belgium) and separated using a 2695 Alliance HPLC equipped with a 15-cm Pico-Tag column, 2487 UV/Vis detector, and Empower software (all from Waters Corp., Milford, MA) using previously described conditions [18]. Amino acid concentrations are expressed in g/100 g of nettle leaf.
2.6. Fatty Acid Analysis
Fatty acid methyl esters (FAMEs) were prepared by treating raw and processed samples with ethyl chloride and absolute methanol as described [19]. Fatty acid methyl esters were analyzed by gas chromatography using an Agilent 6890 N GC system (Agilent Technologies), equipped with a HP-INNOWax column (30 m × 0.32 mm I.D. × 0.5 μm film thickness) and flame ionization detector. Peaks were identified against retention times for a known FAME and quantified by the aid of heptadecanoic acid (17:0) included as an internal standard. The concentration of each fatty acid is presented as a percentage of total saponifiable oil in sample.
2.7. Statistical Analysis
One-way analysis of variance (ANOVA) using the Analyst function in SAS (version 9.2 for Windows, SAS Institute, Cary, NC) was performed to compare the effects of blanching and cooking on stinging nettle quality and nutritive value. Treatments were treated as independent variables, and data for fall 2011 and spring 2012 were analyzed separately. Tukey's HSD (P < 0.05) was used to separate treatment means within season.
3. Results and Discussion
3.1. Yield of Edible Portion in U. dioica
Actively growing stinging nettle shoots are ideally harvested before flowering for consumption as a potherb or spinach alternative. Leaves on stems were found to be tender enough for use as a vegetable up to 25 cm from the growing point, but stems become woody about 4 cm away from the growing point necessitating destemming after harvest to separate the tender tip (approx. 4 cm and leaves) from the woody stem. Our results show that the woody stem portion accounts for 23%–30% of total biomass with edible portion comprising of 70% or more of harvested material (Table 1). Yield (edible portion) was higher in fall than in spring samples because of seasonal differences in U. dioica growth characteristics. Consistent with published observations [20], U. dioica displays two distinct phenological stages when grown in south-central Virginia: reproductive growth up to late spring, limited development during summer, and mostly vegetative growth in the fall.
Table 1.
Edible portion (leaf) yield as a percentage of total biomass in stinging nettle (Urtica dioica L.) harvested from field plots in the fall of 2011 and spring of 2012. Actively growing shoots (20 ± 2 cm) were harvested and processed by de-stemming.
| Season | Shoot wt. (g) | Stem wt. (g) | Leaf wt. (g) | Loss (%) |
|---|---|---|---|---|
| Fall 2011 | 203 ± 1.73a | 46 ± 3.5 | 157 ± 4.69 | 23 ± 1.8 |
| Spring 2012 | 199 ± 5.5 | 55 ± 7.9 | 144 ± 10.3 | 28 ± 4.2 |
aMean (n = 3) ± standard deviation.
3.2. Effect of Blanching and Boiling on Proximate Composition, Vitamin, and Mineral Content in U. dioica
After draining, there was not much difference in moisture content between raw and processed samples in the fall of 2011, while there was slightly more moisture in processed samples in the spring of 2012, likely due to differences in draining time. There was a slight reduction in crude protein, ash, and fat after blanching or cooking in both fall and spring samples. In both cases, the most significant reductions were observed with longer exposure to heat and also to salt. The same applies to dietary fiber, carbohydrate content, and calorie value. Samples harvested in the spring contained significantly higher values for all parameters measured and showed higher decline after processing (Table 2). Preparation and cooking generally result in deterioration of vegetable quality. For example, cooking significantly reduces ash, carbohydrate content, and calorific value in Cocoyam (Colocasia esculenta) leaves [21], while chopping amaranth (Amaranthus sp.) leaves before cooking can result in increased loss of vitamins and minerals [22]. Our results show that vitamin A, calcium, and iron contents in U. dioica leaf are similarly affected by cooking. Sodium content was low and was not affected by cooking, but the salt added to cooking water in one of the treatments significantly (P < 0.05) increased sodium content in drained samples (Table 2). Salt addition for seasoning or preservation has been reported to affect vegetable quality through dilution of minerals and other chemical changes [23]. Cooking led to changes in the fatty acid profile of U. dioica with more saturated fat being converted into mono-unsaturated and polyunsaturated forms (Table 3) or lost into solution. Saponifiable oil content in raw and processed U. dioica samples (3.2%–4.7% in the spring; 3.2%–4.1% in the spring) was comparable to that in wild asparagus (Asparagus acutifolius) and black bryony (Tamus communis), edible wild greens common to Mediterranean diets [24].
Table 2.
Proximate composition, vitamins, minerals, and fatty acid profile of raw and processed stinging nettle (Urtica dioica L.) shoots harvested from field plots in the fall of 2011 and spring of 2012.
| Fall 2011 | Spring 2012 | |||||||
|---|---|---|---|---|---|---|---|---|
| Raw | Blanched | Cooked | Cooked + salt | Raw | Blanched | Cooked | Cooked + salt | |
| Proximate analysis | ||||||||
| Moisture (%) | 89.0 ± 1.4a | 87.2 ± 0.9a | 87.7 ± 0.7a | 88.6 ± 0.5a | 75.1 ± 1.5c | 84.6 ± 2.5b | 85.6 ± 0.8b | 91.7 ± 0.9a |
| Protein (%) | 3.7 ± 0.5a | 3.6 ± 0.4ab | 3.6 ± 0.3a | 2.7 ± 0.2b | 6.3 ± 0.3a | 4.1 ± 0.2b | 3.8 ± 0.3b | 2.2 ± 0.2c |
| Fat (%) | 0.6 ± 0.1a | 0.4 ± 0.1b | 0.4 ± 0.0b | 0.2 ± 0.0b | 1.4 ± 0.3a | 1.1 ± 0.1a | 1.1 ± 0.2a | 0.6 ± 0.1b |
| Ash (%) | 2.1 ± 0.3a | 1.8 ± 0.3ab | 1.5 ± 0.3b | 1.5 ± 0.1b | 3.4 ± 0.2a | 1.4 ± 0.1b | 1.2 ± 0.1c | 1.0 ± 0.1c |
| Fiber, total dietary (%) | 6.4 ± 0.4a | 4.2 ± 0.1b | 3.5 ± 0.3c | 3.6 ± 0.3bc | 9.7 ± 1.0a | 5.4 ± 0.9b | 4.9 ± 1.0b | 4.2 ± 0.2c |
| Carbohydrates, total (%) | 7.1 ± 1.7a | 6.6 ± 1.4ab | 6.3 ± 0.8b | 6.2 ± 1.2b | 16.5 ± 1.6a | 8.9 ± 0.7b | 8.1 ± 1.1b | 4.2 ± 0.6c |
| Other carbohydrates (%) | 2.7 ± 0.2ab | 2.9 ± 0.3a | 2.5 ± 0.1b | 2.7 ± 0.1a | 6.2 ± 1.0a | 3.5 ± 0.7b | 3.3 ± 0.5b | 2.0 ± 0.1c |
| Calories, total (kcal/100 g) | 45.7 ± 3.1a | 42.6 ± 2.1a | 44.7 ± 2.5a | 36.5 ± 2.3b | 99.7 ± 2.5a | 62.0 ± 1.0b | 57.3 ± 1.5c | 32.0 ± 1.0d |
| Calories from fat (kcal/100 g) | 5.0 ± 1.0a | 4.3 ± 0.6ab | 2.7 ± 0.5bc | 2.3 ± 0.6c | 12.3 ± 1.6a | 10.0 ± 1.0ab | 8.7 ± 3.1b | 4.0 ± 1.0c |
| Vitamins and minerals | ||||||||
| Vitamin A, total (IU/100 g) | 4935 ± 104a | 4851 ± 56a | 4548 ± 53b | 4362 ± 78b | 11403 ± 1333a | 6470 ± 222bc | 6021 ± 90c | 7872 ± 354b |
| Vitamin A, as β-carotene (IU/100 g) | 5035 ± 213a | 4689 ± 37b | 4549 ± 130b | 4062 ± 39c | 7860 ± 460a | 4811 ± 88b | 5028 ± 65b | 4154 ± 148c |
| Vitamin C (mg/100 g) | 1.1 ± 0.1a | 0.6 ± 0.1b | 0.6 ± 0.1b | 0.5 ± 0.1b | 0.5 ± 0.0a | 0.5 ± 0.0a | 0.5 ± 0.0a | 0.5 ± 0.0a |
| Calcium (mg/100 g) | 278 ± 9c | 441 ± 12a | 376 ± 9ab | 318 ± 52bc | 788 ± 41a | 464 ± 10b | 430 ± 10b | 316 ± 7c |
| Iron (mg/100 g) | 1.2 ± 0.1c | 1.8 ± 0.2b | 2.6 ± 0.1a | 2.5 ± 0.3a | 3.4 ± 0.3a | 2.1 ± 0.2b | 2.1 ± 0.3b | 1.6 ± 0.1c |
| Sodium (mg/100 g) | 5.7 ± 0.1b | 6.3 ± 0.4b | 6.5 ± 0.3b | 87.7 ± 6.0a | 5.5 ± 0.6b | 7.0 ± 0.2b | 6.7 ± 0.2b | 81.1 ± 2.9a |
| Fatty acid profile | ||||||||
| Saturated fat (%) | 35.5 ± 2.6a | 25.7 ± 2.5b | 23.6 ± 4.1 c | 21.7 ± 1.9d | 32.7 ± 2.8a | 16.5 ± 1.5bc | 17.3 ± 1.2b | 15.7 ± 1.4c |
| Monounsaturated (%) | 2.7 ± 0.2c | 3.3 ± 0.2a | 4.8 ± 0.3a | 3.2 ± 0.1b | 7.5 ± 0.6a | 5.3 ± 0.3b | 5.8 ± 1.1b | 4.6 ± 0.2c |
| Polyunsaturated (%) | 61.8 ± 3.5c | 71.0 ± 2.0b | 71.6 ± 1.2c | 75.1 ± 1.9a | 59.8 ± 2.7d | 78.2 ± 4.4b | 76.9 ± 2.5c | 79.7 ± 2.6a |
| Cholesterol (mg/100 g) | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0a |
aMean (n = 3) ± standard deviation. Values within a year followed by different letters are significantly different at P < 0.05 (Tukey's HSD).
Table 3.
Fatty acid contenta in raw and processed stinging nettle (Urtica dioica L.) shoots harvested from field plots in the fall of 2011 and spring of 2012.
| Total fat (%) | Fatty acidb (% of total fat) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | 20:0 | 20:1 | 22:0 | 22:1 | 24:0 | ||
| Fall 2011 | ||||||||||||
| Raw | 3.15 ± 0.12c | 17.06 ± 0.05a | 2.54 ± 0.04b | 1.86 ± 0.01a | 2.18 ± 0.01c | 23.30 ± 0.20a | 49.55 ± 0.10d | 0.83 ± 0.01a | 0.03 ± 0.01a | 1.37 ± 0.02a | 0.06 ± 0.01b | 1.23 ± 0.03a |
| Blanched | 4.72 ± 0.05ab | 14.91 ± 0.12b | 2.54 ± 0.02b | 1.41 ± 0.02c | 2.23 ± 0.02b | 21.58 ± 0.20b | 54.42 ± 0.37c | 0.67 ± 0.01c | 0.06 ± 0.01a | 1.11 ± 0.01c | 0.09 ± 0.02ab | 0.98 ± 0.01b |
| Cooked | 4.65 ± 0.10b | 14.83 ± 0.09b | 2.45 ± 0.02c | 1.60 ± 0.01b | 1.91 ± 0.03d | 20.96 ± 0.10c | 55.48 ± 0.20b | 0.69 ± 0.01b | 0.03 ± 0.01a | 1.13 ± 0.01b | 0.05 ± 0.01b | 0.88 ± 0.01d |
| Cooked + salt | 4.78 ± 0.14a | 14.22 ± 0.11c | 2.62 ± 0.01a | 1.35 ± 0.01d | 2.54 ± 0.01a | 19.67 ± 0.2d | 56.70 ± 0.34a | 0.67 ± 0.01bc | 0.05 ± 0.01a | 1.13 ± 0.01b | 0.14 ± 0.01a | 0.91 ± 0.01c |
| Spring 2012 | ||||||||||||
| Raw | 3.17 ± 0.01d | 16.30 ± 0.04a | 1.88 ± 0.01b | 1.76 ± 0.01a | 2.99 ± 0.02a | 23.89 ± 0.07a | 48.06 ± 0.09d | 1.12 ± 0.01a | 0.25 ± 0.01a | 1.63 ± 0.02a | 0.53 ± 0.01a | 1.61 ± 0.01a |
| Blanched | 4.27 ± 0.04b |
14.58 ± 0.06b | 1.70 ± 0.01d | 1.64 ± 0.01c | 2.95 ± 0.01a | 21.56 ± 0.27b | 53.25 ± 0.30c | 1.05 ± 0.02b | 0.20 ± 0.01c | 1.48 ± 0.01b | 0.38 ± 0.01b | 1.26 ± 0.01d |
| Cooked | 4.50 ± 0.15a | 14.07 ± 0.21d | 1.93 ± 0.03a | 1.64 ± 0.03c | 2.30 ± 0.07c | 20.78 ± 0.35c | 55.59 ± 0.41a | 0.99 ± 0.04c | 0.21 ± 0.01b | 1.47 ± 0.03b | 0.38 ± 0.01b | 1.35 ± 0.12c |
| Cooked + salt | 3.58 ± 0.06 c | 14.29 ± 0.05 c | 1.84 ± 0.05c | 1.67 ± 0.03 b | 2.39 ± 0.01b | 20.19 ± 0.47d | 54.44 ± 0.37b | 1.04 ± 0.02b | 0.18 ± 0.01d | 1.50 ± 0.01b | 0.36 ± 0.01c | 1.50 ± 0.03b |
aMethylated samples were analyzed for total fatty acid content using gas chromatography.
bPalmitic acid (16:0); palmitoleic acid (16:1); stearic acid (18:0); oleic acid (18:1); linoleic acid (18:2); α-linoleic acid (18:3); gadoleic acid (20:1); behenic acid (22:0); erucic acid (22:1); lignoceric acid (24:0).
cMean (n = 3) ± standard deviation. Column values followed by different letters within season are significantly different at P < 0.05 (Tukey's HSD).
3.3. Effect of Cooking on Fatty and Amino Acid Composition in U. dioica Tissue Samples
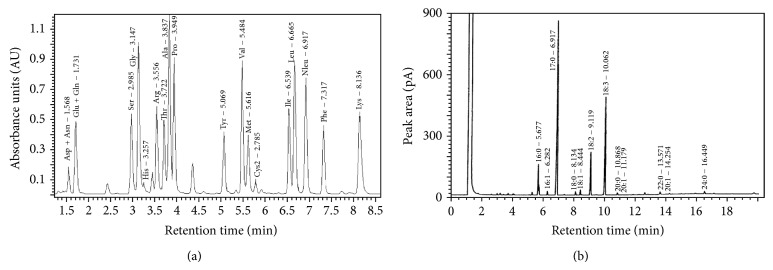
Data on individual amino and fatty acid content in stinging nettle shows that the species can supply significant quantities of oleic (18:1), linoleic (18:2), and α-linoleic (18:3) acids and is a good source of unsaturated fatty acids. Considerable amounts of palmitic acid (16:0), a saturated fatty acid, were found in the leaf (Table 3; Figure 1). There were no significant differences in fatty acid content between samples collected from fall and spring growth. Similarly blanching and cooking with or without salt did not affect fatty acid content within season except for a general trend showing an increase in unsaturated fatty acid content and a corresponding decrease in the concentration of saturated fatty acids (Table 3). Similarly, high levels of linoleic and α-linoleic acids in young and mature leaves and the presence of relatively high concentrations of the same oils in U. dioica seed, stem, and roots portions have been reported [8], with the seed containing up to 15% saponifiable oil.
Figure 1.
Representative chromatograms showing peaks and retention times for different amino (a) and fatty (b) acids in raw and processed stinging nettle (Urtica dioica L.) leaf samples.
In terms of omega-3 fatty acid content, U. dioica compares favorably with frozen spinach (Spinacia oleracea L.) pretreated by steaming, blanching, or autoclaving [25]. Relative to other commonly consumed wild plants, it contains a higher concentration of omega-3 fatty acids than borage (Borago officinalis), and about the same level as water-blinks (Montia fontana) [26], watercress (Rorippa nasturtium-aquaticum), sheep sorrel (Rumex acetosella), and sorrel (Rumex induratus) [27]. However, carbohydrate content (including total sugars) was significantly lower in raw and processed U. dioica (4.2%–16.5%) than in the four species above reported to constitute 66.6%–78.9% total carbohydrates [27]. These results show that processing by blanching and cooking has a minimal impact on U. dioica fatty acid composition, implying that it can be a good source of essential fatty acids when eaten as a leafy vegetable.
With regard to individual amino acids, tissue content was similarly not affected by season. Our results show that U. dioica can supply considerable amounts of essential amino acids including threonine, valine, isoleucine, leucine, phenylalanine, and lysine, along with lower concentrations of histidine and methionine (Table 4; Figure 1). Amino acid content was largely unchanged in the spring as compared with fall growth though asparagine, glutamine, leucine, and histidine levels were generally lower in samples from spring growth. There were slight to significant increases in amino acid content after blanching or cooking in fall samples, but no similar observation was made for samples collected in the spring (Table 4). There may be differences between and within species in response to postharvest handling and processing conditions. In one study, a significant increase in amino acid content was recorded after cooking relative to raw spinach [28], while the opposite was true for cooked and frozen versus raw Brussels sprouts [29].
Table 4.
Amino acid content in raw and processed stinging nettle (Urtica dioica L.) shoots harvested from field plots in the fall of 2011 and spring of 2012.
| Amino acid (g/100 g) | Fall 2011 | Spring 2012 | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw | Blanched | Cooked | Cooked + salt | Raw | Blanched | Cooked | Cooked + salt | |
| Isoleucine | 0.90 ± 0.17b | 1.13 ± 0.20ab | 1.30 ± 0.10a | 1.39 ± 0.06a | 1.04 ± 0.08a | 1.04 ± 0.08a | 1.06 ± 0.09a | 0.97 ± 0.05a |
| Leucine | 1.65 ± 0.27b | 2.09 ± 0.033ab | 2.37 ± 0.18a | 2.56 ± 0.18a | 1.79 ± 0.38a | 1.91 ± 0.06a | 1.91 ± 0.08a | 1.75 ± 0.03a |
| Lysine | 1.11 ± 0.21a | 1.37 ± 0.11a | 1.37 ± 0.30a | 1.48 ± 0.17a | 1.16 ± 0.38a | 1.33 ± 0.20a | 1.19 ± 0.30a | 1.10 ± 0.19a |
| Methionine | 0.24 ± 0.05a | 0.31 ± 0.04a | 0.33 ± 0.05a | 0.35 ± 0.06a | 0.23 ± 0.15a | 0.19 ± 0.13a | 0.17 ± 0.07a | 0.20 ± 0.13a |
| Tyrosine | 0.75 ± 0.13b | 0.95 ± 0.13ab | 1.11 ± 0.10ab | 1.18 ± 0.14a | 0.97 ± 0.20a | 0.90 ± 0.10a | 0.93 ± 0.12a | 0.91 ± 0.13a |
| Phenylalanine | 1.03 ± 0.19b | 1.27 ± 0.17ab | 1.43 ± 0.15a | 1.51 ± 0.03a | 1.15 ± 0.23a | 1.14 ± 0.05a | 1.13 ± 0.04a | 1.06 ± 0.04a |
| Threonine | 1.00 ± 0.17a | 1.08 ± 0.05a | 1.12 ± 0.15a | 1.24 ± 0.08a | 1.03 ± 0.24a | 0.75 ± 0.07a | 0.84 ± 0.11a | 0.75 ± 0.14a |
| Valine | 1.11 ± 0.19b | 1.40 ± 0.23ab | 1.60 ± 0.11a | 1.72 ± 0.16a | 1.30 ± 0.24a | 1.28 ± 0.12a | 1.32 ± 0.15a | 1.22 ± 0.10a |
| Histidine | 0.42 ± 0.09b | 0.53 ± 0.11ab | 0.64 ± 0.06ab | 0.68 ± 0.11a | 0.32 ± 0.15a | 0.30 ± 0.12a | 0.37 ± 0.08a | 0.22 ± 0.12a |
| Total essential amino acids | 8.23 ± 1.36b | 10.13 ± 1.39ab | 11.26 ± 1.00a | 12.11 ± 1.60a | 8.95 ± 2.14a | 8.83 ± 0.39a | 8.93 ± 0.29a | 8.20 ± 0.59a |
| Arginine | 1.22 ± 0.21b | 1.57 ± 0.27ab | 1.79 ± 0.16a | 1.97 ± 0.14a | 1.55 ± 0.42a | 1.43 ± 0.26a | 1.56 ± 0.21a | 1.52 ± 0.24a |
| Aspartic acid + asparagine | 0.85 ± 0.32a | 1.01 ± 0.25a | 0.88 ± 0.40a | 1.01 ± 0.04a | 0.60 ± 0.37a | 0.47 ± 0.09a | 0.49 ± 0.10a | 0.39 ± 0.14a |
| Glutamic acid + glutamine | 1.69 ± 0.39a | 2.13 ± 0.19a | 1.97 ± 0.62a | 2.22 ± 0.26a | 1.49 ± 0.72a | 1.25 ± 0.26a | 1.42 ± 0.13a | 1.14 ± 0.27a |
| Serine | 0.85 ± 0.14b | 1.06 ± 0.15ab | 1.14 ± 0.13ab | 1.26 ± 0.10a | 1.00 ± 0.29a | 0.82 ± 0.12a | 0.96 ± 0.15a | 0.82 ± 0.20a |
| Proline | 0.90 ± 0.15b | 1.11 ± 0.17ab | 1.31 ± 0.13ab | 1.41 ± 0.20a | 1.24 ± 0.17a | 1.06 ± 0.22a | 1.19 ± 0.28a | 1.07 ± 0.16a |
| Glycine | 0.92 ± 0.15b | 1.13 ± 0.16ab | 1.26 ± 0.10ab | 1.39 ± 0.17a | 1.14 ± 0.23a | 0.98 ± 0.22a | 1.12 ± 0.22a | 0.97 ± 0.12a |
| Alanine | 1.20 ± 0.19b | 1.40 ± 0.13ab | 1.54 ± 0.11ab | 1.66 ± 0.16a | 1.54 ± 0.29a | 1.24 ± 0.22a | 1.38 ± 0.26a | 1.21 ± 0.14a |
| Total amino acids | 17.46 ± 2.88b | 21.58 ± 3.40ab | 22.87 ± 2.21ab | 24.76 ± 0.96a | 19.40 ± 5.00a | 17.77 ± 1.83a | 18.73 ± 1.44a | 16.97 ± 1.63a |
| Dry matter (g/100 g edible portion) | 11.0 | 12.8 | 12.3 | 11.4 | 14.9 | 15.4 | 14.4 | 8.3 |
aMean (n = 3) ± standard deviation. Row values followed by different letters within season are significantly different at P < 0.05 (Tukey's HSD).
Data from this experiment show that both raw and cooked U. dioica can be important sources of dietary protein. The species can supply higher concentrations of essential amino acids than Brussels sprouts [29] and has a better amino acid profile than most other leafy vegetables. Although similar to S. oleracea in terms of total amino acid content, U. dioica contains higher levels of all essential amino acids except leucine and lysine. Some of the published recipes incorporating U. dioica leaf flour in bread, pasta, and noodle dough suggest that it can be used as a protein-rich supplement in starchy diets associated with poor and undernourished populations. This is because on a dry weight basis, U. dioica leaf is better than almond (dry) and is comparable to common bean (Phaseolus vulgaris) and chicken (Gallus gallus) as a source of essential amino acids [30]. The agronomic properties of U. dioica including perennial growth, quick response to fertilization, and high biomass yield make it an excellent candidate for low-cost mass production for such a purpose.
3.4. Labeling Information for Processed U. dioica
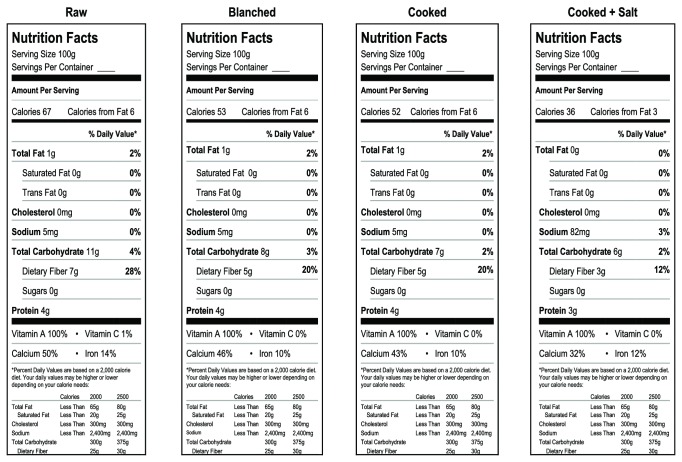
Results from this study show that U. dioica retains a significant portion of minerals, vitamins, and essential nutrients after pre-treatment by blanching or cooking prior to frozen storage. Processing may be the most effective approach to availing the nutritional benefits of U. dioica to consumers discouraged by the stinging quality of live or fresh nettle. The nutritional information in Figure 2, representing means of data from both spring and fall growth, can be used to label frozen raw and processed U. dioica leaf. However, lower vitamin A and higher carbohydrate content and other data reported for blanched U. dioica samples collected from the wild [31] show that more work is required to evaluate the properties of U. dioica products as affected by interactions between landrace, environment, harvesting time, and processing conditions.
Figure 2.
Suggested food labeling information for raw and processed stinging nettle (Urtica dioica L.).
4. Conclusions
Although the usage of U. dioica as a leafy vegetable is widespread, there is little information on processing potential, and the impact of different processing methods on nutritive and functional value. The results presented in this report show that U. dioica retains significant amounts of minerals, vitamins, and other functional values after blanching or cooking. We recommend processing and selling of U. dioica leaf as a highly functional and nutritive food.
Acknowledgments
The authors are grateful to Mr. Robert Kraemer and Mr. Landon West, VSU Farm Manager and Assistant Farm Manager, respectively, for field support and to Dr. Ngowari Jaja for assistance with sample preparation. This is a contribution of Virginia State University Research Station Article No. 303.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.di Tizio A., Łuczaj J. Ł., Quave C. L., Redzic S., Pieroni A. Traditional food and herbal uses of wild plants in the ancient South-Slavic diaspora of Mundimitar/Montemitro (Southern Italy) Journal of Ethnobiology and Ethnomedicine. 2012;8, article 21 doi: 10.1186/1746-4269-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uprety Y., Poudel R. C., Shreshta K. K., et al. Diversity of use and local knowledge of wild edible plant resources in Nepal. Journal of Ethnobiology and Ethnomedicine. 2012;8, article 16 doi: 10.1186/1746-4269-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Łuczaj Ł., Pieroni A., Tardío J., et al. Wild food plant use in 21st century Europe: the disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Societatis Botanicorum Poloniae. 2012;81(4):359–370. [Google Scholar]
- 4.Lopatkin N., Sivkov A., Walther C., et al. Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms—a placebo-controlled, double-blind, multicenter trial. World Journal of Urology. 2005;23(2):139–146. doi: 10.1007/s00345-005-0501-9. [DOI] [PubMed] [Google Scholar]
- 5.Mittmann P. Randomized, double-blind study of freeze-dried Urtica dioica in the treatment of allergic rhinitis. Planta Medica. 1990;56(1):44–47. doi: 10.1055/s-2006-960881. [DOI] [PubMed] [Google Scholar]
- 6.Minina S. A., Ozhigova M. G. Development of granulation technology for lipophilic extracts. Pharmaceutical Chemistry Journal. 2010;44(4):213–215. doi: 10.1007/s11094-010-0434-5. [DOI] [Google Scholar]
- 7.Bacci L., Baronti S., Predieri S., di Virgilio N. Fiber yield and quality of fiber nettle (Urtica dioica L.) cultivated in Italy. Industrial Crops and Products. 2009;29(2-3):480–484. doi: 10.1016/j.indcrop.2008.09.005. [DOI] [Google Scholar]
- 8.Guil-Guerrero J. L., Rebolloso-Fuentes M. M., Torija Isasa M. E. Fatty acids and carotenoids from Stinging Nettle (Urtica dioica L.) Journal of Food Composition and Analysis. 2003;16(2):111–119. doi: 10.1016/S0889-1575(02)00172-2. [DOI] [Google Scholar]
- 9.Pinelli P., Ieri F., Vignolini P., Bacci L., Baronti S., Romani A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. Journal of Agricultural and Food Chemistry. 2008;56(19):9127–9132. doi: 10.1021/jf801552d. [DOI] [PubMed] [Google Scholar]
- 10.Karabacak S., Bozkurt H. Effects of Urtica dioica and Hibiscus sabdariffa on the quality and safety of sucuk (Turkish dry-fermented sausage) Meat Science. 2008;78(3):288–296. doi: 10.1016/j.meatsci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Kaban G., Aksu M. I., Kaya M. Behavior of Staphylococcus aureus in sucuk with nettle (Urtica dioica L.) Journal of Food Safety. 2007;27(4):400–410. doi: 10.1111/j.1745-4565.2007.00090.x. [DOI] [Google Scholar]
- 12.Turhan S., Sagir I., Temiz H. Oxidative stability of brined anchovies (Engraulis encrasicholus) with plant extracts. International Journal of Food Science and Technology. 2009;44(2):386–393. doi: 10.1111/j.1365-2621.2008.01777.x. [DOI] [Google Scholar]
- 13.Menendez-Baceta G., Aceituno-Mata L., Tardío J., Reyes-García V., Pardo-de-Santayana M. Wild edible plants traditionally gathered in Gorbeialdea (Biscay, Basque Country) Genetic Resources and Crop Evolution. 2012;59:1329–1347. [Google Scholar]
- 14.Alibas I. Energy consumption and color characteristics of nettle leaves during microwave, vacuum and convective drying. Biosystems Engineering. 2007;96(4):495–502. doi: 10.1016/j.biosystemseng.2006.12.011. [DOI] [Google Scholar]
- 15.Özcan M. M., Ünver A., Uçar T., Arslan D. Mineral content of some herbs and herbal teas by infusion and decoction. Food Chemistry. 2008;106(3):1120–1127. [Google Scholar]
- 16.Kara D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chemistry. 2009;114(1):347–354. doi: 10.1016/j.foodchem.2008.09.054. [DOI] [Google Scholar]
- 17.Albin D. M., Wubben J. E., Gabert V. M. Effect of hydrolysis time on the determination of amino acids in samples of soybean products with ion-exchange chromatography or precolumn derivatization with phenyl isothiocyanate. Journal of Agricultural and Food Chemistry. 2000;48(5):1684–1691. doi: 10.1021/jf990599q. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S. A., Meys M., Tarvin T. L. The Pico-Tag Method: A Manual of Advanced Techniques for Amino Acid Analysis. Milford, Mass, USA: Waters Division of Millipore; 1989. [Google Scholar]
- 19.Lepage G., Roy C. C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. Journal of Lipid Research. 1984;25(12):1391–1396. [PubMed] [Google Scholar]
- 20.Šrůtek M. Growth responses of Urtica dioica to nutrient supply. Canadian Journal of Botany. 1995;73(6):843–851. [Google Scholar]
- 21.Lewu M. N., Adebola P. O., Afolayan A. J. Effect of cooking on the proximate composition of the leaves of some accessions of Colocasia esculenta (L.) Schott in KwaZulu-Natal province of South Africa. African Journal of Biotechnology. 2009;8(8):1619–1622. [Google Scholar]
- 22.Funke O. M. Evaluation of nutrient contents of amaranth leaves prepared using different cooking methods. Food and Nutrition Sciences. 2011;2:249–252. [Google Scholar]
- 23.Rickman J. C., Bruhn C. M., Barrett D. M. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. Journal of the Science of Food and Agriculture. 2007;87(7):1185–1196. doi: 10.1002/jsfa.2824. [DOI] [Google Scholar]
- 24.Martins D., Barros L., Carvalho A. M., Ferreira I. C. F. R. Nutritional and in vitro antioxidant properties of edible wild greens in Iberian Peninsula traditional diet. Food Chemistry. 2011;125(2):488–494. doi: 10.1016/j.foodchem.2010.09.038. [DOI] [Google Scholar]
- 25.Cho E., Lee J., Park K., Lee S. Effects of heat pretreatment on lipid and pigments of freeze-dried spinach. Journal of Food Science. 2001;66(8):1074–1079. [Google Scholar]
- 26.Tardío J., Molina M., Aceituno-Mata L., et al. Montia fontana L. (Portulacaceae), an interesting wild vegetable traditionally consumed in the Iberian Peninsula. Genetic Resources and Crop Evolution. 2011;58:1105–1118. [Google Scholar]
- 27.Pereira C., Barros L., Carvalho A. M., Ferreira I. C. F. R. Nutritional composition and bioactive properties of commonly consumed wild greens: potential sources for new trends in modern diets. Food Research International. 2011;44(9):2634–2640. [Google Scholar]
- 28.Lisiewska Z., Kmiecik W., Gebczynski P., Sobczynska L. Amino acid profile of raw and as-eaten products of spinach (Spinacia oleracea L.) Food Chemistry. 2011;126(2):460–465. [Google Scholar]
- 29.Lisiewska Z., Słupski J., Skoczeń-Słupska R., Kmiecik W. Content of amino acids and the quality of protein in Brussels sprouts, both raw and prepared for consumption. International Journal of Refrigeration. 2009;32(2):272–278. [Google Scholar]
- 30.FAO. Amino-acid content of foods and biological data on proteins. FAO Food and Nutrition Series no. 21, Rome, Italy, 1970. [PubMed]
- 31.U.S. Department of Agriculture, Agricultural Research Service. USDA Nutrient Database for Standard Reference. Release 24, 2011, http://ndb.nal.usda.gov/ndb/foods/show/7593.