Abstract
Background
The value of adjuvant chemotherapy for patients with positive lymph nodes (+LNs) after induction therapy and resection of esophageal cancer is controversial. This study assesses survival benefit of adjuvant chemotherapy in this cohort.
Methods
We analyzed our single institution database for patients with +LNs after induction therapy and resection of primary esophageal cancer between 2000 and 2013. Factors associated with survival were analysed using a Cox proportional hazards model.
Results
101 of 764 esophagectomy patients received induction and had +LNs on final pathology. Forty-five also received adjuvant therapy: 37/45 (82%) received chemotherapy alone, 1/45 (2%) received radiation alone, and 7/45 (16%) received both. Pathological stage was IIB in 21 (47%), IIIA in 19 (42%), and IIIB in 5 (11%). In 56 node-positive patients with induction but not adjuvant therapy, pathological stage was IIB in 28 (50%), IIIA in 18 (32%), IIIB in 7 (13%), and IIIC in 3 (5%). Neither age nor comorbidity score differed between cohorts. Adjuvant patients experienced a shorter hospital length of stay (LOS) [10 days (6–33) vs. 11 (7–67); p=.03]. Median survival favored the adjuvant group: 24.0 months (95% CI, 16.6 – 32.2) versus 18.0 months (95% CI, 11.1 – 25.0) (p=.033). Multivariate Cox regression identified adjuvant therapy, LOS, and number of +LNs as influential for survival.
Conclusions
Optimal management of node-positive patients after induction therapy and esophagectomy remains unclear, but in this series, adjuvant therapy, LOS, and number of +LNs impacted survival. A prospective trial may reduce potential bias and guide the evaluation of adjuvant therapy in this patient population.
Keywords: Esophageal cancer, Chemotherapy of esophagus, Radiation therapy, Esophagus
For patients with esophageal cancer, a complete pathologic response following preoperative chemoradiation and surgical resection has been associated with decreased risk of recurrence and improved overall survival. [1–3] However, a poorly characterized, but clearly identifiable subset of patients has persistent nodal disease following esophagectomy. Further characterization of this group is necessary to help establish prognosis and guide treatment options.
The role of induction therapy in patients with esophageal cancer is generally accepted in clinical practice and well-founded by evidence. The Medical Research Council randomized and controlled trial, OEO2, showed that induction chemotherapy prior to esophageal resection was superior to surgery alone, with 23% 5-year survival for the chemotherapy plus surgery cohort vs. 17.1% for patients undergoing surgery alone (HR=0.84, 95% CI, 0.72–0.98, P = 0.03). [4] Additionally, a meta-analysis of 11 randomized and controlled trials totaling 2,051 patients indicated a clinically important and statistically significant 44% increase in survival in the induction chemotherapy group at 5 years compared to surgery alone (p = 0.02). [5] The prospective and randomized Chemoradiotherapy for Oesophageal Cancer (CROSS) trial showed that a combined regimen of carboplatin and paclitaxel with concurrent radiotherapy produced a significantly higher median overall survival of 49.4 months in the chemoradiotherapy group versus 24.0 months in the surgery alone group (HR=0.657; 95% CI=0.495 to 0.871; P=.003). [6] Considering that combined chemoradiation is also the standard of care in the nonoperative setting, [7] initial chemoradiation therapy appears beneficial to both operative and nonoperative esophageal cancer patients.
In contrast, the role of adjuvant chemotherapy is not well established. The reason for the difference is the predictable decline in performance status that occurs with most esophageal cancer patients after esophagetomy. Many postoperative patients have experienced complications from the surgery and they endure increased levels of frailty and dependency for many weeks after the operation. Adding chemotherapy at this vulnerable time seems, for many patients, to be a high-risk intervention with uncertain therapeutic value.
Based on a retrospective analysis of 96 patients with persistent nodal disease following induction therapy and esophagectomy, Stiles et al. suggested that postoperative chemotherapy conferred no additional survival benefit to patients. [8] The JCOG 9905 study of over 300 patients with squamous cell carcinoma concluded that preoperative chemotherapy was superior to postoperative chemotherapy. [9]
It is clear from the previous work of many that node positive patients after induction therapy face a much higher risk of recurrence and cancer death than patients with complete response. Optimal management of patients with positive lymph nodes following induction therapy and resection remains unclear. The purpose of this study is to assess for the presence and magnitude of survival benefit associated with adjuvant chemotherapy in patients with positive lymph nodes following induction chemoradiotherapy and esophagectomy.
PATIENTS AND METHODS
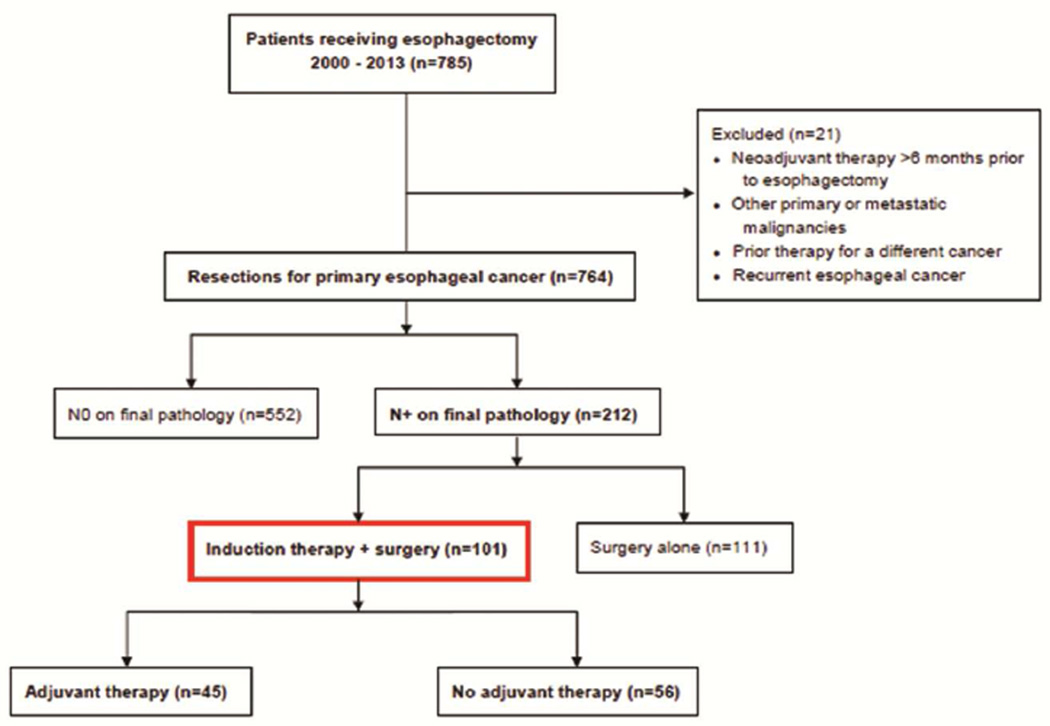
All patients undergoing resection for primary esophageal cancer between January 2000 and July 2013 at Barnes-Jewish Hospital were identified from our expanded version of the STS database and retrospectively analyzed in accordance with a protocol approved by the institutional review board at the Washington University School of Medicine (Figure 1). Inclusion criteria included esophagectomy for esophageal cancer, positive lymph nodes on pathology, and the use of induction therapy. Exclusion criteria included (1) neoadjuvant therapy more than 6 months before the date of surgery (i.e. salvage esophagectomy) (2) other primary or metastatic malignancies; (3) prior therapy for a different cancer; and (4) recurrent esophageal cancer. Four patients for whom adjuvant therapy status could not be verified were excluded. Pathology results were classified according to the AJCC 7th edition staging system. [10] Adult Comorbidity Evaluation 27 (ACE27) scores served as estimates for comorbidity. We supplemented chart reviews for the ascertainment of death by screening for vital status using the social security death index search engine and supplementary internet searches. Because all patients had induction therapy, follow up times were calculated from date of surgery to date of expiration or last known follow up.
Figure 1.
Consort diagram of patient population
Surgery was performed at the discretion of the surgeon as this was routine clinical practice and many approaches were used. Follow up was typically every three months for one year and every six months thereafter. Similar follow up routines took place with a medical oncologist and radiation oncologist. There was no protocol on obtaining imaging, but CT and PET were used liberally during the first three years postoperatively.
Patients with +LNs following resection who received adjuvant therapy were compared to similar patients who received no further treatment. Descriptive statistics were expressed as mean ± standard deviation unless otherwise specified. Median and range of values are displayed for non-normally distributed data. Categorical data were expressed as counts and proportions. Comparisons were made using paired, two-tailed t tests for means of normally distributed continuous variables and the Mann-Whitney U test for skewed continuous data. Chi-square or Fisher’s exact tests were used to analyze differences among the categorical data. Univariate survival analysis was done with Cox regression for continuous variables and Kaplan-Meier (KM) for binary variables. Clinically relevant variables as well as variables with p<0.1 on univariate survival analysis were included in a multivariate model. Cox proportional hazards regression was used for multivariate analysis of independent factors associated with death in the studied patient population including age, pathologic T status, length of hospital stay, number of positive nodes resected, total number of nodes resected, and adjuvant therapy status. KM graphs were used to demonstrate survival. Survival comparisons between groups of patients were completed using the Mantel-Cox log rank test. All p values less that 0.05 were considered to be statistically significant. Data analysis was performed using SPSS (SPSS 21.0 for Windows; SPSS Inc., Chicago, IL).
RESULTS
A total of 764 patients received an esophagectomy for primary esophageal cancer during the study period. 101 (13%) of these patients met inclusion criteria with one or more positive LNs on pathology following induction therapy and esophagectomy. The specific induction chemotherapeutic agent or strategy for 10 patients was not clear. Of the remaining 91, 97% of patients underwent a multidrug regimen, with cisplatin and 5-fluorouracil (5-FU) as the most common drug combination (n=43/91, 47%). Additional agents used included paclitaxel (n=21, 23%), carboplatin (n=19, 21%), and panitumumab (n=5, 5%). Eight of 101 (8%) patients did not receive induction radiotherapy, while the remaining received a median of 5020 cGy (range, 3240-5940cGy) of radiation over 5–6 weeks.
Sixty-seven of the 101 patients had been staged with EUS, 19/101 were known not to have had EUS, while the EUS status was uncertain for 15/101. Pre-operative CT scan was performed for 96% of patients and PET scan was performed for 100% of patients. Additionally, 6/101 (6%) were diagnosed with esophageal squamous cell carcinoma, 85/101 (84%) had adenocarcinoma, and 10/101 (10%) had esophageal cancer not otherwise specified. Forty-five of these 101 patients received postoperative adjuvant therapy: 37 (82%) received adjuvant chemotherapy alone, 1 (2%) received adjuvant radiation alone, and 7 (16%) received both. The specific adjuvant chemotherapeutic agents were unknown in 11 patients. Of the remaining 33, agents included 5-fluorouracil (n=9, 27%), EOX (n=9, 27%), FOLFOX (n=9, 27%), carboplatin (n=8, 24%), docetaxel (n=5, 15%), and cisplatin (n=3, 9%).
Table 1 contrasts the age, gender, resected LNs, hospital stay, comorbidity, histology, and clinical staging characteristics of the patients who received induction, resection and adjuvant therapy (adjuvant, n=45) with the characteristics of those who only received induction and resection (no adjuvant, n=56). The cohorts did not differ in age, gender, or comorbidity.
Table 1.
Patient Characteristics
| Adjuvant (n=45) | No adjuvant (n=56) | P value | Test | |
|---|---|---|---|---|
| Age | 57.6 ± 11.7 | 59.5 ± 9.5 | 0.37 | t test |
| Male sex | 39 (87) | 50 (89) | 0.69 | Pearson’s χ2 |
| Clinical T status | ||||
| cT1 | 0 | 1 (2) | ||
| cT2 | 9 (20) | 17 (30) | ||
| cT3 | 35 (78) | 37 (66) | 0.36 | Pearson’s χ2 |
| cT4 | 1 (2) | 0 | ||
| cTx | 0 | 1 (2) | ||
| Clinical N status | ||||
| cN0 | 12 (27) | 13 (23) | ||
| cN1 | 32 (71) | 39 (70) | ||
| cN2 | 1 (2) | 1 (2) | 0.63 | Pearson’s χ2 |
| cN3 | 0 | 2 (4) | ||
| cNx | 0 | 1 (2) | ||
| Mean ACE27 score | 1.02 ± 0.78 | 1.20 ± 0.99 | 0.34 | t test |
| Histological subtype | ||||
| Adenocarcinoma | 37 (82) | 48 (86) | ||
| Squamous cell | 3 (7) | 3 (5) | 0.89 | Pearson’s χ2 |
| Other | 5 (11) | 5 (9) | ||
Data are n (%) or mean ± SD
Operative characteristics including total lymph nodes resected, number of positive nodes, pathologic stage, length of hospital stay, total follow up, and recurrence are shown in Table 2. Median length of stay after resection (LOS) was one day shorter for the adjuvant cohort [10 days (6–33) vs. 11 (7–67); p=.03]. Overall median follow up time was 18.8 months (range, 0.3–170.1). Follow up was longer in those who received adjuvant therapy [21.1 months (range, 2.5–170.1) vs. 17.4 (0.3–142.4); p=.04]. Recurrence was identified in 56 of 91 patients (62%) with known recurrence status. Two year freedom from recurrence did not differ between the two groups, with 39% in the adjuvant group and 46% in the group that did not receive adjuvant (p=0.85). Patients who did not receive adjuvant therapy recurred earlier (6.2 months vs. 9.4 months, p=0.41) though this difference was not statistically significant.
Table 2.
Operative Characteristics
| Adjuvant (n=45) |
No adjuvant (n=56) |
P value | Test | |
|---|---|---|---|---|
| Procedure type | ||||
| Ivor Lewis | 20 (44) | 26 (46) | ||
| Transhiatal | 21 (47) | 21 (38) | 0.44 | Pearson’s χ2 |
| Three hole | 2 (4) | 7 (13) | ||
| Minimally invasive | 2 (4) | 2 (4) | ||
| Median # nodes resected | 15 (5–46) | 17 (2–47) | 0.87 | Mann-Whitney U Test |
| Median # positive nodes | 2 (1–7) | 2 (1–14) | 0.56 | Mann-Whitney U Test |
| Median LN ratio | 0.16 (.02–80) | 0.19 (.02–69) | 0.98 | Mann-Whitney U Test |
| Pathologic T status | ||||
| pT0 | 9 (20) | 8 (14) | ||
| pT1 | 5 (11) | 5 (9) | ||
| pT2 | 6 (13) | 15 (27) | 0.45 | Pearson’s χ2 |
| pT3 | 25 (56) | 25 (45) | ||
| pT4 | 0 | 2 (4) | ||
| pTx | 0 | 1 (2) | ||
| Pathologic N status | ||||
| pN1 | 36 (80) | 43 (77) | ||
| pN2 | 9 (20) | 10 (18) | 0.43 | Pearson’s χ2 |
| pN3 | 0 | 2 (4) | ||
| Final pathologic stage | ||||
| IIB | 21 (47) | 28 (50) | ||
| IIIA | 19 (42) | 18 (32) | 0.36 | Pearson’s χ2 |
| IIIB | 5 (11) | 7 (13) | ||
| IIIC | 0 | 3 (5) | ||
| Median LOS in days months | 10 (6–33) | 11 (7–67) | 0.03 | Mann-Whitney U Test |
| Median follow-up in months | 21.1 (2.5–170.1) | 17.4 (0.3–142.4) | 0.04 | Mann-Whitney U Test |
| 2 year freedom from recurrence | 39% | 46% | 0.85 | Kaplan-Meier log rank |
Data are n (%) or median (range)
LOS, length of hospital stay
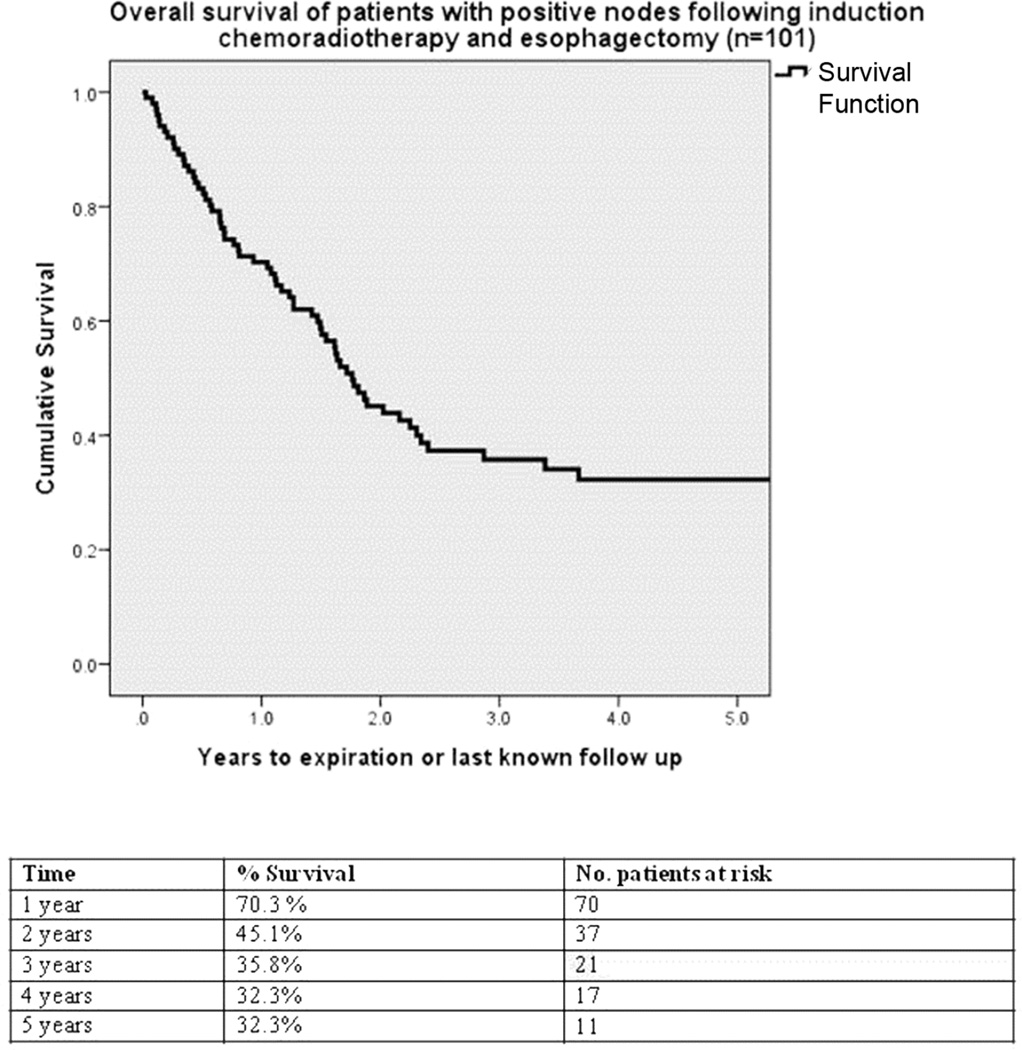
The median survival for all 101 patients was 21.1 months (95% CI 16.8 to 25.4 months; Figure 2). Survival was significantly longer for patients who received adjuvant therapy compared to those who did not (Figure 3). Five year survival estimate was 41% in the adjuvant group and 25% in the no adjuvant group (p=.033). More than three positive lymph nodes resected was associated with significantly worse overall survival, with 37% survival at 5 years for those with ≤3 positive nodes versus 17% for patients with 4 or more positive nodes (p=.013, Figure 4).
Figure 2.
Kaplan-Meier survival analysis of all patients with positive lymph nodes following induction therapy and esophagectomy
Figure 3.
Kaplan-Meier survival analysis of adjuvant therapy for patients with positive lymph nodes following induction therapy and esophagectomy
Figure 4.
Kaplan-Meier survival analysis of patients based on number of positive lymph nodes resected during esophagectomy following induction therapy
Age, total number of nodes resected, and pathologic T status were selected as clinically relevant factors for inclusion in a multivariate model of variables affecting survival. Additionally, univariate analysis using p<0.1 as a cutoff demonstrated that use of adjuvant therapy, number of positive nodes, and LOS were relevant predictors of mortality (Table 3). These six variables were added to a Cox regression multivariate analysis, which showed that use of adjuvant therapy, number of positive lymph nodes resected, and LOS were independently associated with survival (Table 4).
Table 3.
Univariate analysis of factors affecting overall survival
| Variable | P-value | Test |
|---|---|---|
| Number of positive nodes resected | <.001 | Cox Regression |
| ACE27 score | .392 | Cox Regression |
| Length of stay (days) | .008 | Cox Regression |
| Gender | .869 | KM |
| Adjuvant therapy | .033 | KM |
| cT status (T3-4 vs. T0-2) | .678 | KM |
KM, Kaplan-Meier
Table 4.
Cox proportional hazard regression multivariate model of factors predicting survival
| Variable | Hazard Ratio | Confidence Interval | P-value |
|---|---|---|---|
| Adjuvant therapy (Y/N) | 0.576 | 0.334 – 0.993 | .047 |
| Length of stay (days) | 1.029 | 1.009 – 1.050 | .004 |
| Number of positive nodes resected | 1.211 | 1.085 – 1.352 | .001 |
| Total number of nodes resected | 0.991 | 0.963 – 1.019 | .529 |
| pT status (T3-4 vs. T0-2) | 1.283 | 0.744 – 2.212 | .369 |
| Age (years) | 1.011 | 0.984 – 1.038 | .429 |
COMMENT
While most accept the value of induction chemoradiotherapy prior to esophagectomy and naïve patients are more commonly fit enough to tolerate adjuvant chemoradiotherapy, the utility of adjuvant therapy for patients with positive nodes and who have already experienced the burdens of both induction therapy and esophagectomy remains poorly defined.
Previously reported data has shown that adjuvant therapy may improve survival in some select populations, [11–13] though none of these studies included patients who had already received induction therapy. A retrospective study similar to our current work described 96 patients treated by Stiles and colleagues [8] who evaluated predictors of survival in a population with positive nodes following induction chemotherapy and resection of esophageal cancer. These Cornell University investigators concluded that clinical stage, pathologic T classification, and number of positive lymph nodes resected independently predicted survival.
However, in contrast to our study, they found that adjuvant therapy did not confer additional survival benefit. While they primarily used carboplatin (56%) and paclitaxel (55%) for induction chemotherapy, our patients were more likely to receive cisplatin and 5-FU with a concurrent 5–6 weeks of induction radiation. Since 92% of our patients also received induction radiation versus none in their study, it is possible that patients in our cohort would be less fit for additional therapy postoperatively. However, the Cornell cohort of patients may be less fit following surgery since 91% underwent a radical en bloc esophagectomy. The Cornell authors suggested that a survival benefit may not have occurred in their population receiving adjuvant therapy because they typically only received a single cycle of chemotherapy in the adjuvant setting. Due to inconsistent approaches over the 13 years of our series, it is unknown how many cycles of chemotherapy each patient received adjuvantly. It is possible that, in contrast to the Stiles et al. study, the patients in our series received an amount of chemotherapy more likely to produce a survival benefit.
The two most common operative approaches were the Ivor Lewis (IL n=46, 46%) and transhiatal (THE n=42, 42%) techniques. Multiple previous studies [8, 14] have found improved survival following en bloc resection compared with transhiatal resection after induction therapy for esophageal cancer. We found no difference in survival between the two types of resection in our likely under-powered patient population, with 5 year survival estimates of 30.4% for IL vs. 40.2% for THE (p=.43).
We did not find total lymph nodes resected to be a significant predictor of survival. In contrast, one retrospective study concluded that the total number of resected nodes is a significant determinant of survival in node-positive patients treated by surgery alone. [15] These findings were bolstered by an extensive international study of the Surveillance, Epidemiology, and End Results (SEER) database, which combined data from 9 centers and found increased survival in those with higher total negative node counts and higher total node counts. [16] A notable difference is that patients in these two studies did not receive any perioperative chemoradiotherapy, whereas in our series all patients received induction therapy and some also received adjuvant therapy.
As seen in multiple prior studies, [8, 17–18] we did find the number of positive resected lymph nodes to be independently associated with survival. Whereas Gu et al. [17] and Rizk et al. [18] determined the presence of more than one positive node to be associated with significantly worse survival, our series produced the same conclusion as Stiles et al. [8] that greater than 3 positive nodes resulted in significantly worse overall survival in our series, 17% vs. 36.5% survival at five years (Figure 4, p=.013). Exploratory analysis setting cutoffs at 4, 5, 6, and 7 positive nodes also produced statistically significant predictors of survival, with the strongest being ≤7 positive nodes vs. >7 nodes, with 5 year overall survival of 0% vs. 33% (p<.001). This analysis is intuitive but has extremely limited significance since only 6 patients in our series had greater than 7 positive nodes.
After determining 3 positive nodes to be a statistically significant cutoff, we analyzed a subset of 27 patients with greater than 3 positive nodes to assess the survival benefit of adjuvant therapy. Of these, 13 received adjuvant therapy, while 14 did not. Survival was significantly higher in the adjuvant group, with 69% vs. 21% survival at one year and 43% vs. 11% at two years (p=.007). While small sample size hinders this subset analysis, the option of administering adjuvant therapy to a subset of patients with a worse prognosis should be further explored. If evidence does not support administering adjuvant therapy to every patient with positive nodes following induction therapy and surgery, it may be beneficial for certain subsets such as the patients with >3 positive nodes in our series.
Conversely, we explored the group least likely to gain benefit from adjuvant therapy, namely those with a low ratio of positive lymph nodes to total lymph nodes resected. The median LN ratio in our overall series was 0.16 (0.02 – 0.80) in the adjuvant group and 0.19 (0.02 – 0.69) in the no adjuvant group. Setting an arbitrary cutoff of LN ratio < 0.10, we identified 37 patients with relatively lower LN ratios. 23/37 (62%) were final stage IIB, while the other 14/37 (38%) were stage IIIA. 9/37 (24%) had two positive nodes while the remaining 28/37 (76%) patients had one positive node. The median total nodes resected in this subset was 21 (range, 11 – 47). Sixteen of these 37 patients (43%) received adjuvant therapy. There was no survival benefit to adjuvant therapy appreciated in this subset analysis, with a two year survival of 46.3% in the adjuvant group versus 64.3% in the group that did not receive adjuvant (p=0.91). With an increased sample size, this trend could become significant as we would expect lower LN ratio to confer lesser benefit or even harm from administration of adjuvant therapy.
Interestingly, KM analysis of two year freedom from recurrence did not differ between cohorts despite the overall survival advantage for the adjuvant group. Larger sample sizes remain necessary to adequately explore differences in both survival and recurrence in these two cohorts. The difference in survival but not recurrence may indicate that the patients in the group not receiving adjuvant therapy were more morbid and did not survive long enough to realize a higher rate of recurrence as compared to the patients who did receive adjuvant therapy. Our analysis used ACE27 scores and preoperative staging as indicators of morbidity but it remains possible that these metrics did not fully characterize the conditions of each cohort.
Molecular targets are currently being evaluated as modes of personalized treatment for these patients with persistent disease following induction therapy and surgery. An Academic and Community Cancer Research United (ACCRU) trial begun in September 2014 compares cohorts receiving adjuvant regorafenib versus placebo for patients with persistent nodal disease after neoadjuvant chemoradiation therapy and surgery. [19] While molecular targets are being considered for treating many patient populations with esophageal cancer, the personalized nature of this treatment may be especially beneficial for the cohort of patients in this series, for whom treatment options are limited and prognosis is poor. There is also an impression that targeted therapy can be given with less toxicity, albeit at potentially higher cost.
Our study is limited by its small sample size and retrospective nature. Most notably, it is subject to selection bias by only selecting patients who are clearly fit enough to receive adjuvant therapy. This study included unmeasured covariates. Some factors for patients not receiving adjuvant therapy were according to physician preference and not captured in the database. Additionally, some patients fit for adjuvant therapy declined to receive it.
As seen on Figure 3, the survival curves diverge the most within the first year, with 82% survival in the adjuvant group at one year compared to 61% in the no adjuvant group. This may be explained by the adjuvant group of patients having less comorbidity or fewer negative physical consequences of the esophagectomy operation. Although the ACE27 scores were not significantly different between cohorts, it is still possible that the group not receiving adjuvant was simply less fit after surgery. Comparing survival data, at least two patients in the no adjuvant group died within 30 days of operation, one at 10 days and one at 30. In total, 22/56 (39%) of the no adjuvant group died within the first year postoperatively, compared to 8/45 (18%) of the adjuvant group.
We performed additional exploratory analyses of survival excluding patients who died in the first 30 days and 90 days. Five year survival estimates after excluding the two early deaths were still 41% in the adjuvant group and 26% in the no adjuvant group though the difference was no longer statistically different (p=.052). An analysis excluding patients with 90 day mortality (n=1 in adjuvant cohort, n=5 in the no adjuvant cohort) showed a five year survival of 42% in the adjuvant group and 27% in the no adjuvant group (p=.075). These analyses indicate that the difference in survival may be due, in part, to the patients that did not receive adjuvant being sicker, rather than a direct effect of adjuvant therapy. As stated, a larger sample size remains necessary to draw strong enough conclusions to change practice in this population.
Our series suggests an apparent survival benefit for those patients receiving adjuvant therapy after induction therapy and surgery, as well as a survival benefit in those receiving adjuvant therapy among a subset of patients with 4 or more positive lymph nodes following induction therapy and surgery (n = 13 vs. 14, p=.007). The collection of additional retrospective data from multiple institutions is currently being gathered and pooled to further assess the conclusions from this article. If additional studies confirm these findings, a prospective trial might better assess the survival benefit of adjuvant therapy for this poorly defined patient population.
Acknowledgments
Grant support
Varun Puri -NIH K07CA178120, K12CA167540-02 (Paul Calabresi award)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 2.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinmo of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 4.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol. 2009;27 doi: 10.1200/JCO.2009.22.2083. 30.5062-7. [DOI] [PubMed] [Google Scholar]
- 5.Malthaner RA, Wong RK, Rumble RB, Zuraw L. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med. 2004;2:35. doi: 10.1186/1741-7015-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 8.Stiles BM, Christos P, Port JL, Lee PC, Paul S, Saunders J, Altorki NK. Predictors of survival in patients with persistent nodal metastases after preoperative chemotherapy for esophageal cancer. J Thorac Cardio Surg. 2010;139.2:387–394. doi: 10.1016/j.jtcvs.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Tabusaki H, Aoyama N, Kurita A. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy with Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 10.Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual:Esophagus and Esophagogastric Junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 11.DeMeester SR. Adenocarcinoma of the esophagus and cardia: a review of the disease and its treatment. Ann Surg Oncol. 2006;13:12–30. doi: 10.1245/ASO.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Bedard EL, Inculet R, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423–2430. [PubMed] [Google Scholar]
- 13.Rice TW, Adelstein D, Chidel MA, Rybicki LA, DeCamp MM, Murthy SC, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590–1596. doi: 10.1016/s0022-5223(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 14.Rizzetto C, DeMeester S, Hagen JA, Peyre CG, Lipham JC, DeMeester TR. En block esophagectomy reduces local recurrence and improves survival compared with transhiatal resection after neoadjuvant therapy for esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2008;135:1228–1236. doi: 10.1016/j.jtcvs.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 15.Altorki NK, Zhou X, Lee PC, Port JL, Paul S, Stiles B, Port JL, Paul S, Lee PC, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248:221–226. doi: 10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]
- 16.Peyre CG, Hagen J, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–556. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Swisher S, Ajani JA, Correa AM, Hofstetter WL, Liao Z, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer. 2006;106:1017–1025. doi: 10.1002/cncr.21693. [DOI] [PubMed] [Google Scholar]
- 18.Rizk NP, Venkatraman E, Bains MS, Park B, Flores R, Tang L, et al. American Joint Committee on Cancer. American Joint Committee on Cancer. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 19.Study of Adjuvant Regorafenib vs Placebo in Patients with Node Positive Esophageal Cancer that Completed Pre-operative Therapy. Academic and Community Cancer Research United. < https://clinicaltrials.gov/ct2/show/NCT02234180>.