Abstract
Chondromas usually affect the small bones of hand and feet and account for only 0.5% of all intracranial tumors. We present a case of a giant, supratentorial meningeal chondroma in a 19-year old male patient and discuss the preoperative diagnostic findings as well as the appropriate treatment options. A 19-old male presented with headache, new onset of focal seizures and paresis of left upper extremity. Magnetic resonance imaging revealed a large right parietal tumor in the precentral region with local mass effect. The patient underwent right parietal craniotomy and gross total resection of the tumor. The histopathological report revealed a chondroma. Intradural supratentorial chondromas are extremely rare. As with other slow growing intracranial masses, they often reach a relatively large size before generating symptoms. Maximal surgical resection is the treatment of choice and if this is achieved no adjuvant therapy is necessary.
Key words: Chondroma, supratentorial, eloquent
Introduction
Intracranial chondromas account for only 0,5% of all primary intracranial tumors. They usually arise in the skull base and especially in the sphenopetrosal, sphenoclival or petroclival junctions.1-3 They tend to engulf cranial nerves or major arteries and become symptomatic with cranial nerve palsies or headaches. They grow slowly and are considered benign as opposed to their malignant relatives the chondrosarcomas. The histological differentiation between the two can be challenging and has enormous significance regarding their therapy.
Case Report
A 19-year old male was admitted with complains of headache, hyperventilation and new onset of focal seizures. There was no significant family history, especially no history of tumor. The patient received no medication and had no history of radiation therapy. On clinical examination higher mental functions were intact. The patient was in postictal recovery and presented a paresis of the left upper extremity.
Magnetic resonance imaging (MRI) scan revealed a giant mass in the right precentral gyrus measuring 5×6×6 cm (Figure 1A and B). The corticospinal fibers as well as the motoric areas in the precentral gyrus were displaced anteriorly and posteriorly around the tumor (Figure 1C-E) On MRI imaging the tumor mass showed intermediate signal intensity on T1 weighted and high signal intensity on T2 weighted images as well as heterogeneous enhancement with typical honeycomb appearance. The patient underwent surgical treatment via a right parietal craniotomy. The dura overlying the tumor mass was irregularly distended. The partially involved dura and the tumor were dissected from the cortical surface without difficulty. The intracranial mass was completely removed in a piecemeal fashion. Since the bone did not seem to be involved in the tumor process we drilled the inner table of the calvaria but did not remove the bone completely. The removed tumor mass was macroscopically multinodular, ivory colored with a very cartilaginous consistency (Figure 2). The patient had postoperatively no motor or sensory deficits. The histological diagnosis revealed a chondroma (Figure 3). The postoperative computed tomography (CT) brain scan confirmed the total excision of the tumor (Figure 4A). The patient was placed on appropriate antiepileptic drugs and made a good recovery. He was discharged on the 12th postoperative day. Following gross total resection and after establishment of the histological diagnosis no adjuvant therapy was needed. The whole body scintigraphy did not show further tumors (Figure 4B and C).
Figure 1.
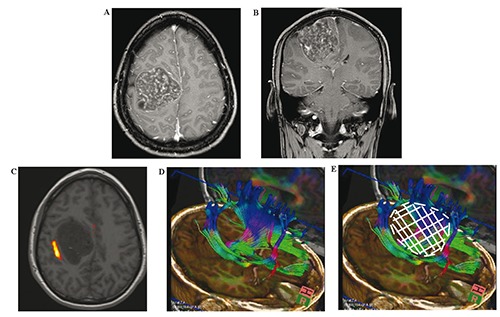
A) Axial T2WI of the brain showing a tumor with a honeycomb appearance; B) Coronal section of the tumor (T2WI); C) Functional magnetic resonance imaging illustrating the motor area of the left hand. The tumor seems to grow in the precentral gyrus pushing fibers of the motor cortex aside; D) Fibertracking image shows the corticospinal fibers pushed anteriorly and posteriorly from the tumor; E) The white area illustrates the tumor.
Figure 2.
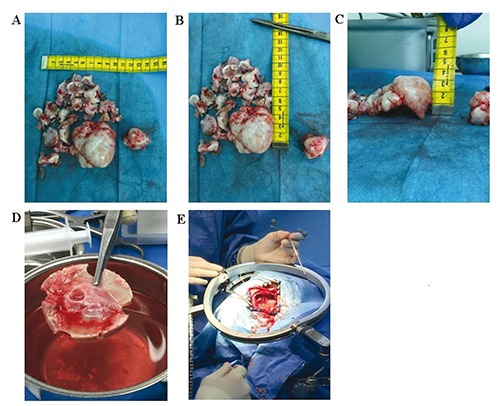
Macroscopical image of the resected tumor. The tumor was resected piecemeal as shown in A-C; D) Tumor growth into the calvaria. The tumor seems only to grow towards the calvaria and not to originate from it. The dura on the other side was infiltrated with tumor; E) Intraoperative image of the brain after tumor resection.
Figure 3.
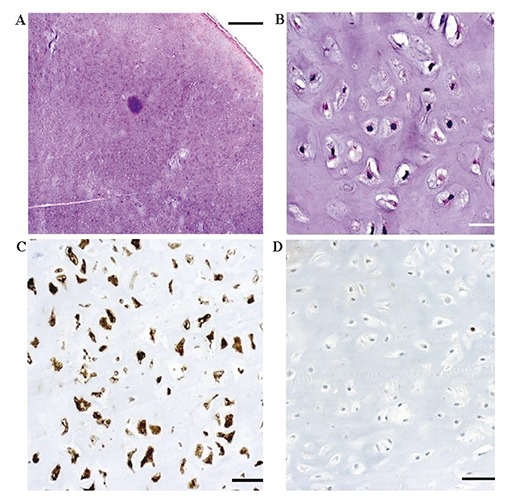
Histopathological analysis reveals a monomorphous tumor of low-moderate cellularity surrounded by a fibrous pseudocapsule [A: hematoxylin and eosin (H&E); scale bar = 1000 μm]. The mononucleated cells are embedded within a chondroid matrix, without signs of atypia (B: H&E; scale bar = 50 μm). The tumor cells are strongly positive for S100 (C: scale bar = 100 μm) and negative for cytokeratine, GFAP, and EMA (not shown). Proliferative activity as determined by immunohistochemistry for Ki67 is almost absent (D: scale bar = 100 μm).
Figure 4.
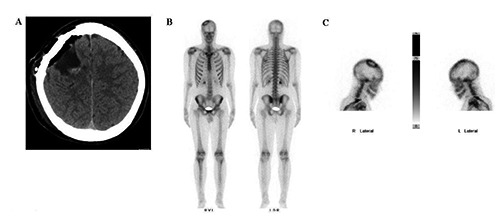
A) Postsurgical computed tomography showing tumor removal; B and C) Whole body scintigram showing no further tumor dissemination.
Discussion
Chondromas are recognized as a dyschondroplasia caused by developmental errors in enchondral ossification and are slow-growing, benign tumors of cartilaginous origin.1,3 They originate in the skull base and especially arise from the sphenopetrosal, sphenoclival or petroclival junctions. Rarely, they can be seen in the parasellar region, at the falx or arousing from the dura convexity. Multiple theories have been suggested to explain the genesis of these tumors. In their paper Colpan and colleagues listed four of them:4 i) metaplasia of meningeal fibroblasts; ii) multipotential or perivascular mesenchymal cells in the dura mater; iii) aberrant nests of cartilage forming cells in the dura mater; iv) traumatic displacement of cartilage.
They can be found as solitary lesions or as part of Ollier’s disease (multiple polysystemic enchondromatosis) or Maffuci’s syndrome5,6 (multiple enchondromatosis associated with soft tissue angiomas). The publications regarding convexity chondromas are shown in Table 1.2,7-28 A given predominance of the left hemisphere can be seen. The frontoparietal region is the most common localization of the tumor. The oldest patient that has been described was 55 years old, whereas most of the patients had symptoms in a young age. There was no difference among men and women. The tumor reached in most of the cases a size of 6 cm. The neurological symptoms that the patients developed depended on the localization of the tumor and were usually epileptic seizures or motor deficits. Delgado-Lopez et al. published a patient with Noonan’s syndrome and chondroma,7 a combination which was never described before. All authors agree that operative excision of the tumor is the best treatment option. No adjuvant therapy is then needed. Since intracranial chondromas are rare tumor entities, there are no grade 1 recommendations regarding their treatment. In all patients that were surgically treated and where the tumor was completely resected, no recurrence occurred. In our patient and after macroscopically complete tumor resection we planned the next MRI scan 6 months postoperatively. The further MRI scans will then be scheduled in one, two and five years or earlier, if symptoms appear.
Table 1.
Literature review with patients with convexity chondromas.
| Publication | Age/Gender | Localization | Histologic type | Tumor dimensions |
|---|---|---|---|---|
| Berkmen and Blatt8 (1968) | 33 m | Right frontoparietal | N/A | 550 g |
| Wu and Lapi9 (1970) | 32 f | Left parietal | N/A | 8.5×6.5×4 cm |
| Hardy et al.10 (1978) | 22 f | Left frontoparietal | N/A | 250 g |
| Matz et al.11 (1981) | 20 m | Left frontoparietal | I | 7×8 cm |
| Sebbag et al.12 (1990) | 25 m | Left frontal | N/A | 4×7 cm |
| Nakazawa et al.13 (1993) | 16 f | Left parietal | II | 5×4 cm |
| Salazar-Calderon Perrigo et al.14 (1993) | 27 f | Right frontoparietal | II | 3×4 cm |
| Lacerte et al.15 (1996) | 32 f | Right frontal | II | 8×6×6 cm |
| Khosrovi et al.16 (2000) | 14 m | Left frontoparietal | II | N/A |
| Nakayama et al.17 (2001) | 47 f | Right frontal | II | N/A |
| Colpan et al.18 (2003) | 40 f | Right frontoparietal | I | 5×3.5×2.5 cm |
| Bergmann et al.19 (2004) | 30 f | Bifrontal | N/A | 8×8×5 cm |
| Cosar et al.20 (2005) | 21 f | Left frontal | I | 2×2×2 cm |
| Hong et al.21 (2005) | 18 m | Left frontal | I | 3×3×4 cm |
| Erdogan et al.22 (2006) | 14 m | Left frontal | II | 6×7×4 cm |
| Delgado-Lopez et al.7 (2007) | 18 m | Left frontoparietal | N/A | N/A |
| Laghmari23 (2007) | 50 m | Frontal parasaggital | N/A | N/A |
| Kawabata et al.24 (2010) | 48 f | Left frontal | N/A | 1.5×1.5×6.3cm |
| Maheshwari et al.25 (2011) | 40 m | Left frontal | N/A | 8 cm |
| Uddin et al.26 (2012) | 23 m | Left frontal | N/A | 10.0×7.5×1.4cm |
| Yeung et al.27 (2012) | 22 f pregn | Right frontal | N/A | 4.2×3.2×3.1 cm |
| Park et al.28 (2013) | 55 f | Left frontal | N/A | 5.9×3.5 cm |
| Atalay et al.2 (2014) | 52 f | Left frontal | N/A | 2×1 cm |
N/A, not available.
Imaging
The diagnostic work- up includes MRI and CT sequences. In the contrast enhanced MRI images the tumor appears ringlike with intratumoral nodular enhancement and no edema.7 In the computed tomography the tumor appears as an irregulary lobulated mass with obvious calcification. Nonetheless, a definitive diagnosis can only be made after histological examination of the tumor.
Differential diagnosis and immunhistochemistry
Intracranial chondromas present rare benign tumors of the central nervous system. The differential diagnosis primarily includes chondrosarcomas. In our case the initial diagnosis was suspicious for chondrosarcoma and therefore the histological specimen was sent to a specialized reference center. Histopathological analysis revealed a monomorphous tumor of low-moderate cellularity surrounded by a fibrous pseudocapsule. The mononucleated cells were embedded within a chondroid matrix, without signs of atypia. The tumor cells were strongly positive for S100 and negative for cytokeratine, GFAP, and EMA. Proliferative activity as determined by immunohistochemistry for Ki67 was almost absent. Therefore, the final diagnosis was chondroma. Other tumors that have to be considered in the differential diagnosis are meningiomas,2 hemangiopericytomas, gliomas and metastases. Immunohistochemistry is an important tool in establishing the diagnosis. Since chondromas are benign tumors and grow slowly, they are only then symptomatic, when the mass effect affects vital functions of the brain. Sugiura et al. performed a molecular analysis in their case of convexity chondroma which revealed wild type IDH1/2 and expression of HMGA2.29
Therapy
Our patient presented with symptoms of focal mass effect and high intracranial pressure as well as seizures. As a result, we preferred a gross total resection over a possible stereotactic biopsy in order to alleviate the symptoms and to obtain a permanent histological diagnosis. Because of its huge size we also did not consider biopsy and radiosurgery as an option. Since the tumor was located in the eloquent precentral region, we preoperatively performed a digital tractography (fibertracking) and a functional MRI, which showed displacement of the fibers posteriorly and anteriorly to the tumor. Since the tumor was well defined and did not diffusely infiltrate the neighboring fibers gross total resection was feasible.
Conclusions
Supratentorial dural chondromas are rare lesions that grow slowly and cause symptoms due to local mass effect or increased intracranial pressure. If they are located in eloquent brain areas, a thorough diagnostic imaging should be performed including functional imaging and fibertracking in order to pick the best possible approach with no or minimum manipulation of the critical structures. Aim of the operation should be gross total resection. The histological diagnosis can be challenging, but once established no adjuvant therapy has to be undertaken.
References
- 1.Geng S, Zhang J, Zhang LW, et al. Diagnosis and microsurgical treatment of chondromas and chondrosarcomas of the cranial base. Oncol Lett 2014;8:301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atalay FO, Ozgun G, Tolunay S, Bekar A. Intracranial extra-axial chondroma: a case report. J Nippon Med Sch 2014;81:35-9. [DOI] [PubMed] [Google Scholar]
- 3.Abeloos L, Maris C, Salmon I, et al. Chondroma of the dural convexity: a case report and literature review. Neuropathology 2012;32:306-10. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabortty S, Tamaki N, Kondoh T, et al. Maffucci’s syndrome associated with intracranial enchondroma and aneurysm: case report. Surg Neurol 1991;36:216-20. [DOI] [PubMed] [Google Scholar]
- 5.De Coene B, Gilliard C, Grandin C, et al. Unusual location of an intracranial chondroma. AJNR Am J Neuroradiol 1997;18:573-5. [PMC free article] [PubMed] [Google Scholar]
- 6.Duan F, Qiu S, Jiang J, et al. Characteristic CT and MRI findings of intracranial chondroma. Acta Radiol 2012;1;53:1146-54. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-López PD, Martín-Velasco V, Galacho-Harriero AM, et al. Large chondroma of the dural convexity in a patient with Noonan’s syndrome. Case report and review of the literature. Neurocirugia (Astur) 2007;18:241-6. [PubMed] [Google Scholar]
- 8.Berkmen YM, Blatt ES. Cranial and intracranial cartilaginous tumours. Clin Radiol 1968;19:327-33. [DOI] [PubMed] [Google Scholar]
- 9.Wu WQ, Lapi A. Primary non-skeletal intracranial cartilaginous neoplasms: report of a chondroma and a mesenchymal chondrosarcoma. J Neurol Neurosurg Psychiat 1970;33:469-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy RW, Benjamin SP, Gardner WJ. Prolonged survival following excision of dural chondroma. J Neurosurg 1978;48:125-7. [DOI] [PubMed] [Google Scholar]
- 11.Matz S, Israeli Y, Shalit MN, Cohen ML. Computed tomography in intracranial supratentorial osteochondroma. J Comput Assist Tomogr 1981;5:109-15. [DOI] [PubMed] [Google Scholar]
- 12.Sebbag M, Schmidt V, Leboucq N, et al. Chondrome dure-merien. A propos d’un cas et revue de la literature. J Radiol 1990;71:495-8. [PubMed] [Google Scholar]
- 13.Nakazawa T, Inoue T, Suzuki F, et al. Solitary intracranial chondroma of the convexity dura: case report. Surg Neurol 1993;40:495-8. [DOI] [PubMed] [Google Scholar]
- 14.Salazar-Calderon Perriggo VH, Oommen KJ, Sobonya RE. Silent solitary right parietal chondroma resulting in secondary mania. Clin Neuropathol 1993;12:325-9. [PubMed] [Google Scholar]
- 15.Lacerte D, Gagne F, Copty M. Intracranial chondroma. Report of two cases and review of the literature. Can J Neurol Sci 1996;23:132-7. [DOI] [PubMed] [Google Scholar]
- 16.Khosrovi H, Sadrolhefazi A, El-Kadi H, et al. Intradural convexity chondroma: a case report and reviewof diagnostic features. W V Med J 2000;96:612-6. [PubMed] [Google Scholar]
- 17.Nakayama M, Nagayama T, Hirano H, et al. Giant chondroma arising from the dura mater of the convexity. J Neurosurg 2001;94:331-4. [DOI] [PubMed] [Google Scholar]
- 18.Colpan E, Attar A, Erekul S, Arasil E. Convexity dural chondroma: a case report and review of the literature. J Clin Neurosci 2003;10:106-8. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann M, Pinz W, Blasius S, et al. Chondroid tumors arising from the meninges-report of 2 cases and review of the literature. Clin Neuropathol 2004;23:149-53. [PubMed] [Google Scholar]
- 20.Cosar M, Iplikcioglu AC, Bek S, Gokduman CA. Intracranial falcine and convexity chondromas: two case reports. Br J Neurosurg 2005;19:241-3. [DOI] [PubMed] [Google Scholar]
- 21.Hong JT, Lee SW, Son BC, et al. Delayed occurrence of intracranial supratentorial chondroma following compound depressed skull fracture. Acta Neurochir 2005;147:343-5. [DOI] [PubMed] [Google Scholar]
- 22.Erdogan S, Zorludemir S, Erman T, et al. Chondromas of the falx cerebri and dural convexity: report of two cases and review of the literature. J Neurooncol 2006;80:21-5. [DOI] [PubMed] [Google Scholar]
- 23.Laghmari M, Metellus P, Fuentes S, et al. Cranial vault chondroma: a case report and literature review. Neurochirurgie 2007;53:491-4. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata Y, Miyake H, Horikawa F. A solitary convexity dural chondroma: the proposed role of diffusion-weighted mr imaging in the differential diagnosis of intracranial chondroma and meningioma. A Case Report. Neuroradiol J 2010;23:496-500. [DOI] [PubMed] [Google Scholar]
- 25.Maheshwari V, Mehdi G, Varshney M, et al. Intracranial chondroma: a rare entity. BMJ Case Rep 2011;2011:pii:bcr0320114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin MM, Ashraf J, Memon AA, Ali J. Intracranial cystic chondroma: a case report. J Med Case Rep 2012;6:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung JT, Krznarich TS, Moreno EA, et al. Intracranial parafalcine chondroma in a pregnant patient. Surg Neurol Int 2012;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Jeun SS. An intracranial chondroma with intratumoral hemorrhage: a case report and review of the literature. Brain Tumor Res Treat 2013;1:42-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura Y, Nagaishi M, Takano I, et al. Convexity dural chondroma: a case report with pathological and molecular analysis. Clin Neuropathol 2015;34:13-8 [DOI] [PubMed] [Google Scholar]


