Summary
Following acute exposure to crocidolite asbestos fibers, flaxseed lignans, enriched in secoisolariciresinol diglucoside (SDG), significantly reduced peritoneal inflammation, proinflammatory/profibrogenic cytokine release and oxidative/nitrosative stress in mice. Our findings support the potential role of SDG, which is safe and well-tolerated, in the chemoprevention of malignant mesothelioma.
Abstract
Malignant mesothelioma (MM), linked to asbestos exposure, is a highly lethal form of thoracic cancer with a long latency period, high mortality and poor treatment options. Chronic inflammation and oxidative tissue damage caused by asbestos fibers are linked to MM development. Flaxseed lignans, enriched in secoisolariciresinol diglucoside (SDG), have antioxidant, anti-inflammatory and cancer chemopreventive properties. As a prelude to chronic chemoprevention studies for MM development, we tested the ability of flaxseed lignan component (FLC) to prevent acute asbestos-induced inflammation in MM-prone Nf2+/mu mice. Mice (n = 16–17 per group) were placed on control (CTL) or FLC-supplemented diets initiated 7 days prior to a single intraperitoneal bolus of 400 µg of crocidolite asbestos. Three days post asbestos exposure, mice were evaluated for abdominal inflammation, proinflammatory/profibrogenic cytokine release, WBC gene expression changes and oxidative and nitrosative stress in peritoneal lavage fluid (PLF). Asbestos-exposed mice fed CTL diet developed acute inflammation, with significant (P < 0.0001) elevations in WBCs and proinflammatory/profibrogenic cytokines (IL-1ß, IL-6, TNFα, HMGB1 and active TGFß1) relative to baseline (BL) levels. Alternatively, asbestos-exposed FLC-fed mice had a significant (P < 0.0001) decrease in PLF WBCs and proinflammatory/profibrogenic cytokine levels relative to CTL-fed mice. Importantly, PLF WBC gene expression of cytokines (IL-1ß, IL-6, TNFα, HMGB1 and TGFß1) and cytokine receptors (TNFαR1 and TGFßR1) were also downregulated by FLC. FLC also significantly (P < 0.0001) blunted asbestos-induced nitrosative and oxidative stress. FLC reduces acute asbestos-induced peritoneal inflammation, nitrosative and oxidative stress and may thus prove to be a promising agent in the chemoprevention of MM.
Introduction
Asbestos refers to six naturally occurring kinds of fibrous minerals, including crocidolite, amosite, tremolite, actinolite, anthophyllite and chrysotile and is mainly used in construction and the shipbuilding industries. In addition to those individuals contacting asbestos in their occupational environment, other population groups working or living close to asbestos-producing or -using areas that are poorly controlled (1) have also been exposed. Exposed individuals can develop non-malignant manifestations (such as pleural plaques and benign asbestos pleural effusion) or malignancies, including mesothelioma, lung cancer, ovarian cancer and laryngeal cancer (2).
Pleural malignant mesothelioma (MM) is currently thought to develop when inhaled asbestos fibers work their way into the lung and ultimately to the pleural surface (3). Peritoneal MM, on the other hand, is caused when inhaled asbestos fibers reach the peritoneum through the lymphatic system (4). Once in the pleural space or peritoneum, asbestos fibers are taken up by tissue phagocytes, primarily macrophages. This stimulates production of intracellular reactive oxygen species (ROS) and activates NF-κB, inducing the release of numerous cytokines and mutagenic ROS (5). Recent work suggests that asbestos also activates the NALP3 inflammasome thus promoting the release of cytokines such as IL-1β and IL-18 (6). Mice exposed to asbestos exhibit recruitment of activated macrophages to mesothelium interacting with asbestos fibers (7). Macrophages are also thought to directly interact with and phagocytize asbestos (8). It is hypothesized that macrophages and mesothelial cells exposed to asbestos undergo frustrated phagocytosis of elongated fibers; this process is thought to cause chronic production of ROS and cytokines, which contribute to DNA damage and ultimately in transformation of mesothelial cells (9,10).
Our group has extensively studied the effects and mechanisms of flaxseed (FS) (11–14) and its lignan component (15,16) in mitigating inflammation and oxidative/nitrosative stress in tissues. Flaxseed lignans have antioxidant properties and act by inhibiting lipid peroxidation in tissues (17). Secoisolariciresinol diglucoside (SDG), the main FS lignan, has strong direct antioxidant properties in vitro without the need for metabolic activation (14). Since oxidative stress is implicated in the etiology of cancer, the chemopreventive use of FS-derived lignans has been considered in some tumors. Lignans were shown to reduce chemically-induced mammary and colon tumorigenesis in rats (18,19) and experimental metastasis of melanoma cells in mice (20). The FS lignan SDG is now emerging as a potential anticarcinogenic agent (21) and found to modulate growth factor-mediated cell signaling, cell cycle gene expression and apoptosis.
Given the proposed importance of chronic inflammation and production of ROS/reactive nitrogen species in the pathogenesis of asbestos-induced MM and the criteria needed for effective chemoprevention (22,23), the anti-inflammatory properties of FLC coupled with its direct antioxidant activity suggested the hypothesis that it could function as a safe, non-toxic chemopreventive agent in asbestos-induced MM. To begin to test this hypothesis, we first evaluated its effects on acute asbestos-induced peritoneal inflammation in a mouse model (with genetic alterations in the NF2 genes) of accelerated MM development that recapitulates many of the molecular, genetic and cell signaling features of human MM after asbestos injection (24).
Materials and methods
Asbestos exposure
Nf2+/mut mice (24) were exposed via intraperitoneal (IP) injection to UICC crocidolite (SPI Supplies) asbestos fibers that were baked overnight, resuspended in phosphate-buffered saline at a stock concentration of 800 µg/ml and sonicated for 30min prior to injection. Male mice 7 or 13 weeks of age were injected IP with 400 μg of crocidolite fibers in 500 µl of phosphate-buffered saline and euthanized at days 3 and 9 post injection. A group of mice (n = 8) was studied without IP injection with crocidolite fibers to determine baseline values.
Study design and dietary treatments
Mice were placed on control (CTL; n = 16) or flaxseed lignan complex (FLC; n = 17) supplemented diets 7 days prior to asbestos exposure. FLC diet consists of 3.37g/kg of FS lignans (providing 1.18g of SDG/kg diet) and has been described previously (15,16). All mice were evaluated 3 days (unless otherwise described in text) after injection of asbestos for abdominal inflammation and proinflammatory cytokine release (see Figure 2A). The Nf2 +/mut model was selected because it develops an accelerated form of MM when exposed to asbestos. Mice were used at 7 or 13 weeks of age under animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Fox Chase Cancer Center and the University of Pennsylvania. Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12 h–12h day–night light cycle. Animals had free access to water and experimental diets.
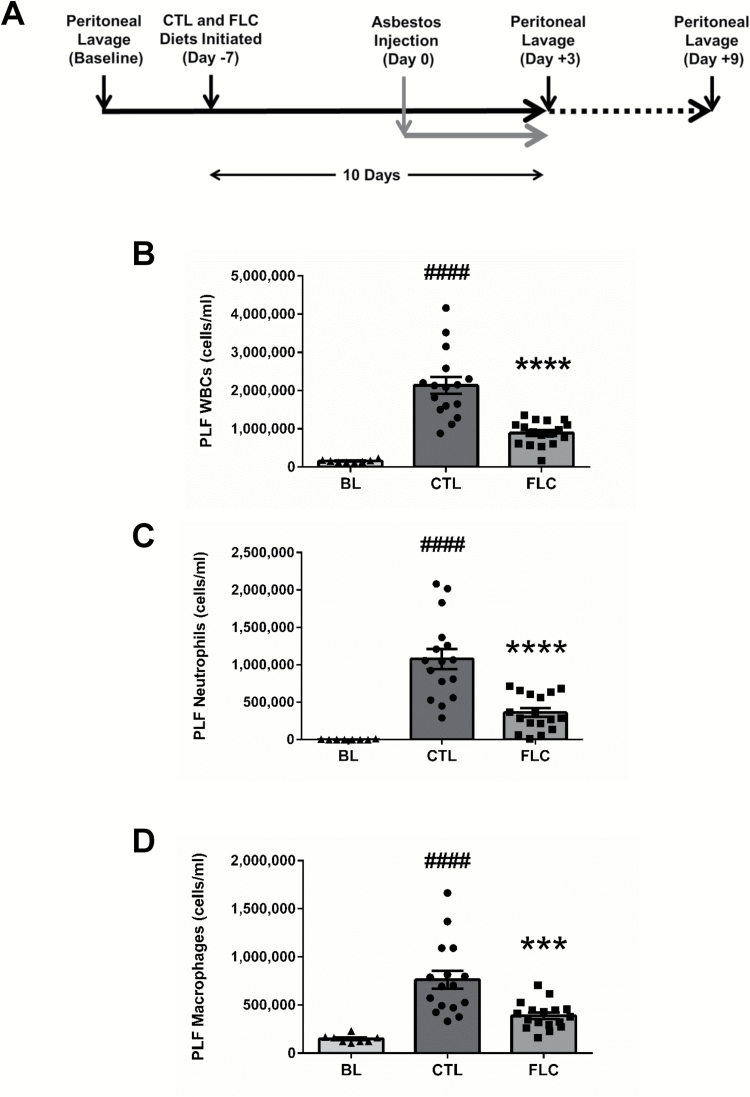
Figure 2.
Flaxseed lignan diet reduces peritoneal inflammation post asbestos exposure. Mouse cohorts (13 weeks old) were fed CTL (n = 16) or FLC (n = 17) diets for 7 days prior to asbestos exposure and remained on test diets throughout the course of the study (A). Mice were exposed to 400 µg of crocidolite asbestos fibers and PLF was harvested 3 days post asbestos exposure and evaluated for total WBCs (B), neutrophils (C) and macrophages (D). # indicates a statistically significant difference (####P < 0.0001) from BL and asterisks indicate a statistically significant difference from CTL-fed mice (***P < 0.001 and ****P < 0.0001).
Analytical evaluation of lignan content in murine plasma
Circulating plasma levels of the FS enterolignans, enterodiol (ED) and enterolactone (EL), were determined at the time of tissue harvest by liquid chromatography/tandem mass spectrometry (LC/MS/MS) as described earlier (25) using commercially available standards in 95% purity (Chromadex, Inc., Santa Ana, CA). Plasma FS lignan metabolite levels were evaluated in three randomly selected mice per cohort.
Peritoneal lavage fluid analysis
Mice were euthanized using an overdose of ketamine (160mg/kg) and xylazine (25mg/kg) at 3 and 9 days following asbestos exposure. Peritoneal lavage was then performed through a 20-gauge angiocatheter (BD Pharmingen, San Diego, CA), with IP instillation of 5ml of phosphate-buffered saline using a 5ml syringe. The peritoneal cavity of the mouse was gently massaged and lavage fluid was removed through the 20-gauge angiocatheter by moving the catheter around the peritoneal cavity to avoid clogging of the catheter by adipose or other organ tissues. Approximately 4.5–5ml of lavage fluid was retrieved and immediately placed on ice. An aliquot of peritoneal lavage fluid (PLF) was immediately separated to measure total white blood cell (WBC) counts (cells/ml PLF) using a Coulter Cell and Particle Counter (Beckman Coulter, Miami, FL). The PLF was spun on a Shandon Cytospin-3 cell preparation system (Thermo Electron, Waltham, MA) at 1500 revolutions per minute (rpm) for 10min and stained with a standard Diff-Quick (Hemacolor) protocol from EM Diagnostic Systems (Gibbstown, NJ) as described previously (25). The concentration of PLF neutrophils, macrophages, eosinophils and lymphocytes was also determined. The remaining lavage fluid was centrifuged at 1200rpm for 10min, and the cell-free supernatant was frozen at –80°C for cytokine and protein analysis. Pelleted WBCs were frozen and kept at −80°C for evaluation of mRNA gene expression.
Determination of proinflammatory and profibrotic cytokines in PLF
Levels of proinflammatory and profibrotic cytokines, interleukin-1ß (IL-1ß), IL-6, tumor necrosis factor alpha (TNFα), high-mobility group box 1 (HMGB1) and active transforming growth factor beta 1 (TGFß1) were determined in PLF 3 days post asbestos exposure using enzyme-linked immunosorbent assays (ELISA). ELISA kits were purchased from BD biosciences (BD OptEIA Mouse TNFα and IL-1ß ELISA Kit), R&D systems (mouse IL-6 Quantikine ELISA Kit), Chondrex, Inc. (HMGB1 Detection Kit) and BioLegend (Free Active TGFβ1 ELISA Kit). PLF samples were run undiluted in duplicate and assays were performed according to manufacturer’s instructions. Baseline (BL) values for PLF cytokine levels were obtained from untreated, naïve Nf2 +/mu mice. Levels of IL-1ß, IL-6, TNFα and active TGFß1 are reported as pg/ml of PLF, and levels of PLF HMGB1 are reported as ng/ml.
Evaluation of oxidative and nitrosative stress in PLF
Malondialdehyde (MDA), an indicator of oxidative stress (26) was measured in PLF using a commercially available kit (TBARS Assay Kit, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. Specifically, levels of thiobarbituric acid reactive substances (TBARS) were quantified by measuring the fluorescence of MDA-thiobarbituric acid adducts in PLF samples. According to manufacturer instructions, MDA-thiobarbituric acid adducts were formed via acid hydrolysis at 100°C and measured fluorometrically with an excitation wavelength of 530nm and an emission wavelength of 550nm. Levels of lipid peroxidation in PLF are reported as the concentration (nM) of MDA (16).
Levels of nitrite were determined in PLF using the Greiss Reagent System supplied by Promega (Madison, WI). The Greiss Reagent System determines nitrite (a stable breakdown product of nitric oxide) concentration in biologic specimens. PLF samples were run undiluted and the assay was performed according to manufacturer’s instructions, with values reported as PLF nitrite concentration (µM).
RNA isolation and gene expression analysis
Total RNA was isolated from PLF WBCs using a commercially available kit, RNeasy Plus Mini Kit, supplied by Qiagen (Valencia, CA). Total RNA was quantified using a NanoDrop 2000 apparatus (ThermoFisher Scientific, Waltham, MA). Reverse transcription of RNA to cDNA was then performed on a Veriti® Thermal Cycler using the high capacity RNA to cDNA kit supplied by Applied Biosystems Quantitative polymerase chain reaction (qPCR) and performed using TaqMan® probe-based gene expression assays supplied by Applied Biosystems, Life Technologies (Carlsbad, CA). Individual TaqMan gene expression assays were selected for proinflammatory and profibrotic cytokines and cytokine receptors (IL-1ß, IL-6, TNFα, HMGB1, TGFß1, TNFαR1 and TGFßR1), for inducible nitric oxide synthase and for relevant cytoprotective and antioxidant enzymes [heme oxygenase-1 (HO-1), NADPH:quinone oxidoreductase-1 (Nqo1) and glutathione s-transferase mu 1 (Gstm1)]. Quantitative real-time PCR was performed using 25ng of cDNA per reaction well on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Gene expression data were normalized to 18S ribosomal RNA housekeeping gene and calibrated to the control samples according to the ΔΔCT method as described previously (15).
Statistical analysis
Results for total WBC counts, WBC differentials and PLF cytokines are reported as mean ± the standard error of the mean (SEM). Levels of target gene mRNA are reported as the mean fold change from CTL-fed mice ± SEM. Statistically significant differences between BL, CTL and FLC cohorts were determined by one-way analysis of variance, followed by Tukey’s multiple comparisons tests using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA, www.graphpad.com. Statistically significant differences in WBC mRNA levels between CTL and FLC cohorts were determined using unpaired t-tests. Statistically significant differences were determined at P value of 0.05. Asterisks shown in figures indicate significant differences between groups (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001). # shown in figures indicate significant differences between unexposed (BL) and asbestos-exposed groups (#P < 0.05, ##P < 0.01, ###P < 0.005 and ####P < 0.001).
Results
To determine the chemopreventive role of FS lignan complex (which is enriched in the plant lignan SDG) in the development of MM, we used a well-established mouse model of MM by injecting asbestos fibers in solution into the peritoneum of Nf2 +/mu mice and determined acute levels of inflammation, cytokine release, WBC mRNA changes and parameters of oxidative and nitrosative stress in the PLF.
Differences in asbestos-induced peritoneal inflammation are associated with time post-exposure and mouse age
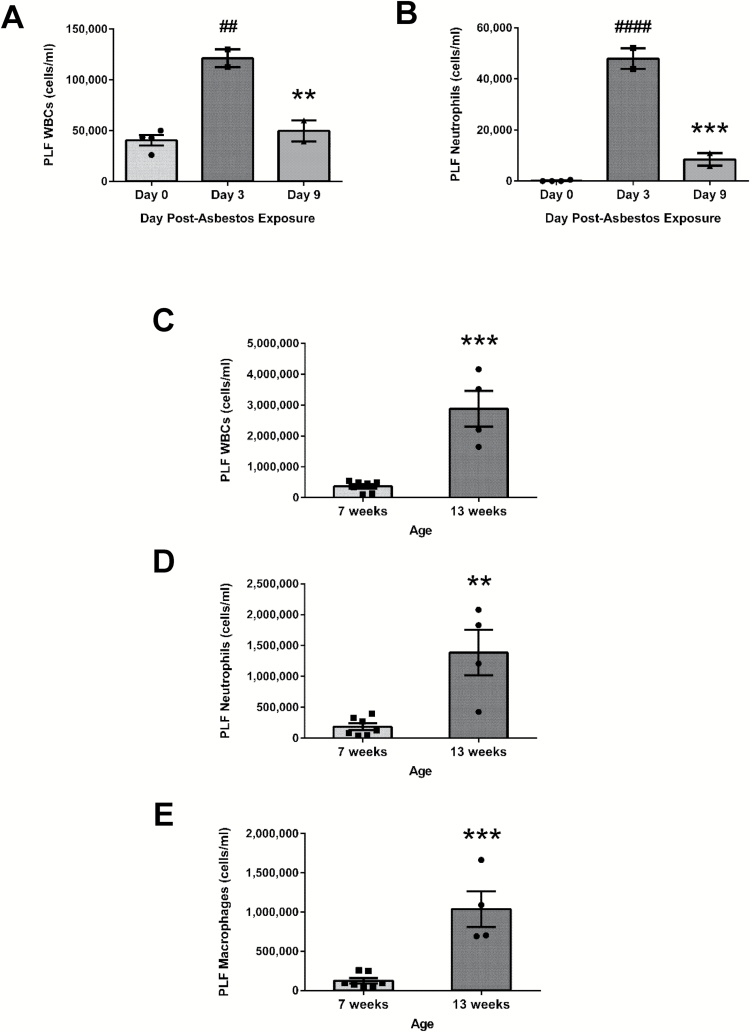
The chemopreventive properties of FS lignans were ultimately evaluated in a mouse model of asbestos-induced peritoneal inflammation using 13-week-old mice and harvesting PLF 3 days post asbestos exposure (Figure 2A). We performed several preliminary experiments to determine the appropriate time of tissue harvest post asbestos exposure and the appropriate mouse age to elicit a significant and meaningful inflammatory response. We observed a significant increase (P < 0.01) in WBC (Figure 1A) and neutrophil (Figure 1B) concentrations in PLF collected 3 days post asbestos exposure (121 250±8750 cells/ml compared to a baseline WBC concentration of 40 563±5120 cells/ml at day 0) that was significantly reduced by day 9 (49 625±10 375 cells/ml) consistent with a previous report (7). In addition, we observed a significant difference in the inflammatory response according to the age of the mouse used in the model (7 weeks old compared to 13 weeks old). Importantly, we observed a significant (P < 0.001) 7.8-fold increase in total PLF WBCs among older mice relative to mice 6 weeks younger (2 882 500±579 028 cells/ml versus 368 143±68 211 cells/ml, respectively). When evaluated 3 days postasbestos exposure, total WBCs (Figure 1C), neutrophils (Figure 1D) and macrophages (Figure 1E) were significantly elevated in 13-week-old mice compared to 7-week-old mice. We thus chose to study 13-week-old mice at the 3-day time point.
Figure 1.
Age- and time-dependent peritoneal inflammatory response following asbestos exposure. The inflammatory response following asbestos exposure was evaluated 3 and 9 days post asbestos IP injection. PLF was evaluated in 7-week-old mice following IP asbestos exposure for total WBCs (A) and neutrophil content (B). # indicates a statistically significant difference (##P < 0.01 and ####P < 0.0001) from day 0, and asterisks indicate a statistically significant difference (**P < 0.01 and ***P < 0.001) between days 3 and 9. Additionally, comparisons were made between 7-week-old mice and 13-week-old mice exposed to 400 µg of asbestos. PLF was evaluated 3 days post asbestos exposure for total WBCs (C), neutrophils (D) and macrophages (E). Asterisks indicate a statistically significant difference from 7-week-old mice (**P < 0.01 and ***P < 0.001).
Detection of the mammalian lignans ED and EL in mice-fed FS lignan complex enriched in SDG
A FS lignan formulation, enriched in the lignan SDG (FLC), was given in mouse chow. The FLC diet contained 0.118% SDG, comparable to SDG levels found in a 10% wholegrain FS diet. Plant lignans, such as the lignan SDG, are metabolized by intestinal bacteria to the mammalian lignans ED and EL that can be readily detected in plasma. Plasma levels of ED and EL were determined 3 days post asbestos exposure at the time of PLF harvest (having been on the diet for 10 days—see Figure 2A). Although plasma levels of ED and EL were not detectable in mice-fed CTL diet, mice-fed FLC diet had detectable levels of ED (461.8±52.19ng/ml) and EL (53.12±17.36ng/ml). The levels were comparable to those reported in earlier studies (15,16).
Peritoneal inflammation associated with exposure to asbestos is decreased by FS lignans
Inflammation is routinely evaluated by determining inflammatory cell influx in the PLF. Peritoneal inflammation following exposure to a single bolus of 400 µg of crocidolite asbestos was evaluated by quantifying PLF total WBCs and neutrophil infiltration. Mice-fed FLC diet had statistically significantly (P < 0.0001) less inflammation, with a 58.3% decrease in mean PLF total WBCs (Figure 2B) compared to CTL-fed mice (890 588±76 957 cells/ml for FLC-fed mice compared to 2 134 000±220 076 cells/ml for CTL-fed mice). Similarly, the average neutrophil (Figure 2C) and macrophage (Figure 2D) concentrations in PLF were significantly (P < 0.001) lower (66.5 and 49.0%, respectively) in the mice-fed FLC diet.
Importantly, we also tested WT mice which launched a robust acute inflammatory response following asbestos exposure. This was significantly (P < 0.01) reduced among mice-fed FLC diet (368 000±236 199 cells/ml for FLC-fed mice compared to 5 140 000±315 087 cells/ml for CTL-fed mice). Additionally, FLC diet significantly (P < 0.05) decreased the level of neutrophils and macrophages in the PLF by 35.3 and 23.9%, respectively (data not shown).
Flaxseed lignans reduce asbestos-induced proinflammatory cytokine release and gene expression levels in WBCs
The inflammatory response was further characterized by determining the levels of proinflammatory cytokines (IL-1ß, IL-6, HMGB1 and TNFα) in PLF. To further elucidate possible mechanisms associated with asbestos-induced inflammation, we also evaluated the mRNA levels of proinflammatory cytokines and cytokine receptors in PLF WBCs.
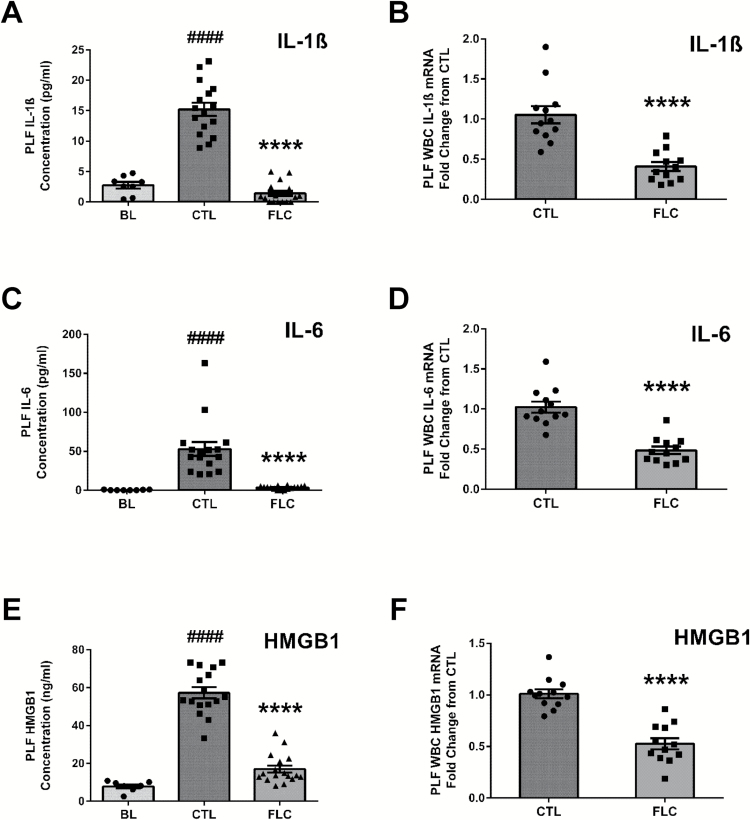
Compared to BL levels (2.73±0.55 pg/ml), mice exposed to asbestos fibers and fed CTL diet had increased levels of PLF IL-1ß (15.22±1.09 pg/ml). The asbestos-induced increase in PLF IL-1ß was significantly (P < 0.0001) blunted in FLC-fed mice (1.41±0.41 pg/ml) (Figure 3A). Levels of IL-1ß mRNA from PLF WBCs were decreased 61.4% in mice that were fed with the FLC diet (Figure 3B). Similarly, compared to BL (0.31±0.14 pg/ml), asbestos-exposed mice fed CTL diet had elevated levels of IL-6 (52.80±8.95 pg/ml) (Figure 3C), which were significantly (P < 0.0001) reduced among asbestos-exposed FLC-fed mice (3.47±0.55 pg/ml). We determined a 52.7% decrease in IL-6 mRNA levels from PLF WBCs of FLC-fed mice (Figure 3D). PLF levels of HMGB1 (Figure 3E) were elevated following asbestos exposure in CTL-fed mice (57.33±2.94ng/ml) compared to BL (7.89±0.93ng/ml). FLC-fed mice had significantly (P < 0.0001) lower PLF HMGB1 levels (17.01±1.87ng/ml) compared to CTL-fed mice, along with a 47.9% decrease in HMGB1 mRNA levels from PLF WBCs (Figure 3F).
Figure 3.
Flaxseed lignan diet ameliorates asbestos-induced WBC proinflammatory cytokine release and gene expression levels. PLF levels (pg/ml for IL-1ß and IL-6 and ng/ml for HMGB1) of proinflammatory cytokines IL-1ß (A), IL-6 (C) and HMGB1 (E) were evaluated 3 days post asbestos exposure. PLF WBC mRNA expression of IL-1ß (B), IL-6 (D) and HMGB1 (F) was determined using qPCR. Levels of target gene mRNA were normalized to 18S ribosomal RNA and values are expressed as fold change from CTL. # indicates a statistically significant difference (####P < 0.0001) from BL and asterisks indicate a statistically significant difference from CTL-fed mice (****P < 0.0001).
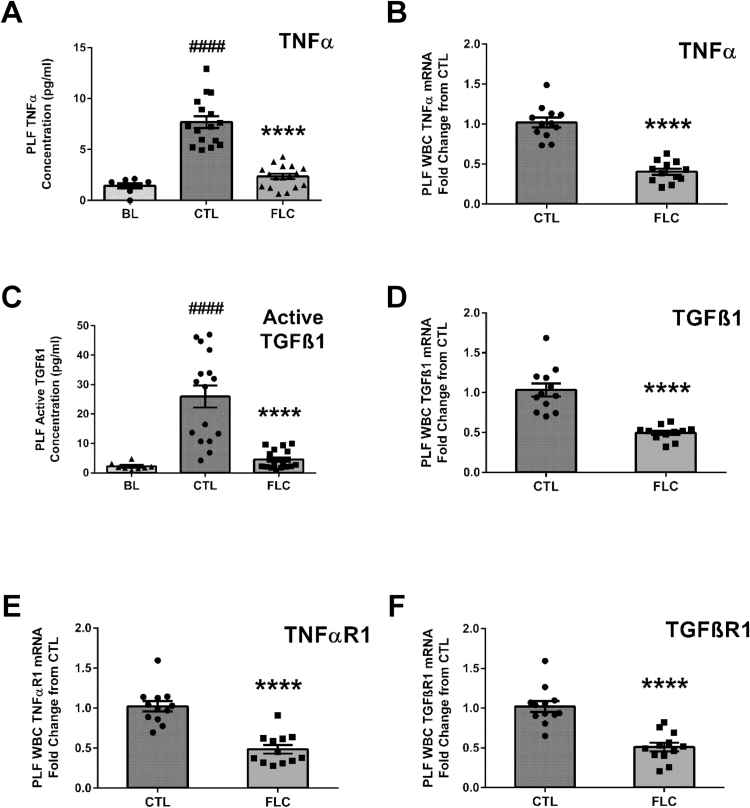
Although asbestos exposure increased the levels of the proinflammatory cytokine TNFα above BL (7.68±0.59 compared to 1.43±0.25 pg/ml at BL), PLF levels of TNFα (2.35±0.26 pg/ml) were significantly lower (P < 0.0001) among FLC-fed mice (Figure 4A). Compared to mice-fed CTL diet, administration of FS lignans led to a 69.3% decrease in PLF TNFα levels. Furthermore, gene expression levels of TNFα were significantly 60.2% decreased (P < 0.0001) in PLF WBCs from mice-fed FLC diet (Figure 4B). We investigated the observed reduction in TNFα levels by FLC further by evaluating the gene expression levels of TNFαR1 (Figure 4E) in PLF WBCs. Gene expression levels of TNFαR1 were significantly 52.6% decreased (P < 0.0001) in PLF WBCs from mice-fed FLC diet.
Figure 4.
Flaxseed lignan diet blunts proinflammatory and profibrogenic cytokine levels induced by asbestos exposure and alters cytokine and cytokine receptor gene expression. PLF levels (pg/ml) of proinflammatory TNFα (A) and profibrogenic free, active TGFß1 (C) were evaluated 3 days post asbestos exposure from mouse cohorts fed CTL or FLC diets. PLF WBC mRNA expression of cytokines, TNFα (B) and TGFß1 (D) and cytokine receptors, TNFαR1 (E) and TGFßR1 (F) was determined using qPCR. Levels of target gene mRNA were normalized to 18S ribosomal RNA and values are expressed as fold change from CTL. # indicates a statistically significant difference (####P < 0.0001) from BL and asterisks indicate a statistically significant difference from CTL-fed mice (****P < 0.0001).
FS lignans reduce asbestos-induced profibrogenic cytokine release and gene expression levels in WBCs
Along with evaluating acute changes in proinflammatory cytokine levels and PLF WBC gene expression changes, we determined acute changes in mRNA levels of profibrogenic TGFß1 and cytokine levels of active TGFß1 resulting from asbestos exposure. First, we detected a significant increase in active TGFß1 in PLF due to asbestos exposure (25.94±3.74 compared to 2.20±0.43 pg/ml at BL). Compared to CTL-fed mice, PLF levels of active TGFß1 were significantly (P < 0.0001) diminished in FLC-fed mice (4.52±0.77 pg/ml) (Figure 4C). We determined a significant reduction in the mRNA levels of TGFß1 (Figure 4D) from PLF WBCs of mice-fed FLC. Compared to CTL-fed mice, administration of FLC led to a significant (P < 0.0001) 52.0% decrease in TGFß1 mRNA levels. Furthermore, we determined a significant reduction in the mRNA levels of TGFß receptor 1 (Figure 4F) from PLF WBCs of mice-fed FLC. Compared to CTL-fed mice, administration of FLC led to a significant (P < 0.0001) 50.1% decrease in TGFßR1 mRNA levels.
FS lignans reduce asbestos-induced acute elevations in nitrosative stress
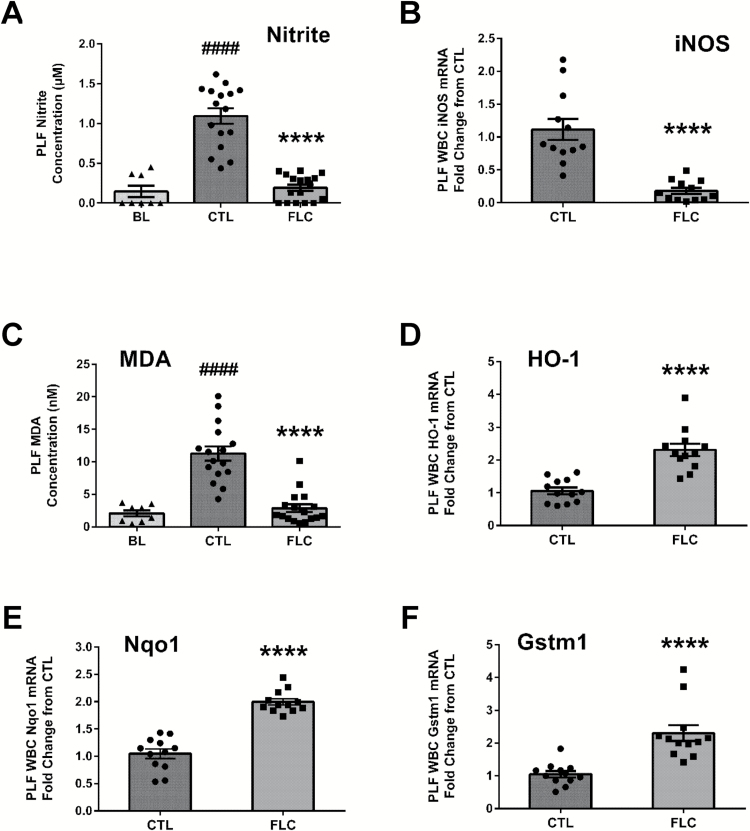
We next evaluated levels of nitrite in the PLF as a marker of nitrosative stress following asbestos exposure. Acute exposure to asbestos led to a significant increase in PLF nitrite concentrations (1.09±0.10 compared to 0.15±0.07 µM at BL) among CTL-fed mice, which was significantly blunted by the administration of FS lignans (0.19±0.04 µM) (Figure 5A). Along with the observed 82.3% decrease in PLF nitrite concentration associated with FLC administration, levels of inducible nitric oxide synthase mRNA in PLF WBCs were significantly (P < 0.0001) decreased by 83.9% among FLC-fed mice (Figure 5B).
Figure 5.
Flaxseed lignan diet abrogates increased nitrosative and oxidative stress from exposure to asbestos fibers. Mouse cohorts (13 weeks old) were fed CTL (n = 16) or FLC (n = 17) diets for 7 days prior to exposure to 400 µg of crocidolite asbestos fibers. PLF was evaluated 3 days following asbestos exposure for nitrite (A) and MDA concentrations (C). PLF WBC mRNA expression of iNOS (B), HO-1 (D), Nqo1 (E) and Gstm1 (F) was determined using qPCR. Levels of target gene mRNA were normalized to 18S ribosomal RNA and values are expressed as fold change from CTL. # indicates a statistically significant difference (####P < 0.0001) from BL and asterisks indicate a statistically significant difference from CTL-fed mice (****P < 0.0001).
Asbestos-induced oxidative stress is decreased by FLC diet
In addition to determining significant asbestos-induced nitrosative stress that is blunted by FLC, we evaluated PLF and PLF WBCs for indicators of oxidative stress. Specifically, lipid peroxidation plays a major role in mediating oxidative damage in tissues, is a qualitative indicator of oxidative stress within tissues and cells, and can be measured by determining the amount of MDA, a product of lipid peroxidation (27). Specifically, in CTL-fed mice, we detected an acute induction of PLF MDA levels (11.26±1.11 compared to 2.08±0.47nM MDA at BL), indicative of lipid peroxidation and oxidative stress following asbestos exposure (Figure 5C). We observed a significant 74.6% reduction in PLF MDA levels associated with FLC administration (2.87±0.60nM MDA). To further investigate the underlying mechanisms behind the decrease in oxidative stress by FLC, we evaluated mRNA expression changes in cytoprotective and antioxidant enzymes (HO-1, Nqo1 and Gstm1), which are important in the detoxification of free radicals and ROS. Compared to CTL-fed mice, mice fed a FLC diet had a statistically significant 2.31-, 2.00- and 2.30-fold increase in PLF WBC mRNA levels of HO-1 (Figure 5D), Nqo1 (Figure 5E) and Gstm1 (Figure 5F), respectively, relative to CTL-fed mice.
Discussion
Acute exposure to asbestos in the peritoneal cavity induced inflammation which was characterized by WBC accumulation and proinflammatory and profibrogenic cytokine release, as well as being associated with gene expression changes in genes of relevance to inflammation and fibrosis. In addition, oxidative and nitrosative stress was detected in the PLF.
The inflammatory and fibrogenic responses induced by asbestos exposure are associated with the location of asbestos fiber deposition and are linked with growth factor release, such as TGFβ and platelet-derived growth factor, HMGB1, a key initiator molecule of inflammation as well as cytokines, such as TNFα and IL-1β, agents that ultimately lead to cell growth and collagen deposition (5,28–31). The chronic production of the above mentioned mediators of inflammation, caused by asbestos exposure, may be critical for malignant transformation and the formation of MM (23,29,32,33) through the induction of DNA damage, defective cell cycle control mechanisms, prolonged angiogenesis, uncontrollable growth signaling and tissue invasion/metastasis (10,34,35). Thus, blocking the release of these mediators might be helpful in preventing the development of mesothelioma.
Our group has reported the protective effect of FS in lung tissue, after repeated radiation and hyperoxia exposure, resulting in significantly decreased bronchoalveolar lavage fluid neutrophils and protein levels, oxidative tissue damage and nitrosative stress as determined by nitrite levels. In addition, lung fibrosis and proinflammatory, profibrogenic cytokine (TGFβ1) gene expression levels were significantly reduced in FS-fed mice (27). We subsequently identified the lignan component in FS (FLC) as the key mediator of these protective effects (15,16). As radiation and hyperoxia exposure are conditions linked to the generation of an inflammatory environment, as in the case of asbestos exposure, we hypothesized that FLC could successfully alter the acute effects of exposure to asbestos. As hypothesized, dietary lignan administration (FLC) prior to asbestos exposure blunted acute inflammation by decreasing the number of WBCs and the release of IL-1ß, IL-6, HMGB1 and TNFα proinflammatory cytokines and profibrogenic, active TGFß1. FLC also decreased cytokine mRNA levels, along with mRNA levels of TNFα and TGFß1 receptor. Additionally, FLC intake reduced asbestos-induced increases in nitrite and MDA, indicators of nitrosative and oxidative stress, respectively, along with decreasing mRNA levels of inducible nitric oxide synthase and increasing gene expression of cytoprotective and antioxidant enzymes, HO-1, Nqo1 and Gstm1.
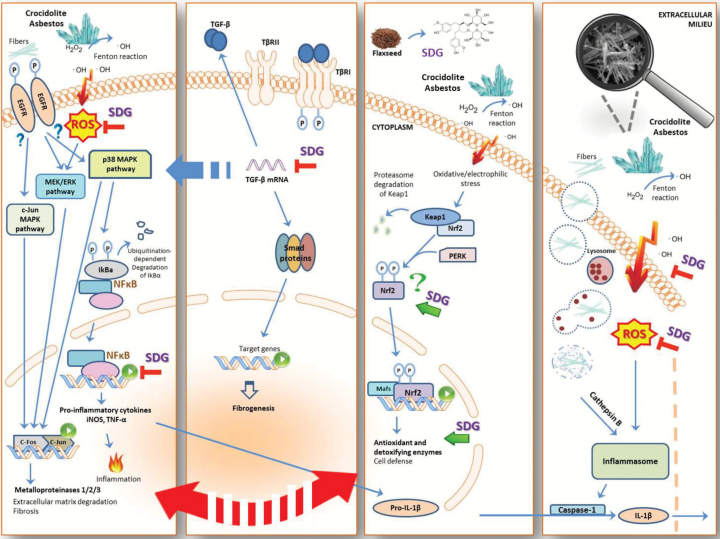
SDG-rich FLC likely acted through multiple mechanisms (see Figure 6). The SDG in FLC can act as a direct antioxidant (36,37). In addition, FLC has been previously reported to activate the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (13,15) the transcription factor of the antioxidant and detoxifying genes, HO-1 and Nqo1, which were also found elevated by this study, and are responsible for the elimination of reactive electrophiles and oxidants which contribute to the mutagenesis and carcinogenesis.
Figure 6.
Role of flaxseed lignans enriched in SDG in ameliorating asbestos-induced inflammation, fibrosis and oxidative and nitrosative stress. Mechanism of asbestos-induced inflammation and amelioration by FLC, enriched in SDG, which inhibits nitrosative and oxidative stress through free radical scavenging and upregulation of antioxidant enzymes. FLC blunts asbestos-induced inflammation and fibrosis through decreased cytokine release and inhibition of proinflammatory and profibrogenic cytokine gene expression.
Given these findings, it seems reasonable to further pursue the use of SDG formulations as a potential chemopreventive agent for the development of MM. Among plant compounds with interesting biological properties, scientific interest in dietary polyphenols is rapidly increasing due to their beneficial effect against diabetes, neurodegenerative and cardiovascular diseases, and cancer (38). Inhibition of growth in a pleural MM model has been shown by the steroidal lactone, withaferin A (39), which acts via blockage of proteasome activity and stimulation of apoptosis. Furthermore, curcumin, a natural ingredient of the spice turmeric, has been shown to inhibit cell cycle progression and to induce apoptosis in preclinical in vitro and in vivo models of pleural MM (40). Another polyphenolic compound, resveratrol, when combined with the chemotherapeutic drug clofarabine has been reported to inhibit activity of the transcription factor Sp1 (41). Importantly, none of these agents has ever been evaluated clinically in MM patients or high-risk individuals exposed to asbestos. Other natural antioxidant agents that have been tested in humans in the context of asbestos exposure include the vitamins A, E and D, but these had no effect on overall survival and progression of asbestos-induced MM (42). Curcumin, also evaluated clinically, albeit not for MM, has poor bioavailability has provided contradictory effects, raising questions of safety in the clinical setting (43).
FS and its lignan SDG have specifically been studied in various cancer models. For example, SDG has been shown to be a successful anticancer agent in vitro against human breast cancer cells (44), as well as a chemopreventive agent against mammary carcinogenesis (45). Moreover, a FS diet has shown anti-estrogenic activity against human breast xenografts in vivo (46), and diminishes tumor growth of human breast MCF-7 xenografts in athymic mice (21). Significantly, FS has proved efficacious in clinical studies of breast cancer patients and reduced the incidence of tumor development in premenopausal women (47). FS has also been associated with reduction of the proliferation rate of prostate cancer, preoperatively (48). Importantly, in our recent pilot clinical study in cystic fibrosis patients (49) we showed that SDG-containing diet achieving comparable plasma levels of SDG metabolites as shown here, resulted in reducing biomarkers of inflammatory and oxidative stress in this chronically ill patient population.
Although asbestos use has been restricted in many western countries, it is still used in many countries around the world and it is estimated that more than 2 million tons were mined in 2008 (50). There will thus likely be a dramatic increase in MM cases in the third world where the use of asbestos has increased with few precautions taken. However, even in the developed world, important exposures still exist. This includes many types of occupations that expose workers to pre-existing asbestos, as well as superfund asbestos hazardous waste sites. Construction workers, plumbers and insulators who work in older buildings where asbestos is present and often needs to be removed are still a high-risk population. Furthermore, asbestos is still legally used in 3000 different consumer products in the United States, such as pipeline wrap, vinyl floor tile, cement shingles, disk brake pads and gaskets. In addition to occupational exposures, individuals in places with known high environmental exposure, such as the Cappadocia region of Turkey (51), New Caledonia (52), eastern Sicily (53), Singapore (54) and southern Nevada (55) are at increased risk of developing mesothelioma. Furthermore, while asbestos use in the United States has declined, consumption has significantly increased in rapidly growing countries, such as China, India and Brazil (56).
A major issue in the link between asbestos and cancer is that inhaled asbestos fibers (especially crocidolite fibers) can persist in the lung for very long periods of time resulting in continuous damage, even if the individual is removed from the exposure. Because of this long latency period (often up to 30–50 years), individuals exposed in the past remain at increased risk of MM and other cancers throughout their lives. Therefore, although reduced use of asbestos is likely to reduce the incidence of MM and asbestos-induced lung cancer in the future, it will have no effects on currently exposed populations. A very large population of exposed patients is therefore currently at risk for the development of MM. Safe, non-toxic agents that can be used as a chemopreventive strategy to mitigate the incidence of MM are thus needed. The purpose of the current paper was to establish a critical ‘first step’. That is, that FS lignans have the ability to blunt acute inflammation caused by asbestos. Now, having shown this, we can move on to the next step which is to study the effects of FS in more chronic exposures.
Future studies will need to evaluate the ability of FS and its lignan SDG to prevent tumor formation in an animal model of MM caused by chronic exposure to asbestos. Although humans are usually exposed to asbestos fibers through inhalation of asbestos, a model of instillation or aerosolization of the asbestos fibers is logistically challenging. The nasal passages of mice are convoluted such that asbestos does not readily enter the airways. Thus, most of the published articles involving inhalation are in rats, not mice. Importantly, it is potentially dangerous for the animal care staff and investigators, it requires stringent environmental controls, and results in a relatively low incidence of MMs after many months. On the other hand, 20% of all human MMs are peritoneal rather than pleural, and there is great similarity between the mesothelial cells in the pleura and peritoneum. For these reasons, we plan to challenge mice via IP injection of asbestos, a well validated model (24,57). Specifically, we plan to pursue these studies using Nf2 +/mut;Cdkn2a +/mut mice. These mice mimic loss of two key tumor suppressors p16INK4a and p14ARF (both encoded by CDKN2A) and NF2/Merlin that are frequently inactivated in MM (58,59). These genetically modified mice recapitulate two of the major somatic changes (CDKN2A and NF2 loss) seen in human MM. These mice also exhibit activation of Akt, ERK, PAK and FAK signaling, which are also seen in human MM (60,61). Mice haploinsufficient (+/−) for either of these tumor suppressor genes develop MM at significantly accelerated rates compared to wild-type littermates when exposed to asbestos by IP injection (24,57).
In summary, we have found that administration of a well-tolerated diet enriched in the FS lignan SDG is able to blunt the acute inflammatory response induced by the IP administration of asbestos. These studies support further research to test the ability of FS lignan to prevent the formation of MM after asbestos exposure with the ultimate goal of finding a safe, effective, well-tolerated chemopreventive agent for individual exposed to asbestos and thus at high risk for mesothelioma.
Funding
NIH-R01 CA133470 (M.C.S.), NIH-1R21AT008291-02 (M.C.S.), NIH-R03 CA180548 (M.C.S.), 1P42ES023720-01 (M.C.S.) and by pilot project support from 1P30 ES013508-02 awarded to M.C.S. (its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH).
Conflict of Interest Statement: M.C.S. reports grants from the NIH during the conduct of the study. In addition, M.C.S. has patents No. PCT/US14/41636 and No. PCT/US15/22501 pending and has a founders equity position in LignaMed, LLC. J.R.T. reports grants from NIEHS and NCI during the conduct of the study; personal fees were also received for consultation regarding genetic aspects of mesothelioma, outside the submitted work. In addition, J.R.T. has a pending patent application on BAP1 mutation testing. All other coauthors report no actual, potential, or perceived conflict of interest with regard to this manuscript.
Glossary
Abbreviations
- BL
baseline
- CTL
control
- ED
enterodiol
- EL
enterolactone
- ELISA
enzyme-linked immunosorbent assay
- FS
flaxseed
- FLC
flaxseed lignan component
- Gstm1
glutathione s-transferase mu 1
- HO-1
heme oxygenase-1
- IL-6
interleukin-6
- IL-1ß
interleukin-1ß
- IP
intraperitoneal
- MDA
malondialdehyde
- MM
malignant mesothelioma
- Nqo1
NADPH:quinone oxidoreductase-1
- PLF
peritoneal lavage fluid
- ROS
reactive oxygen species
- SDG
secoisolariciresinol diglucoside
- TGFß
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
References
- 1. Douglas W.H., et al. (2011) The history of asbestos utilization and recognition of asbestos-induced diseases. In Asbestos. CRC Press, pp. 1–22. [Google Scholar]
- 2. Frank A.L., et al. (2014) The global spread of asbestos. Ann. Glob. Health, 80, 257–262. [DOI] [PubMed] [Google Scholar]
- 3. Yang H., et al. (2008) Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr. Treat. Options Oncol., 9, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudd R.M. (2010) Malignant mesothelioma. Br. Med. Bull., 93, 105–123. [DOI] [PubMed] [Google Scholar]
- 5. Carbone M., et al. (2006) The pathogenesis of mesothelioma. Semin. Diagn. Pathol., 23, 56–60. [DOI] [PubMed] [Google Scholar]
- 6. Dostert C., et al. (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science, 320, 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow M.T., et al. (2012) NLRP3 promotes inflammation-induced skin cancer but is dispensable for asbestos-induced mesothelioma. Immunol. Cell Biol., 90, 983–986. [DOI] [PubMed] [Google Scholar]
- 8. Carbone M., et al. (2012) Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res., 18, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos-Nino M.E., et al. (2006) Cellular and molecular parameters of mesothelioma. J. Cell. Biochem., 98, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heintz N.H., et al. (2010) Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am. J. Respir. Cell Mol. Biol., 42, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christofidou-Solomidou M., et al. (2014) Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed. Cancer Biol. Ther., 15, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christofidou-Solomidou M., et al. (2011) Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer, 11, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J.C., et al. (2008) Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol., 294, L255–L265. [DOI] [PubMed] [Google Scholar]
- 14. Lee J.C., et al. (2009) Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther., 8, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christofidou-Solomidou M., et al. (2012) Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res., 178, 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pietrofesa R., et al. (2013) Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer, 13, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue J.Y., et al. (1992) Antioxidant activity of two dibenzocyclooctene lignans on the aged and ischemic brain in rats. Free Radic. Biol. Med., 12, 127–135. [DOI] [PubMed] [Google Scholar]
- 18. Serraino M., et al. (1991) The effect of flaxseed supplementation on early risk markers for mammary carcinogenesis. Cancer Lett., 60, 135–142. [DOI] [PubMed] [Google Scholar]
- 19. Serraino M., et al. (1992) Flaxseed supplementation and early markers of colon carcinogenesis. Cancer Lett., 63, 159–165. [DOI] [PubMed] [Google Scholar]
- 20. Yan L., et al. (1998) Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer Lett., 124, 181–186. [DOI] [PubMed] [Google Scholar]
- 21. Chen J., et al. (2009) Flaxseed and pure secoisolariciresinol diglucoside, but not flaxseed hull, reduce human breast tumor growth (MCF-7) in athymic mice. J. Nutr., 139, 2061–2066. [DOI] [PubMed] [Google Scholar]
- 22. Neri M., et al. (2012) Chemoprevention of asbestos-linked cancers: a systematic review. Anticancer Res., 32, 1005–1013. [PubMed] [Google Scholar]
- 23. Hillegass J.M., et al. (2010) Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann. N. Y. Acad. Sci., 1203, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altomare D.A., et al. (2005) A mouse model recapitulating molecular features of human mesothelioma. Cancer Res., 65, 8090–8095. [DOI] [PubMed] [Google Scholar]
- 25. Kinniry P., et al. (2006) Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J. Nutr., 136, 1545–1551. [DOI] [PubMed] [Google Scholar]
- 26. Esterbauer H., et al. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med., 11, 81–128. [DOI] [PubMed] [Google Scholar]
- 27. Pietrofesa R.A., et al. (2014) Flaxseed mitigates acute oxidative lung damage in a mouse model of repeated radiation and hyperoxia exposure associated with space exploration. J. Pulm. Respir. Med., 4,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang H., et al. (2010) Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA, 107, 12611–12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qi F., et al. (2013) Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol., 183, 1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Altomare D.A., et al. (2009) Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl. Acad. Sci. USA, 106, 3420–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang H., et al. (2006) TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl. Acad. Sci. USA, 103, 10397–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balkwill F., et al. (2001) Inflammation and cancer: back to Virchow? Lancet, 357, 539–545. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal B.B., et al. (2009) Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res., 15, 425–430. [DOI] [PubMed] [Google Scholar]
- 34. Mossman B.T., et al. (2011) Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health. B. Crit. Rev., 14, 76–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shukla A., et al. (2011) ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. Int. J. Cancer, 129, 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mishra O.P., et al. (2013) Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett., 23, 5325–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishra O.P. et al. > (2014) Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA From gamma radiation damage. Radiat. Res., 182, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez-Mateos A., et al. (2014) Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol., 88, 1803–1853. [DOI] [PubMed] [Google Scholar]
- 39. Yang H., et al. (2012) Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo . PLoS One, 7, e41214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y., et al. (2011) Curcumin suppresses growth of mesothelioma cells in vitro and in vivo, in part, by stimulating apoptosis. Mol. Cell. Biochem., 357, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee Y.J., et al. (2013) Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food Chem. Toxicol., 52, 61–68. [DOI] [PubMed] [Google Scholar]
- 42. Robinson C., et al. (2014) Dietary vitamin D supplementation does not reduce the incidence or severity of asbestos-induced mesothelioma in a mouse model. Nutr. Cancer, 66, 383–387. [DOI] [PubMed] [Google Scholar]
- 43. Gupta S.C., et al. (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J., 15, 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen J., et al. (2003) Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro . Breast Cancer Res. Treat., 80, 163–170. [DOI] [PubMed] [Google Scholar]
- 45. Serraino M., et al. (1992) The effect of flaxseed supplementation on the initiation and promotional stages of mammary tumorigenesis. Nutr. Cancer, 17, 153–159. [DOI] [PubMed] [Google Scholar]
- 46. Bergman Jungeström M., et al. (2007) Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo . Clin. Cancer Res., 13, 1061–1067. [DOI] [PubMed] [Google Scholar]
- 47. Mason J.K., et al. (2014) Flaxseed and its lignan and oil components: can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl. Physiol. Nutr. Metab., 39, 663–678. [DOI] [PubMed] [Google Scholar]
- 48. Demark-Wahnefried W., et al. (2008) Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol. Biomarkers Prev., 17, 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turowski J.B., et al. (2015) Flaxseed modulates inflammatory and oxidative stress biomarkers in cystic fibrosis: a pilot study. BMC Complement. Altern. Med., 15, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. British Geological Survey. (2004. –2008) World Mineral Production 2004 to 2008. Nottingham: British Geological Survey. [Google Scholar]
- 51. Carbone M., et al. (2011) Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA, 108, 13618–13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baumann F., et al. (2015) Posterolateral fusion in acute traumatic thoracolumbar fractures: a comparison of demineralized bone matrix and autologous bone graft. Acta Chir. Orthop. Traumatol. Cech., 82, 119–125. [PubMed] [Google Scholar]
- 53. Paoletti L., et al. (2000) Unusually high incidence of malignant pleural mesothelioma in a town of eastern Sicily: an epidemiological and environmental study. Arch. Environ. Health, 55, 392–398. [DOI] [PubMed] [Google Scholar]
- 54. Lim J.W., et al. (2011) Preventive measures to eliminate asbestos-related diseases in singapore. Saf. Health Work, 2, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baumann F., et al. (2015) The Presence of Asbestos in the Natural Environment is Likely Related to Mesothelioma in Young Individuals and Women from Southern Nevada. J. Thorac. Oncol., 10, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. LE G.V., et al. (2011) Asbestos use and asbestos-related diseases in Asia: past, present and future. Respirology, 16, 767–775. [DOI] [PubMed] [Google Scholar]
- 57. Altomare D.A., et al. (2011) Losses of both products of the Cdkn2a/Arf locus contribute to asbestos-induced mesothelioma development and cooperate to accelerate tumorigenesis. PLoS One, 6, e18828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng J.Q., et al. (1994) p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res., 54, 5547–5551. [PubMed] [Google Scholar]
- 59. Bianchi A.B., et al. (1995) High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA, 92, 10854–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Menges C.W., et al. (2014) Tumor suppressor alterations cooperate to drive aggressive mesotheliomas with enriched cancer stem cells via a p53-miR-34a-c-Met axis. Cancer Res., 74, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shapiro I.M., et al. (2014) Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci. Transl. Med., 6, 237ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]