Abstract
Factors determining who develops PTSD following trauma are not well understood. The €4 allele of the apolipoprotein E (apoE) gene is associated with dementia and unfavorable outcome following brain insult. PTSD is also associated with dementia. Given evidence that psychological trauma adversely affects the brain, we hypothesized that the apoE genotype moderates effects of psychological trauma on PTSD pathogenesis. To investigate the moderation of the relationship between PTSD symptoms and combat exposure, we used 172 participants with combat trauma sustained during the Vietnam War. PTSD symptoms were the dependent variable and number of combat experiences, apoE genotype, and the combat experiences × apoE genotype interaction were predictors. We also examined the outcome of a diagnosis of PTSD (n = 39) versus no PTSD diagnosis (n = 131). The combat × apoE genotype interaction was significant for both PTSD symptoms (P = .014) and PTSD diagnosis (P = .009). ApoE genotype moderates the relationship between combat exposure and PTSD symptoms. Although the pathophysiology of PTSD is not well understood, the €4 allele is related to reduced resilience of the brain to insult. Our results are consistent with the €4 allele influencing the effects of psychological trauma on the brain, thereby affecting the risk of PTSD.
Keywords: posttraumatic stress disorder, apoE, genetics, G×E interaction
Introduction
Exposure to a traumatic event is necessary, but not sufficient, for the development of posttraumatic stress disorder (PTSD). About 80% of the population of the United States has experienced at least one event that would satisfy the DSM-IV criteria for a “traumatic event” [;Breslau, 2009]; however, less than 15–20% of those exposed develop PTSD. Thus, there is considerable variation in susceptibility to PTSD. Genetic factors play a significant role in explaining such individual variation [True et al, 1993].
Genes typically do not influence psychological outcomes independent of environmental factors. Rather, a dynamic interplay frequently exists between genes and environment. Gene–environment interaction (G × E) is a phenomenon by which one's genotype influences the effect of exposure to an environmental factor, and has proven relevant for understanding mental disorders [Moffitt et al., 2005]. Because PTSD can only develop after exposure to an environmental trauma and genetic factors influence susceptibility to PTSD, it lends itself well to analysis as a G×E phenomenon. In this regard, several studies have identified specific GxE interactions with respect to PTSD [Amstadter et al, 2009; Binder et al., 2008; Kilpatrick et al, 2007; Koenen et al., 2009; ;Kolassa et al., 2010a; ;Kolassa et al., 2010b; Nelson et al., 2009; Xie et al., 2009; Xie et al., 2010; Xie et al., 2012]. Two studies reported a three-way interaction of trauma, social support, and genotype [Amstadter et al., 2009; Kilpatrick et al., 2007]. Koenen et al. [2009] reported an interaction between serotonin transporter (SLC6A4) genotype and group-level aspects of the social environment. Two studies [Binder et al., 2008; Nelson et al., 2009] examined the relationship of childhood trauma and adult PTSD or childhood adversity and PTSD at any age. Binder et al. [2008] found an interaction with the FKBinding protein (FKBP5) genotype and Nelson et al. [2009] found an interaction with the gamma-aminobutyric acid receptor, alpha-2 (GABRA2) genotype. Four reports found a two-way G×E interaction of adult trauma exposure and adult PTSD [Kolassa et al., 2010a; ;Kolassa et al., 2010b; Xie et al., 2009, ;2012]. Among survivors of the Rwandan Genocide [Kolassa et al., 2010a; ;Kolassa et al., 2010b] the serotonin transporter gene (SLC6A4) promoter polymorphism and COMT (catechol-O-methyltransferase) genotype interacted with lifetime exposure to traumatic events to influence adult PTSD. Xie et al. found that SLC6A4 interacted with adult trauma to influence adult PTSD and with childhood adversity to influence lifetime risk of PTSD [Xie et al., 2009; Xie et al., 2012]. Boscarino et al. [2012] examined alleles of FKBP5, COMT, CHRNA5, and CRHR1 that had been suggested in previous research to be related to the risk of PTSD. They found that the total count of risk alleles interacted with trauma exposure to predict PTSD.
There are conflicting results regarding the issue of whether genetic factors that influence exposure to trauma also influence susceptibility to developing PTSD following trauma exposure. For example, Sartor et al. [2012] found that the correlation between genetic influences on exposure to ‘high risk trauma’ (defined as rape, sexual molestation, physical attack or assault, childhood physical abuse, and serious childhood neglect) and genetic influences on the risk of PTSD given trauma exposure was quite substantial (r = .89, CI 0.78–0.99). However, Amstadter et al. [2012] observed an approximately one fifth overlap between familial liabilities for trauma exposure and PTSD symptoms. They concluded that the hypothesis that PTSD is etiologically similar to exposure to a traumatic event was not supported. Clearly, further research will be required to explicate the basis for such disparate findings.
Several observations support the possible relevance of the apolipoprotein E(apoE) genotype to risk of PTSD: (1) apoE genotype is related to how resilient the brain is to physical and psychosocial insult; (2) apoE genotype is related to the risk of dementia; and (3) PTSD increases risk of dementia. ApoE is important for neuronal repair. ApoE affects neuronal plasticity through the transport of cholesterol and other lipids to neuronal sites undergoing remodeling [White et al., 2001] and exists in three isoforms: €2, €3, and €4. Whereas the €2 and €3 alleles are associated with enhanced regeneration and sprouting, the €4 allele inhibits the outgrowth of neurites [Teter et al., 1999] and is associated with impaired ability to cope with neurodegeneration and neurologic insult. The €4 isoform is also associated with mitochondrial dysfunction, inflammation, impairment of the antioxidative defense system, increased intracellular calcium, disruption of cholinergic transmission, dys-regulation of the neuronal signaling pathways, and apoptosis [Dardiotis et al., 2010].
The apoE €4 allele is related to difficulty in recovering from brain damage caused by head injuries [Kutner et al, 2000; Teasdale et al, 1997]. Studies of professional boxers and football players suggest that cognitive status following repeated head trauma may be influenced by apoE genotype [Jordan et al., 1997; Kutner et al., 2000]. Similarly, the €4 allele is a significant risk factor for cognitive impairment after stroke [;Wagle et al, 2009]. ApoE €4 is a susceptibility allele for Alzheimer's disease, associated with hippocampal atrophy and beta amyloid plaques [Juottonen et al., 1998].
There is evidence from large epidemiological studies that PTSD is associated with increased risk of dementia, supporting the hypothesis that PTSD is associated with untoward changes in the brain [Yaffe et al., 2010]. Additionally, there is substantial evidence for neurobiological consequences of psychological trauma exposure other than dementia [Nutt and Malizia, 2004]. For example, animal models demonstrate a relationship between experimentally-induced stress and hippocampal damage [Sapolsky et al, 1985]. Human studies indicate that psychological stress may disrupt hypothalamic-pituitary-adrenal axis activity, resulting in altered cortisol levels and cellular damage or tissue volume reductions in various brain regions [Kremen et al., 2010; Lupien et al., 1998; Lupien et al., 2005].
Taken together, these findings raise the question of whether the apoE genotype may influence susceptibility to PTSD following trauma. Only one study has examined the relationship of apoE genotype and PTSD. Freeman et al. investigated the effect of apoE genotype on PTSD symptoms among a sample in which all participants had PTSD [Freeman et al., 2005], so the findings are not informative about the risk of developing PTSD. Contrary to expectations, their subjects with the €2 allele had more severe re-experiencing symptoms. To our knowledge, no studies have examined the effect of apoE on risk of PTSD or its interaction with psychological trauma. However, Lee et al. reported an interaction between apoE genotype and psychosocial stressors [Lee et al, 2011]. Specifically, individuals with one or two €4 alleles were more sensitive to the adverse effects on cognition of neighborhood environmental characteristics associated with heightened vigilance, alarm, or fear that could produce a biological stress response. In a study of older adults, Petkus et al. [2012] found that apoE genotype moderated the relationship of a history of sexual assault with declines in executive functioning; the effect of sexual assault on the measure of cognitive functioning was worse if the individual had an €4 allele. These studies demonstrated that, like the effect of physical insults (e.g., stroke and head trauma), psychosocial factors can interact with apoE genotype to influence psychological characteristics reflecting brain functioning.
The Vietnam Era Twin Study of Aging (VETSA) provides an opportunity to investigate how well-defined traumatic events (combat experiences) interact with apoE genotype to affect PTSD. There are concerns about candidate gene × environment interaction studies because of the widespread and troubling failure to replicate results [Duncan and Keller, 2011]. Although the reasons for this are complex, it is likely that effect sizes of individual polymorphisms are small, and studies have therefore been under-powered. Also, multiple hypotheses have often been tested without adjusting for multiple testing which may result in a high proportion of false positive findings or type I errors [Ioannidis, 2005]. While it is important to address the issue of type I error, studies of psycho-pathology, especially those that include rigorous information about putative risk factors, often face pragmatic constraints on sample size and the probability that effect sizes attributable to any single genetic risk factor are likely to be small. Draconian measures to control type I error rates may cause serious concerns about the risk of type II errors. Replications are one of the best safeguards against false positive findings, but are not always practical.
Another way to attempt to reduce type I errors is to require that candidate gene studies provide a strong biological rationale for the choice of the gene. We believe that the documented associations: (1) among trauma, PTSD, and changes in the brain: (2) between apoE genotype and response of the brain to physical and psychosocial trauma/insult; (3) between apoE genotype and dementia; and (4) between dementia and PTSD provide a compelling rationale for investigating the moderating effect of apoE on response to trauma. Another common problem in G × E studies is multiple testing, that is, a large number of genotypes, environmental risk factors, and/or clinical phenotypes may be investigated in a hypothesis-generating approach. We therefore examined only one genotype, one environmental risk factor, and one clinical phenotype in a very clear and specific hypothesis-testing approach.
Method
Participants
Data were drawn from three previous studies of the Vietnam Era Twin Registry (VETR), a nationally distributed sample of male twin pairs who served in the military between 1965 and 1975 [Goldberg et al, 1987]. Data on combat exposure were drawn from the Survey of Health (SOH), a mailed questionnaire study conducted in 1987. Diagnostic data about PTSD were drawn from the Harvard Drug Study (HDS) in which VETR members were administered the Diagnostic Interview Schedule Version-III-Revised (DIS-III-R) by telephone in 1992. ApoE genotype data were obtained from 1237 VETSA participants with an average age 55 years. Participants in each study were selected without investigator knowledge of PTSD, combat experience, or any other characteristics. The racial/ethnic composition of VETSA participants is 92.3% White, 4.2% African-American, and 3.5% “other.” VETR members are representative of all twins who served in the military during the Vietnam War on a variety of sociodemographic variables [Goldberg et al, 1987]. VETSA participants are very similar to men in their 50s from U.S. Census and Center for Disease Control data in regard to education, median self-income, marital status, employment, and prevalence of various medical conditions.
Materials and Procedure
Combat Experiences Scale
In the SOH, the VETR twins were asked about combat experiences in a questionnaire [Goldberg et al., 1987] that yielded a combat exposure score. Specifically, each veteran was asked whether he was ever engaged in any of 18 various combat experiences, such as receiving incoming fire or being wounded. A combat exposure index score was calculated by summing positive responses. This scale has been described elsewhere and has demonstrated good internal consistency and test-retest reliability; validity is supported by a strong association with being awarded a military combat medal [Janes et al., 1991].
Diagnostic Interview Schedule-III-Revised (DIS-III-R)
Participants were interviewed using the Diagnostic Interview Schedule Version-III-Revised, a structured psychiatric interview for use in epidemiological research [Robins, 1989] that yields DSM-III-R diagnoses. Experienced interviewers administered the DIS-III-R by telephone. Respondents were screened into the PTSD symptom questions if they acknowledged having had a “terrible experience.” If his terrible experience was a military combat experience or seeing people hurt or killed in combat, we considered him to have combat trauma. We utilized the algorithm developed by the authors of the DIS-III-R to convert the item-level data into the presence or absence of each DSM-III-R symptom. In our primary analyses we utilized the DSM-III-R symptom count.
Genotyping
ApoE genotyping was conducted for the 1,237 VETSA participants who were assessed at one of two testing sites (Boston University and the University of California, San Diego) or, in rare circumstances, elected to have a research assistant travel to them. Informed consent was obtained after the nature and possible consequences of the study were explained. The study was approved by Institutional Review Boards at Boston University and the University of California, San Diego. ApoE genotypes were determined using previously described PCR conditions [Emi et al., 1988] and the HhaI restriction digest method [Hixson and Vernier, 1990]. All genotypes were independently determined twice by laboratory personnel blind to initial genotype and the identity of the co-twin. Due to the low rate of €4 homozygosity, we divided the combat trauma exposed sample into two groups: €4 positive (individuals with one or two €4 alleles) and €4 negative (no €4 alleles). Among these VETSA combat trauma exposed veterans, 24% possessed at least one €4 allele. There were no significant differences among racial/ethnic groups for the frequency of the €4 allele, diagnosis of combat-related PTSD, mean number of combat-related PTSD symptoms, or mean combat exposure scores.
Statistical Analyses
We examined the influence of combat exposure, apoE genotype, and the interaction of combat exposure and apoE genotype on the number of PTSD symptoms and the diagnosis of PTSD using generalized estimating equations (GEE) as implemented in SPSS. GEE is a quasi-likelihood method that allows for correlated error terms between observations. While GEE is used widely to allow for non-independence of repeated observations in time series analysis, we used it here to allow for non-independent measurements within pairs of twins. GEE has been validated for use in twin studies [Carlin et al., 2005] and performs similarly to random coefficient models [Twisk, 2004]. Aside from ability to accommodate correlated error terms, the GEE model used here has the same form as a standard regression model (linear for continuous response variables and logistic for dichotomous response variables) and the effect estimates can be interpreted in the same manner. That is, in the linear GEE model, the β estimate represents the coefficient in the linear model (slope) and the logistic GEE model exp(β) is the odds ratio increase for one unit increase in the predictor variable. Presented significance levels represent each parameter's influence after all other terms have been entered into the equation.
Primary analyses examined the effect of apoE and combat exposure on the number of PTSD symptoms using a linear model. First we fit a model which includes the main effects of combat and the presence of €4. That is, yij = (β0 + β1xij + β21E4ij + eij, where yij is the number of PTSD symptoms for sibling i in twin-pair j, and 1E4ij is the indicator variable of the presence of an €4 allele (1 when the subject ij has no €4 alleles and 0 when the subject has 1 or 2 €4 alleles). This is a standard linear regression model, except for the fact that GEE is used to model a positive correlation in the error term (eij) within each twin pair (subscript j). Next, we used GEE to fit an interaction model to the data (yij = β0 + β1xij + β21E4ij + β3xij1E4ij + eij.). In our secondary analyses, the dependent variable is diagnosis of combat-related PTSD versus no PTSD using a logistic model. That is, if PTSD diagnosis for subject ij is denoted zij, our main-effects and interaction model can be written as log(zij/(1- zij)) = β0 + β1xij+ β21E4ij+ eij and log(zij/(1-zij)) = β0+ β1xij+ β21E4ij+ β3xij1E4ij+eij respectively.
We used number of symptoms of PTSD as the primary outcome because a dimensional approach to PTSD is likely to be the most informative as it captures the full range of severity. Moreover, there is no compelling evidence that the criterion number of symptoms required by the DSM identifies a condition that is qualitatively distinct from those below the threshold. However, because many studies use diagnosis as the primary phenotype, we conducted a secondary, parallel analysis utilizing a DSM-III-R diagnosis of PTSD.
Results
Among VETSA participants who served in Vietnam, 179 reported on the Diagnostic Interview Schedule-III-Revised that they had experienced a combat-related trauma. Participants without a qualifying trauma could not be interviewed about PTSD symptoms—by definition one cannot have PTSD symptoms without a traumatic event. Moreover, some symptoms can only be assessed in terms of the traumatic event (e.g., re-experiencing the traumatic event). We only included participants whose PTSD symptoms were related to combat exposure. Of these 179 participants, 172 also had quantitative combat exposure data and genotypic data. We examined whether apoE genotype moderated the relationship between combat exposure and number of PTSD symptoms. There were 170 participants with complete diagnostic, genotypic, and combat exposure data. There were 39 with combat-related PTSD (22.9%) and 131 participants without PTSD. We examined whether apoE genotype moderated the relationship between the combat exposure and the diagnosis of PTSD.
In step 1 of our primary analyses we examined the relationship between number of PTSD symptoms and number of combat experiences among the 172 Vietnam veterans who reported combat trauma and also had Combat Experiences Scale and genotypic data available. There were main effects for combat exposure (beta = 0.257; P = 0.01) and for apoE genotype (beta =−1.766; P = 0.02). The second step in the analysis demonstrated a significant interaction between combat exposure and apoE genotype (beta = 0.529; P = 0.014;see Table I). Among this group in which everyone had experienced significant combat trauma, the relationship of the level of combat exposure to PTSD symptomatology was moderated by apoE genotype. Figure 1 displays the major trend in these results with separate lines for participants with 1 or 2 vs. 0 €4 alleles (€4+ vs. €4−). The number of PTSD symptoms is graphed for each apoE group, smoothed using a moving average technique with a window size of three for the number of combat experiences. For example, the observed number of symptoms graphed at five combat experiences is the average number of symptoms for all individuals with four to six combat experiences. This compensates for erratic behavior of points with a small number of observations. The solid line is the predicted number of symptoms according to the GEE model allowing for an interaction between APOE status and the number of combat exposures.
Table I.
Regression of Combat-Related PTSD Symptomatology and the Probability of a Diagnosis of PTSD on Number of Combat Experience (Combat Experiences Scale Score), apoE Genotype (0 vs. 1 or 2 €4 Alleles), and the Combat × apoE Genotype Interaction
| PTSD symptom count (n = 172) | Diagnosis of PTSD (n = 170) | |||
|---|---|---|---|---|
|
|
|
|||
| Predictor | Beta | Signif from GEE | Beta | Signif from GEE |
| Step 1: main effects | ||||
| Combat | 0.257 | 0.010 | 1.124 | 0.032 |
| apoE genotype | 1.766 | 0.020 | 1.1265 | 0.585 |
| Step 2: interaction model | ||||
| Combat | 0.160 | 0.122 | 1.052 | 0.404 |
| apoE genotype | −0.255 | 0.813 | 0.120 | 0.056 |
| Combat × apoE genotype | 0.529 | 0.014 | 1.534 | 0.009 |
The analysis includes participants with service in Vietnam who reported combat trauma in the DIS-III-R interview and had Combat Experiences Scale and genotypic data.
Fig. 1.
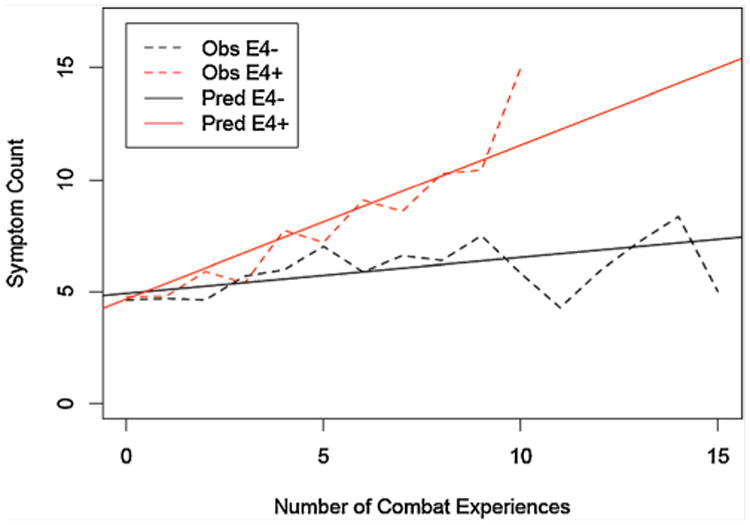
Moving-average smoothed graph of the number of combat-related PTSD symptoms among 172 participants who reported combat trauma by level of combat exposure. The mean number of PTSD symptoms is graphed for each apoE group (i.e., 1 or 2 vs. 0 €4 alleles; “€4+” vs. “€4−”). In addition to graphing the observed (“Obs”) mean, we also include a graph of the mean predicted (“Pred”) by the GEE-interaction model.
In our secondary analyses using hierarchical GEE analysis, we examined the main effects of combat exposure and apoE genotype on risk of PTSD. Among the 170 participants who reported experiencing combat-trauma, there was considerable heterogeneity with regard to combat exposure. (About 16% reported 0 combat experiences, and the highest score was 15 different combat experiences. We assume that the 16% of subjects who reported experiencing a combat-related trauma, but scored a zero on the Combat Experiences Scale, had experienced a combat-related traumatic event that was not included among the 18 experiences included in the scale.) There were 39 participants with combat-related PTSD and 131 participants with no PTSD from any source. In this group with a wide range of combat experiences, the main effect of level of combat was a significant predictor of a diagnosis of PTSD (B = .117; Exp (B) = 1.124; P = .032). The main effect for apoE genotype was not significant (P = .58). In the next step, the interaction of combat exposure and apoE genotype was significant (P = .009) with B = .428; Exp (B) = 1.534 (See Table I). Figure 2 illustrates the interaction by graphing the prevalence of a PTSD diagnosis for each apoE group, again smoothed using a moving average technique with a window size of three.
Fig. 2.
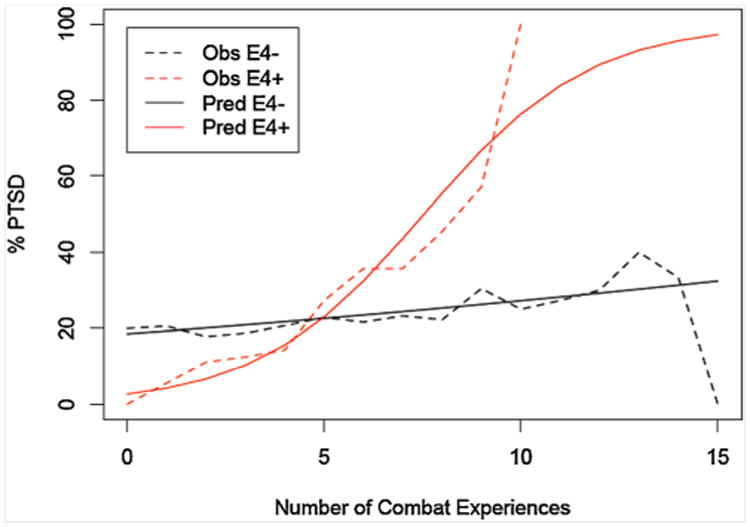
Moving-average smoothed graph of prevalence of combat-related PTSD among 170 participants who served in Vietnam and had either a diagnosis of combat-related PTSD (n = 39) or no diagnosis of PTSD from any type of trauma (n = 131) by level of combat exposure. The prevalence of PTSD is graphed for each apoE group (i.e., 1 or 2 vs. 0 €4 alleles; “€4+” vs. “€4−”). In addition to graphing the observed (“Obs”) prevalence, we also include a graph of the prevalence predicted (“Pred”) by the GEE-interaction model.
To address possible ethnic stratification, we repeated both primary and secondary analyses using only White, non-Hispanic participants. The interaction between combat exposure and apoE genotype remained a significant predictor of the number of PTSD symptoms (P = .008) and a diagnosis of PTSD (P = .001). There were not enough non-White participants to allow meaningful analyses of that group.
Because of the possibility that genetic factors that influence exposure to a traumatic event may overlap with genetic influences on the risk of developing PTSD given exposure to a traumatic event, we compared the rate of exposure to combat trauma among €4+ versus €4− participants. The groups did not differ in their rate of exposure to combat trauma (Fisher's Exact Test significance P = 0.38).
Discussion
The present study aimed to investigate whether apoE genotype moderates the relationship of trauma to the development of PTSD. We found that the apoE genotype interacts with level of combat exposure to predict the number of PTSD symptoms given exposure to combat trauma; that is, the effect of combat is different depending upon apoE genotype. Our secondary analyses, which compared the subset of cases with a diagnosis of combat-related PTSD to the participants exposed to combat-trauma who did not develop PTSD, bolstered the conclusion that the €4 allele makes an individual more vulnerable to the adverse consequences of exposure to combat.
In both our primary and secondary analyses, we found a main effect for combat exposure. There was a significant apoE main effect in the primary analyses using PTSD symptomatology as the outcome, but there was not a significant main effect for apoE genotype in our secondary analyses in which a diagnosis of combat-related PTSD was the outcome. The significant main effect for apoE genotype in our primary analysis reflects the effect of apoE genotype across all exposure levels as this model assumes that the effect is constant across exposure to combat (parallel lines). Moreover, even among this group in which everyone had experienced significant combat trauma, the relationship of the level of combat exposure to PTSD symptomatology was moderated by apoE genotype. Whether we treated PTSD as a categorical outcome or as a dimensional outcome, we found that the presence of an €4 allele makes the individual more vulnerable to the adverse consequences of traumatic exposure.
Our study does not address the mechanism responsible for our results. However, apoE is commonly associated with deficits in recovering from neurodegeneration and brain insult. In contrast to direct physical trauma, psychological trauma might be conceptualized as a type of indirect brain insult, as, for example when stress exposure leads to chronic exposure to high levels of cortisol or cortisol dysregulation which, in turn, may result in hippocampal [Lupien et al., 2005] or frontal lobe damage [Kremen et al., 2012]. Thus, the €4 allele may also impair the capacity to recover from the effects of psychological trauma on the brain and possibly contribute to the pathophysiology of PTSD.
;Wang et al. [2010] demonstrated that PTSD is associated with selective volume loss in subfields of the hippocampus. The finding by Gilbertson et al. [2002] that trauma-unexposed monozygotic co-twins of twins with PTSD had smaller hippocampal volumes compared to controls indicated that smaller hippocampi are, in part, a risk factor for the development of PTSD, rather than just a consequence of trauma exposure and/or PTSD. The €4 allele is associated with memory impairments and hippocampal atrophy, and numerous studies have identified the dysregulation of the processing of memories as a factor contributing to PTSD symptoms. For example, individuals with PTSD have deficits in fear extinction memory compared to individuals who experienced trauma but did not develop PTSD, and less activation in the hippocampus and ventromedial prefrontal cortex was observed during memory extinction tasks among a PTSD group [Milad et al., 2009]. The possible effects of apoE genotype are not limited to the hippocampus. The €4 allele has been associated with frontal and parietal cerebral blood flow reduction [Thambisetty et al., 2010] and frontotemporal gray matter density reductions in healthy adults [Wishart et al., 2006; Fennema-Notestine et al., 2011; Kremen et al., 2012]. These findings indicate that differences in brain structure or function may reflect risk for, or consequences of, illness. In either case, taken together with the results of the present study, the data do appear to support the hypothesis that having an €4 allele increases brain vulnerability in relation to the development of PTSD.
Since all participants were male, the extent to which these results apply to women is unclear. While psychological trauma sustained during combat may be considered a prototypical traumatic event, it is not clear that all types of psychological trauma would interact with apoE genotype in the same way. For example, combat related psychological trauma may be more likely to be associated with physical brain trauma than other types of psychological trauma. On the other hand, precision is gained by that fact that the sample is relatively homogenous, with a fairly narrow age range, all participants being mentally and physically healthy enough before trauma exposure to qualify for military service, and the relative uniformity for the type of trauma experienced. Because life-time diagnostic data were collected about 17 years after the end of the Vietnam War, we could not detect combat-related PTSD with onset after 1992, but there were probably few, if any, such delayed onset cases. This study examined the interactive effect of the severity of the trauma itself (combat)with the relevant genotype and only included diagnoses of PTSD or symptom severity of PTSD that were explicitly related to the trauma that we assessed (i.e., combat). The number of cases meeting full diagnostic criteria for PTSD is relatively small (n = 39), but the conclusions from these analyses are supported by the findings of a significant interaction between apoE genotype and combat trauma on the level of PTSD symptomatology among the 172 subjects who reported experiencing combat trauma.
This study demonstrates that a gene related to structural and functional characteristics of the central nervous system moderates the effect of a psychological risk factor on an individual's health status. Disappointing results so far in identifying risk genes for complex disorders may reflect the fact that many of these disorders are determined by an interaction of genes and the environment. The G×E model in which the individual's sensitivity to environmental factors is determined by his or her genotype is particularly relevant to mental disorders in general and PTSD in particular. However, as mentioned in the Introduction to this paper, there are a number of concerns about findings from GxE analyses and, pending replication, our results must be considered preliminary, especially in light of our relatively small sample. We believe that our study does avoid a number of problems that are often associated with candidate gene × environment studies. The biological plausibility of our findings is supported by the fact that there is considerable evidence that: (1) psychological trauma is associated with PTSD and with changes to the brain; (2) PTSD is associated with structural changes to the brain and with dementia; (3) the €4 allele of the apoE gene is associated with adverse outcomes following physical and psychosocial trauma to the brain; and (4) the €4 allele is associated with dementia. Our study also avoided concerns associated with multiple testing because we investigated only one genotype, one environmental factor, and one clinical phenotype based on an explicit a priori hypothesis.
Acknowledgments
The U.S. Department of Veterans Affairs provides financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Grant sponsor: NIH; Grant numbers: R01 AG018386; R01 AG022982; R01 AG018384; R01 AG022381; K01 MH076100.
Footnotes
No author has any conflict of interest to disclose.
References
- Amstadter AB, Nugent NR, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Gelernter J. Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry. 2009;72(4):360–369. doi: 10.1521/psyc.2009.72.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Aggen SH, Knudsen GP, Reichborn-Kjennerud T, Kendler KS. A population-based study of familial and individual-specific environmental contributions to traumatic event exposure and posttraumatic stress disorder symptoms in a Norwegian twin sample. Twin Res Hum Genet. 2012;15(5):656–662. doi: 10.1017/thg.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Zhang X. Higher FKBP5, COMT, CHRNA5, and CRH1 allele burdens are associated with PTSD and interact with trauma exposure: Implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat. 2012;8:131–139. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: A critical review. Int J Epidemiol. 2005;34(5):1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Dardiotis E, Fountas KN, Dardioti M, Xiromerisiou G, Kapsalaki E, Tasiou A, Hadjigeorgiou GM. Genetic association studies in patients with traumatic brain injury. Neurosurg Focus. 2010;28(1):E9. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- Duncan L, Keller MC. A critical review of the first ten years of measured gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M, Wu LL, Robertson MA, Myers RL, Hegele RA, Williams RR, White R, Lalouel JM. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3(4):373–379. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Panizzon MS, Thompson WK, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, Lyons MJ, Neale MC, Seidman LJ, Tsuang MT, Xian H, Dale AM, Kremen WS. Presence of ApoE e4 allele associated with thinner frontal cortices in middle-age. J Alzheimers Dis. 2011;26(3):49–60. doi: 10.3233/JAD-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WS. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2005;17(4):541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Ascertainment bias. Acta Genet Med Gemellol (Roma) 1987;36(1):67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47(1):80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein Eepsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278(2):136–140. [PubMed] [Google Scholar]
- Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer's disease carrying the apolipoprotein E epsilon4 allele. J Neurol Neurosurg Psychiatry. 1998;65(3):322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169(6):704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Ertl V, Eckart C, Glockner F, Kolassa S, Papassotiropoulos A, Elbert T. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: Evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010a;71(5):543–547. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010b;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kremen WS, O'Brien RC, Panizzon MS, Prom-Wormley E, Eaves LJ, Eisen SA, Franz CE. Salivary cortisol and prefrontal cortical thickness in middle-aged men: A twin study. Neuroimage. 2010;53(3):1093–1102. doi: 10.1016/j.neuroimage.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: Differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62(2):647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner KC, Erlanger DM, Tsai J, Jordan B, Relkin NR. Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery. 2000;47(3):651–657. doi: 10.1097/00006123-200009000-00026. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, James BD, Bandeen-Roche K, Schwartz BS. Neighborhood psychosocial environment, apolipoprotein E genotype, and cognitive function in older adults. Arch Gen Psychiatry. 2011;68(3):314–321. doi: 10.1001/archgenpsychiatry.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Schwartz G, Ng YK, Fiocco A, Wan N, Pruessner JC, Nair NP. The Douglas Hospital Longitudinal Study of Normal and Pathological Aging: Summary of findings. J Psychiatry Neurosci. 2005;30(5):328–334. [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, Madden PA. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14(3):234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(Suppl 1):11–17. [PubMed] [Google Scholar]
- Petkus AJ, Wetherell JL, Stein MB, Liu L, Barrett-Connon E. History of sexual assault is associated with greater declines in executive functioning in older adults with APOE 4. J Gerontol B. 2012;67(6):653–659. doi: 10.1093/geronb/gbr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN. Diagnostic grammar and assessment: Translating criteria into questions. Psychol Med. 1989;19(1):57–68. doi: 10.1017/s0033291700011028. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci. 1985;5(5):1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Bucholz KK, Madden PAF, Heath AC, Martin NG, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69(3):293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350(9084):1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Human apolipoprotein E isoform-specific differences in neuronal sprouting in organotypic hippocampal culture. J Neurochem. 1999;73(6):2613–2616. doi: 10.1046/j.1471-4159.1999.0732613.x. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol. 2010;67(1):93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19(8):769–776. doi: 10.1023/b:ejep.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]
- Wagle J, Farner L, Flekkoy K, Wyller TB, Sandvik L, Eiklid KL, Engedal K. Association between ApoE epsilon4 and cognitive impairment after stroke. Dement Geriatr Cogn Disord. 2009;27(6):525–533. doi: 10.1159/000223230. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67(3):296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8(4):611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67(7):1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for posttraumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie PX, Kranzler HR, Farrer L, Gelernter J. Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood adversity on posttraumatic stress disorder risk: A replication study. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(6):644–652. doi: 10.1002/ajmg.b.32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Marmar C. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


