Abstract
While many studies have reported that individual differences in personality traits are genetically influenced, the neurobiological bases mediating these influences have not yet been well characterized. To advance understanding concerning the pathway from genetic variation to personality, here we examined whether measures of heritable variation in neuroanatomical size in candidate regions (amygdala and medial orbitofrontal cortex) were associated with heritable effects on personality. A sample of 486 middle-aged (mean = 55 years) male twins (complete MZ pairs = 120; complete DZ pairs = 84) underwent structural brain scans and also completed measures of two core domains of personality: positive and negative emotionality. After adjusting for estimated intracranial volume, significant phenotypic (rp) and genetic (rg) correlations were observed between left amygdala volume and positive emotionality (rp = .16, p < .01; rg = .23, p < .05, respectively). In addition, after adjusting for mean cortical thickness, genetic and nonshared-environmental correlations (re) between left medial orbitofrontal cortex thickness and negative emotionality were also observed (rg = .34, p < .01; re = −.19, p < .05, respectively). These findings support a model positing that heritable bases of personality are, at least in part, mediated through individual differences in the size of brain structures, although further work is still required to confirm this causal interpretation.
Keywords: MPQ, Personality, MRI, Amygdala, Orbitofrontal cortex, Genetics, Twins
Introduction
Delineating the etiology of individual differences in personality constitutes a fundamental challenge in the human behavioral sciences. Considerable work in recent decades has demonstrated that genetic influences underpin individual differences in personality (Jang et al., 1996; Loehlin et al., 1998; Riemann et al., 1997; Tellegen et al., 1988); however, comparatively little work has identified the neurobiological substrates mediating these genetic influences (DeYoung and Gray, 2009). Whereas standard approaches to localizing neural correlates of personality typically employ functional imaging methods (Canli et al., 2002), recent work has illustrated that regional gray matter volume is also related to variation in such traits (DeYoung et al., 2010; Kanai and Rees, 2011; Lewis et al., 2012, 2013). However, although examinations of regional gray matter correlates have been combined with genetically-informative designs in prior work examining traits such as cognitive ability (Posthuma et al., 2002), to the best of our knowledge, no study has yet examined whether individual differences in regional brain size share common heritable variation with personality traits. Evidence that these levels of analysis share a common genetic basis would help to significantly advance biological understandings of personality. Accordingly, the current study examined whether two major dimensions of personality – positive emotionality and negative emotionality – were linked to genetic influences on individual differences in regional brain size.
Biological bases of personality: from behavioral genetics to neuroanatomy
Understanding the origins of personality has been an enduring and major endeavor for human behavioral research (Eysenck, 1967; Gray and McNaughton, 2000; McCrae and Costa, 1999; Tellegen, 1985; Zuckerman, 2005). Behavioral genetic methods, including the twin and family designs, have facilitated such research agendas, allowing individual differences in personality to be decomposed into genetic and environmental components, with work in this area robustly showing genetic influences on personality. For instance, approximately 40%–60% of the variance in 11 primary and 3 higher-order factors on Tellegen'sMultidimensional Personality Questionnaire was attributable to genetic influences (Tellegen et al., 1988). Twin analyses examining the Five-Factor Model (FFM) traits reported that individual differences were approximately 50% heritable for all of the FFM traits in adulthood (Jang et al., 1996). This finding is consistent for earlier measures of personality such as Eysenck's Neuroticism and Extraversion scales (Eaves et al., 1998; Floderus-Myrhed et al., 1980), and it has been replicated in several other studies (Loehlin et al., 1998; Riemann et al., 1997), across multiple cultural groupings (Yamagata et al., 2006).
Such work supports a model positing that variation in personality is reflective of individual differences in neurobiology (DeYoung et al., 2010; McCrae and Costa, 1999), especially given the well-established genetic influences on brain measures from twin, and more recently genetic association, studies (Blokland et al., 2012; Kremen et al., 2010a; Schmitt et al., 2007; Toga and Thompson, 2005; Thompson et al., 2014). Although neurobiological models of personality have a long history (Eysenck, 1967), only recently has regional variation in the cortical and subcortical gray matter been associated with variation in personality traits (DeYoung et al., 2010; Kanai and Rees, 2011), with most of this work focusing on the two most central dimensions of personality (Eysenck and Eysenck, 1969; McCrae and Costa, 1999; Tellegen and Waller, 2008): the tendency to express positive affect and proactively engage in the world (extraversion/positive emotionality), and the tendency to express negative affect and to break down under stress (neuroticism/negative emotionality).
In the current study, we also examined these two core dimensions, operationalized here by positive emotionality and negative emotionality, two of the major personality dimensions in the Multidimensional Personality Questionnaire (Tellegen et al., 1988; Tellegen, 1985). These constructs are not isomorphic with extraversion and neuroticism as operationalized in the Big Five or Five-Factor Model of personality (John et al., 2008), but the respective constructs do share important features and are highly correlated (Church, 1994; Clark and Watson, 1999; Klein et al., 2011; Tellegen and Waller, 2008). Research on these two traits has high theoretical importance both to basic personality theory — they are included in most personality lexicons (Eysenck and Eysenck, 1969; McCrae and Costa, 1999; Tellegen and Waller, 2008), as well as because of the translational value that will likely follow a deeper understanding of the underlying biology of these psychiatrically-relevant traits. Indeed, there are robust links between positive and negative emotionality and a range of mood disorders including major depression (Fanous et al., 2007), generalized anxiety disorder (Bienvenu and Stein, 2003), and various personality disorders (Krueger et al., 2012; Saulsman and Page, 2004; Samuel and Widiger, 2008).
Of the studies to address gray matter correlates of positive emotionality/extraversion, specific brain regions are noteworthy as having shown replicable links in independent samples. In particular, medial orbitofrontal cortex (mOFC) has been reported to be larger in individuals with higher scores of extraversion (Cremers et al., 2011; DeYoung et al., 2010; Rauch et al., 2005), with amygdala also showing positive links to extraversion (Cremers et al., 2011; Omura et al., 2005). Broadly similar associations have been reported for neuroticism/negative emotionality, although here smaller amygdala and mOFC volumes have been found to associate with higher scores on such constructs. For instance, individuals with panic disorder, which in turn shows strong links to neuroticism (Kotov et al., 2010), were noted, on average, to have smaller amygdala volumes (Hayano et al., 2009). Similarly, higher neuroticism in a sample of healthy adults has been related to smaller amygdala volume (Omura et al., 2005). Research has also shown a negative association between neuroticism and OFC volume (Jackson et al., 2011; Wright et al., 2006). This work has been complemented by recent research (Fuentes et al., 2012), which reported that scores on the behavioral inhibition system scale (Carver and White, 1994) – a construct with strong links to neuroticism (Smits and Boeck, 2006) – were inversely associated with mOFC volume.
While numerous other regions have shown links to personality (DeYoung et al., 2010), the associations with amygdala and OFC appear to be the most robust findings to date. These associations also seem to cohere with recent work on the function of these brain regions. Indeed, amygdala and orbitofrontal cortex function and their interplay are both linked with sensitivity to reward (Gottfried et al., 2003) – a close analogue of extraversion (Depue and Collins, 1999), and sensitivity to threat and fearfulness (Dolan and Vuilleumier, 2003) – which closely describes trait neuroticism (Eysenck, 1967).
The current study
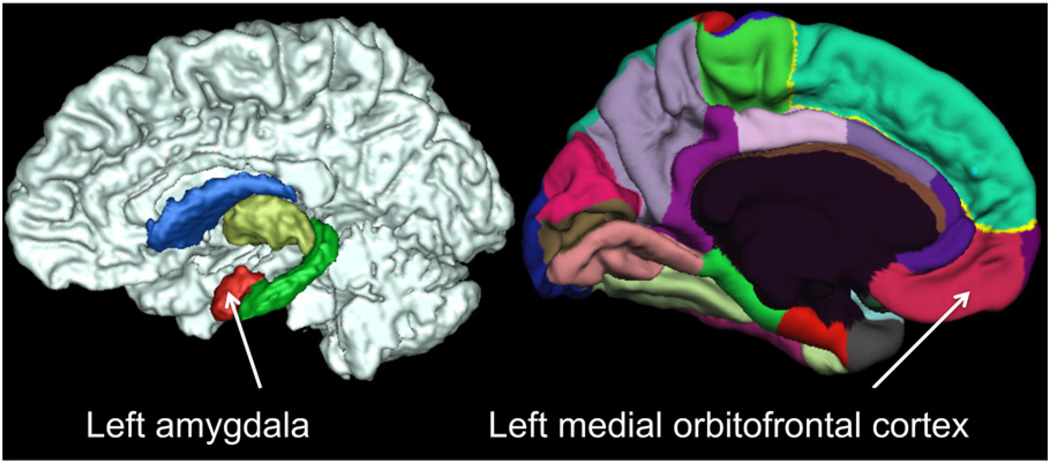
Research demonstrating links between regional brain structure and personality supports the possibility that the pathway from genetic variation to individual differences in personality may be mediated via variation in regional brain structure. To date, however, no work has addressed this possibility. The importance of addressing whether brain structure plays a role in the pathway from genes to personality is of particular salience given that both personality traits (Jang et al., 1996; Tellegen et al., 1988) and regional brain size (Kremen et al., 2010a; Thompson et al., 2014; Toga and Thompson, 2005) have moderate to high heritabilities. To advance understanding on the pathway linking genetic variation and personality, we examined whether size differences in amygdala and mOFC (see Fig. 1) were genetically linked to positive emotionality and negative emotionality. While additional brain regions may be associated with personality, we restricted our analyses to just these brain regions in order to maintain a hypothesis-driven approach given that they are the regions most consistently associated with extraversion/PEM and neuroticism/NEM in the extant literature. Of importance, although most (if not all) prior work in this field has used gray matter volume as a correlate of personality, cortical volume is comprised of two measures – surface area and thickness – that have been demonstrated to be largely genetically dissociable (Panizzon et al., 2009). With this finding in mind, and because of our specific interests in identifying genetic and environmental links between personality and regional brain structure, here we used measures of cortical thickness and surface area to examine variability in mOFC cortex size. Amygdala size was measured as a volume, however, as this subcortical region—not being part of the cortical ribbon—is better characterized in volumetric terms.
Fig. 1.
Anatomical location of left amygdala based on subcortical segmentation and left medial orbital frontal cortex based on cortical parcellation in Freesurfer.
Methods
Participants
Participants were 486 individuals with analyzable MRI and personality data who are part of a larger sample of 1237 twins who participated in wave 1 of the Vietnam Era Twin Study of Aging (VETSA: Kremen et al., 2013). Institutional Review Board approval was obtained at all sites. Written informed consent was obtained from participants after they received a complete description of the study. There were 120 complete monozygotic (MZ) pairs, 84 complete dizygotic (DZ) pairs, and 78 unpaired twins. All participants are male–male twins who both served in the United States military sometime between 1965 and 1975. Seventy-six percent of the twins with MRI data were not exposed to combat. Mean age of the participants was 55.45 (SD = 2.5) years (range: 51–60). A questionnaire that included the NEM and PEM scales was mailed to participants three weeks prior to the MRI assessment and participants returned the completed questionnaire when they arrived at the lab as part of the VETSA wave 1. These variables were not assessed prior to military service. Mean years of education was 13.9 (SD = 2.1), and 85.2% were right-handed. Most participants were employed full-time (74.9%), 4.2% were employed part-time, and 11.2% were retired. There were 88.3% non-Hispanic white, 5.3% African-American, 3.4% Hispanic, and 3.0% “other” participants. Self-reported overall health status was as follows: excellent (14.8%); very good (36.5%); good (37.4%); fair (10.4%); and poor (0.9%). These demographic characteristics did not differ from the full VETSA sample, nor were there significant differences between MZ and DZ twins. Basic demographic and health characteristics of the VETSA sample are comparable to U.S. census data for similarly aged men (Kremen et al., 2006, 2010b). Handedness concordance across zygosity was virtually identical: 75% and 73% for MZ and DZ pairs, respectively.
Measures
Brain structure phenotypes
MRI images were acquired on Siemens 1.5 T scanners (n = 260 at University of California, San Diego; n = 226 at Massachusetts General Hospital: MGH). Sagittal T1-weighted MPRAGE sequences were employed with a TI = 1000 ms, TE = 3.31 ms, TR = 2730 ms, flip angle = 7°, slice thickness = 1.33 mm, voxel size 1.3 × 1.0 × 1.3 mm. Raw DICOM MRI scans (including two T1-weighted volumes per case) were downloaded to the MGH site. Images were automatically corrected for spatial distortion caused by gradient nonlinearity and B1 field inhomogeneity. The two T1-weighted images were registered and averaged to improve signal-to-noise.
Volumetric segmentation (Fischl et al., 2002, 2004a) and cortical surface reconstruction (Dale and Sereno, 1993; Dale et al., 1999; Fischl et al., 1999, 2002, 2004a,b) methods were based on the publicly available FreeSurfer (http://surfer.nmr.mgh.harvard.edu/fswiki; version 3.0.1b) software package. The semi-automated, fully 3D whole-brain segmentation procedure uses a probabilistic atlas and applies a Bayesian classification rule to assign a neuroanatomical label to each voxel (Fischl et al., 2002, 2004a,b). A widely used training atlas has been shown to be comparable to that of expert manual labeling (Fischl et al., 2002, 2004b), but we created a VETSA-specific atlas that further increased accuracy compared to expert manual labeling (Kremen et al., 2010a). Surface area of each parcellation unit was calculated as the sum of the areas of all vertices within that unit, with total surface area calculated as the sum of each of the surface areas. Cortical thickness was calculated as the average distance between the gray/white boundary and the pial surface within each parcellation unit (Fischl and Dale, 2000). Mean cortical thickness was calculated as the weighted average thickness of all parcellation units, weighted by the area of each parcellation unit (for further details see Kremen et al., 2010a; Eyler et al., 2012). Estimated intracranial volume is derived as part of the standard FreeSurfer software. A volume-scaling factor is derived by registration to an atlas template in order to provide an automated correction for head size variation. The method and its validation against manual total intracranial volume measurement have been described in detail by Buckner et al. (2004). 6.5% (n = 34) of participants were excluded from the current analyses because of insufficient quality of the MR scans, leaving 486 participants.
Personality
Positive and negative emotionality were measured by self-report using the appropriate items from the Multidimensional Personality Questionnaire-form NZ (Patrick et al., 2002). The NZ version is considered to be very similar to the Brief Form (Caspi, 2000; Caspi et al., 1997; Krueger et al., 2000; Patrick et al., 2002). Positive emotionality (PEM) was scored as the sum of four primary scales (65 items): social closeness (tapping sociability and the liking of being around others), social potency (tapping forcefulness, decisiveness, and leadership), wellbeing (tapping cheerfulness and how good one feels about themselves), and achievement (tapping enjoyment of challenges and hard work). Negative emotionality (NEM) was scored as the sum of three primary scales (49 items): stress reaction (tapping tenseness and how easily upset one tends to be), aggression (tapping tendencies to discomfort others), and alienation (tapping feelings of being derogated/treated poorly). Psychometric properties of the MPQ are well documented and valid (Krueger et al., 2000; Patrick et al., 2002; Tellegen, 1985) and Cronbach's alpha was good for the current sample: .76 for PEM and .82 for NEM.
Analysis
For all analyses, we: 1) controlled for age and scanning site; 2) controlled amygdala volumes for estimated intracranial volume; 3) and controlled mOFC surface area and thickness for total surface area and weighted mean thickness, respectively. All variables were approximately normally distributed with no evidence of outliers.
Phenotypic analyses
To take into account the hierarchical nature of the data – i.e., that individuals are nested within twin pairs and thus violate the assumption of independence – multilevel models using the lme4 package (Bates and Maechler, 2010) in R (R Core Team, 2012)were used to test whether hypothesized brain structures (i.e., amygdala and mOFC) were associated with PEM and NEM. Family (twins within a pair) was included in the models as a random effect. Other variables were fixed effects in the models with covariates as described below. To test fixed effects for significance we used the likelihood ratio test (comparing a model with and without the fixed effect of interest), in line with recommended practice (Pinheiro and Bates, 2000).
Twin analyses
A popular model for data collected from twins partitions observed variation into three components: additive genetic influences (A), shared-environmental influences (C), and nonshared-environmental influences (E) (Neale and Cardon, 1992). Genetic effects are inferred when monozygotic (MZ) twins are more similar than dizygotic (DZ) twins, while shared-environment effects are inferred when the MZ twin correlation is less than twice that of the DZ twins. Nonshared-environment effects are inferred when MZ twin correlations are less than unity, and this variance component also includes measurement error. Of importance here, this design can be extended to the bivariate case such that not only are the A, C, and E components for both traits estimated (i.e. for brain structure or personality), but also correlations between these variance components for pairs of traits (e.g., for brain structure and personality). As such, genetic (or environmental) correlations can be derived that reflect the magnitude of common heritable (or environmental) influences underlying a given pair of traits.
These bivariate models were fit using full-information maximum-likelihood in OpenMx 1.1 (Boker et al., 2010a,b) running within R 3.0.1 (R Development Core Team, 2013), and nested models were compared using the χ2 ratio test. As detailed in previous publications (Eyler et al., 2012; Kremen et al., 2010a; Panizzon et al., 2009), controlling for age and scanning site, and for normalization of the brain measures was carried out prior to the biometric analyses. We also controlled for effects of age on our personality variables in our biometric analyses.
Results
Phenotypic analyses
Descriptive statistics for each of the variables are presented in Table 1. Positive emotionality (PEM) and negative emotionality (NEM) showed a significant inverse association (r = −.22, p < .01). Mixed effect models were used to examine associations (controlling, where appropriate, for estimated intracranial volume, total surface area, and weighted mean thickness: site and age were included in all models as covariates) between each of the brain variables (see Table 2). Left and right amygdala volumes were associated (β = .70, p < .0001, 95% CI [.63, .78]); left and right mOFC cortical thickness were associated (β = .48, p < .0001, 95% CI[.40, .56]); however, left and right mOFC surface area were not associated (β = .08, p = .08). Estimated intracranial volume was associated with total surface area but not associated with weighted mean thickness: β = .80, p < .0001, 95% CI[.74, .85] and β = .08, p = .08, respectively. Total surface area and weighted mean thickness were uncorrelated (β = −.01, p = .82).
Table 1.
Descriptive statistics for all study variables.
| Mean | SD | |
|---|---|---|
| Amygdala L/R | 1916.73/2055.85 | 206.83/213.41 |
| mOFC thickness L/R | 1.85/1.84 | .15/.16 |
| mOFC surface area L/R | 1558.95/1888.01 | 247.55/299.47 |
| PEM | 37.70 | 9.95 |
| NEM | 10.19 | 7.78 |
Note: mOFC = medial orbitofrontal cortex; L = left hemisphere; R = right hemisphere; PEM = positive emotionality; NEM = negative emotionality. Brain structure values are mm3 for volumes (i.e. amygdala), mm2 for surface area, and mm for thickness.
Table 2.
Associations between brain variables.
| L Amygdala | R Amygdala | L mOFCct | R mOFCct | L mOFCsa | |
|---|---|---|---|---|---|
| R Amygdala | .70*** | ||||
| L mOFCct | −.07 | .01 | |||
| R mOFCct | .01 | −.03 | .48*** | ||
| L mOFCsa | .08* | .04 | .06 | −.01 | |
| R mOFCsa | .06 | −.07 | −.01 | .04 | .08 |
Note. Mixed effects models were used to control for non-independence due to family structure. We controlled for estimated intracranial volume when amygdala volumes were used, total surface area when surface area variables were assessed, and weighted mean thickness when cortical thickness variables were assessed; mOFC = medial orbitofrontal cortex; L = left hemisphere; R = right hemisphere; CT = cortical thickness; SA = surface area;
p < .05;
p < .01;
p < .0001.
We next tested whether our hypothesized brain regions were significantly associated with personality traits. Left and right amygdala volume were both significant predictors of PEM (β = .16 (95% CI[.06, .26]) and β = .11 (95% CI[.004, .21]), respectively). No other hypothesized brain regions showed significant links to personality (also see Table 3).
Table 3.
Genetic and environment effects for candidate brain structures alongside genetic and environmental correlations with PEM and NEM.
| PEM | NEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MZr | DZr | A | C | E | Ar | Er | Pr | Ar | Er | Pr | |
| L Amyg | .62 | .31 | .53 (.13–.69) |
.07 (.00–.44) |
.40 (.30–.50) |
.23* | .06 | .16* | −.12 | .02 | −.06 |
| R Amyg | .67 | .22 | .64 (.53–.72) |
.00 (.00–.34) |
.36 (.28–.46) |
.16 | .02 | .11* | −.15 | .00 | −.09 |
| L mOFCct | .31 | .02 | .35 (.06–.48) |
.00 (.00–.23) |
.65 (.52–.79) |
.10 | .02 | .05 | .34** | −.19* | .04 |
| R mOFCct | .36 | .06 | .42 (.12–.55) |
.00 (.00–.25) |
.58 (.45–.72) |
.15 | −.06 | .03 | −.09 | −.02 | −.05 |
| L mOFCsa | .16 | −.09 | .10 (.00–.52) |
.00 (.00–.40) |
.90 (.86–1.0) |
.02 | −.07 | −.04 | .38 | −.08 | .03 |
| R mOFCsa | .26 | .18 | .21 (.00–.64) |
.05 (.00–.57) |
.74 (.77–.95) |
.12 | −.03 | .02 | .02 | −.05 | −.02 |
Note: Amyg = amygdala; mOFC = medial orbitofrontal cortex; ct = cortical thickness; sa = surface area; MZr = monozyogotic twin pair correlation; DZr = dizyogotic twin pair correlation; PEM = positive emotionality; NEM = negative emotionality; Ar = genetic correlation; Er = nonshared-environment correlation; shared-environment effects were non-significant and estimated at zero or close to zero in all models, thus only AE models are reported here; Pr = phenotypic association; significant associations are starred;
p < .05;
p < .01.
Analyses are adjusted for the appropriate global brain variable (e.g. amygdala analyses control for estimated intracranial volume); A, C, and E are standardized variance components (i.e. they capture the percentage of total variance accounted for by that source of variance).
To check whether the observed associations specifically reflected PEM, in line with the significant correlation between PEM and NEM noted above, we performed additional analyses controlling for NEM by including it in the model. The association between left amygdala and PEM was essentially unchanged when additionally accounting for NEM in the model (β = .15, p = .004, 95% CI[.05, .24]); however, the association between right amygdala volume and PEM showed a trend to significance when NEM was also included as a fixed effect (β = .09, p = .09).
We also examined whether our findings were stable when controlling for the following mental and physical health indicators: current smoking (yes/no), alcohol consumption (0: no drinks in last two weeks; to 4: > three drinks per day over last two weeks), posttraumatic stress disorder (PTSD; yes/no: DSM-III-R diagnosis made in 1992: Robins, 1989), or depressive symptoms (Center for Epidemiologic Studies Depression Scale (Radloff, 1977)). Smoking, alcohol consumption, and depressive symptoms were measured at the same time as the MPQ. PTSD was measured approximately 15 years prior. We ran additional models with each of these additional covariates included simultaneously. Left amygdala was still significantly associated with PEM (p = .002, 95% CI[.05, .24]). For right amygdala, however, controlling for these additional variables reduced the effect of right amygdala on PEM to non-significant (p = .096). This reduction in significance was due to the inclusion of depressive symptoms and PTSD: including just these variables (independently) reduced the association between right amygdala and PEM to trend level (p = .10, and .07, respectively). This is in keeping with the result reported above having controlled for NEM, and is consistent with the significant correlations between NEM and both depressive symptoms and PTSD (r = .63, p < .01 and r = .23, p < .01, respectively). Right amygdala and PEM were, however, still significantly associated when current smoking and alcohol consumption were included in the model as covariates (p = .04, 95% CI [.01, .21]). Finally, we examined whether our results were stable when including handedness as a covariate. This led to virtually no change in the associations reported above: Left and right amygdala volume were both still significant predictors of PEM (β = .16 (95% CI[.06, .26]) and β = .11 (95% CI[.005, .21]), respectively).
Twin analyses
We next turned to genetically-informative analyses. Univariate results for PEM indicated moderate heritable (A = .49) and nonshared-environment influences (E = .51), but no evidence for shared-environment influences (C = .00). Similar observations were seen for NEM, with moderate heritable (A = .43) and nonshared-influences (E = .53), but also modest (albeit non-significant) shared-environment influences (C = .04). Univariate results for candidate brain structures are more extensive so are detailed in Table 3. In summary, we observed moderate-to-large genetic and nonshared-environment influences for each of these variables, with only limited evidence for shared-environment influences.
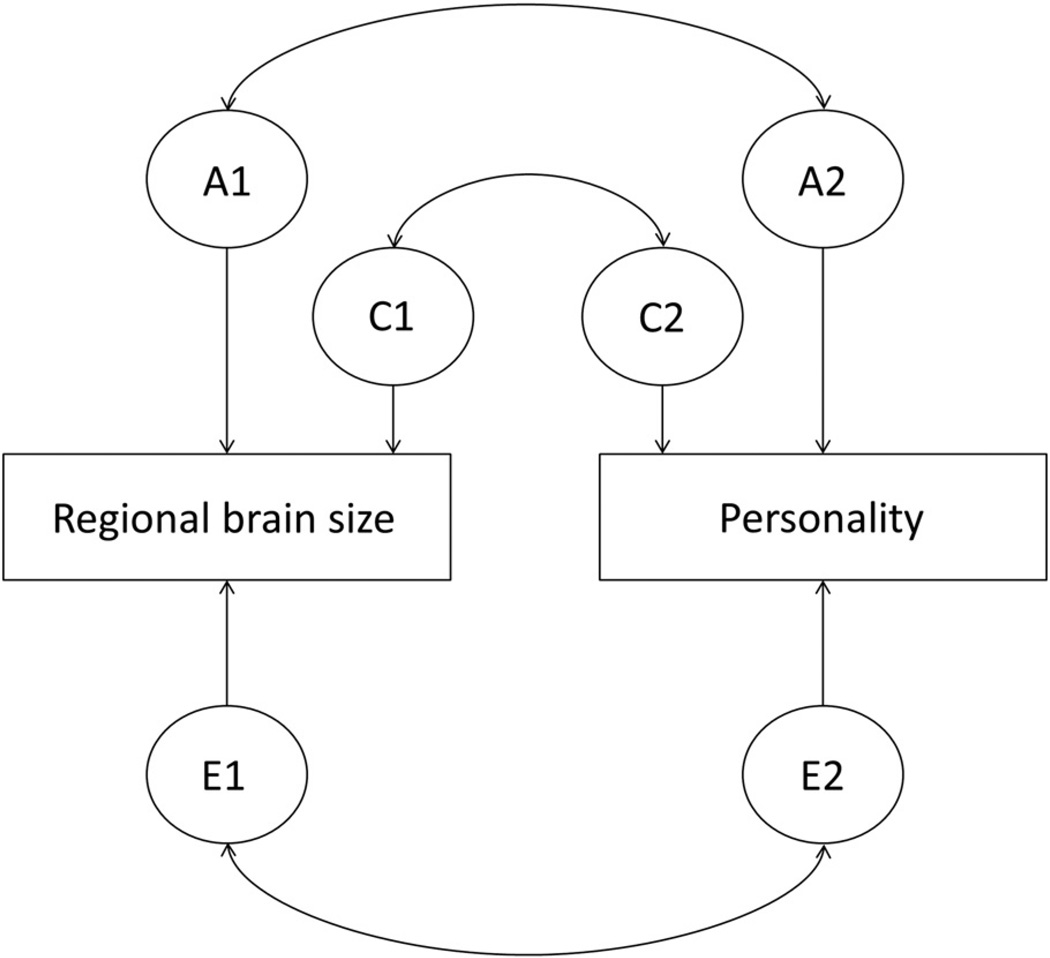
We next fitted a series of bivariate correlated factors models (12 in total) for each of our candidate brain structures with either PEM or NEM to examine whether common genetic or environmental factors were present (see Fig. 2 for an example of this model). We report the genetic (A) and nonshared-environmental (E) correlations from the final bivariate models in Table 3. We first examined whether the effects of the shared-environment (i.e. c1 and c2 in Fig. 2) could be constrained to zero without worsening model fit for each our models. This step demonstrated no significant contribution for a common shared-environment for any model (all Δχ2 (3) < 1.90, p > .59), and thus these paths were removed for subsequent tests. We next tested genetic paths that reflected common bases between brain structure and, depending on which personality trait was included in the model, PEM or NEM. Of these tests, we observed significant and positive genetic correlations between left amygdala and PEM (p = .02), and between left mOFC thickness and NEM (p = .006). (See the discussion section for an explanation of how a positive genetic correlation can emerge despite a non-significant phenotypic correlation.) We also observed a significant negative nonshared-environment association between left OFC thickness and NEM (p = .01). These correlations remained significant when including NEM or PEM, respectively, in the model as an additional covariate.
Fig. 2.
Example of the bivariate correlated factors model for candidate brain regions and personality. Note: A = additive genetic effects; C = shared-environment effects; E = nonshared-environment effects.
Discussion
The current study provides novel evidence that genetic effects on positive emotionality (PEM) are common (in part) with the genetic effects underlying amygdala volume. These findings were broadly mirrored at the phenotypic level, with left and, to a lesser extent, right amygdala volume both positively associated with PEM. Moreover, left mOFC thickness showed a significant and positive genetic association with negative emotionality (NEM). These findings, taken together, support the hypothesis that individual differences in personality are partly grounded in neuroanatomical structures, although reverse causation cannot be ruled out with this data (also see below). For the most part, associations remained significant after controlling for other factors that might be associated with mOFC or amygdala. Controlling for depressive symptoms and PTSD reduced phenotypic correlations between right amygdala and PEM to trend level, but doing so may be an overcorrection because traits such as NEM and PEM share substantial phenotypic and genetic variance with mood and anxiety disorders (Franz et al., 2011; Hettema et al., 2006), and because longitudinal data suggest that traits affect the expression of psychopathology (Naragon-Gainey et al., 2013).
At first glance, the phenotypic link between amygdala and PEM is perhaps curious given more commonly reported links between amygdala and negatively-valenced experiences (Feinstein et al., 2011). However, amygdala activation to happy faces has been associated with extraversion in functional imaging work (Canli et al., 2002), with amygdala function argued in contemporary literature to reflect emotional content more broadly than just negative stimuli (Adolphs, 2010). This result, then, concurs with previous research.
Of interest, we found positive genetic links between left mOFC thickness and NEM, although leftmOFC thickness was phenotypically uncorrelated with NEM. Although this may seem contradictory, it is entirely consistent with theory that a null phenotypic link between two traits can be accompanied by a positive genetic link, or indeed vice versa (Purcell, 2008). That is, genetic etiology need not mirror phenotypic etiology. For example, if genetic and environmental effects on two traits act in different directions (as is the case here for mOFC and NEM: we also saw a negative nonshared-environmental correlation between left mOFC thickness and NEM), these influences can cancel out associations when examined at the phenotypic level. Phenotypic correlations represent composites of genetic and environmental covariances, but in a non-twin study it is not possible to disentangle these effects, highlighting the value of the twin design for understanding etiology. Such results represent an important scientific observation as they inform researchers that the etiology of the variable in question may not be discerned from phenotypic information alone.
Some recommendations for future research are noteworthy. First, as noted earlier, we constrained our analyses here to only the most well supported links between personality and brain structure. As a consequence, we were only able to explain a small proportion of common genetic and phenotypic variance between personality and the current candidate brain regions. Future work should explore additional brain structures in order to more fully characterize the links between regional brain size and personality traits. Second, more research is required to further delineate the nature of the phenotypic and genetic associations observed here between brain structure and personality. While one possible interpretation is that genes influence brain structural development, which in turn affects personality expression, the reverse causal path is also conceivable. Further work, perhaps utilizing a longitudinal genetically informative design (which is not currently available in the VETSA sample), should address this important question. Third, the phenotypic results reported here showed smaller effect sizes than in previous neuroanatomical-personality work. This may reflect differences in methods between this study and other reported research. For example, voxel-based morphometry can identify gray matter structure within broader regions of interest and so may provide a more sensitive test than the pre-segmented regions of interest approach implemented here. Fourthly, while we noted the left hemisphere showed larger associations with personality, we did not have a laterality hypothesis and we think the results for left and right hemisphere are not sufficiently different to make a strong claim for asymmetry. Future work may nonetheless wish to consider this possibility in light of the current results. Finally, the current results are the first to address whether genetic factors are shared between neuroanatomy and personality. As such, while these results can help to guide this field of research herein, additional work is now needed to confirm and extend these initial findings in order to establish whether these results are robust and generalize to females and broader age groups.
In summary, our observations provide novel evidence for genetic links between regional brain structure (specifically, amygdala and medial orbitofrontal cortex) and two core dimensions of personality: positive and negative emotionality. Our results also provide insights into the neuroanatomical bases of important elements of psychopathology, in line with the robust links between positive and negative emotionality and a range of mood disorders.
Acknowledgments
The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry, their families, and the efforts of many staff members. Numerous organizations have provided invaluable assistance in the conduct of the VET Registry, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Sources of support: National Institute on Aging (NIA): NIA R01 AG018386, R01 AG022381, R01 AG022982, R01 AG018384. CESAMH. The Academy of Finland (257075: EV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH or the VA.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann. N. Y. Acad. Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M. lme4: Linear Mixed Effects Models Using S4 ClassesR package version 0.999375 33. 2010 http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Bienvenu OJ, Stein MB. Personality and anxiety disorders: a review. J. Pers. Disord. 2003;17:139–151. doi: 10.1521/pedi.17.2.139.23991. [DOI] [PubMed] [Google Scholar]
- Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res. Hum. Genet. 2012;15:351–371. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2010a;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, et al. OpenMx: multipurpose software for statistical modeling(Version R package version 0.3.1–1246) 2010b http://openmx.psyc.virginia.edu. [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Gotlib IH, Gabrieli JDE. Amygdala activation to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioural activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Caspi A. The child is father of the man: personality continuities from childhood to adulthood. J. Pers. Soc. Psychol. 2000;78:158–172. doi: 10.1037//0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J. Pers. Soc. Psychol. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Church AT. Relating the Tellegen and Five-Factor models of personality structure. J. Pers. Soc. Psychol. 1994;67:898–909. doi: 10.1037//0022-3514.67.5.898. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Temperament: a new paradigm in trait psychology. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. 2nd ed. New York: Guildford; 1999. pp. 399–423. [Google Scholar]
- Cremers H, et al. Extraversion is linked to volume of the orbitofrontal cortex and amygdala. PLoS One. 2011;6:e28421. doi: 10.1371/journal.pone.0028421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I: Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav. Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: explaining individual differences in affect, behavior, and cognition. In: Corr PJ, Matthews G, editors. Cambridge Handbook of Personality. New York: Cambridge University Press; 2009. pp. 323–346. [Google Scholar]
- DeYoung CG, et al. Testing predictions from personality neuroscience: brain structure and the Big Five. Psychol. Sci. 2010;21:820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann. N. Y. Acad. Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Heath AC, Neale MC, Hewitt JK, Martin NG. Sex differences and non-additivity in the effects of genes on personality. Twin Res. 1998;1:131–137. doi: 10.1375/136905298320566267. [DOI] [PubMed] [Google Scholar]
- Eyler LT, et al. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: a magnetic resonance imaging twin study. Twin Res. Hum. Genet. 2012;15:304–314. doi: 10.1017/thg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. The Biological Basis of Personality. Springfield: Thomas; 1967. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Personality Structure and Measurement. London: Routledge; 1969. [Google Scholar]
- Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychol. Med. 2007;37:1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr. Biol. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004a;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Floderus-Myrhed B, Pedersen N, Rasmuson I. Assessment of heritability for personality, based on a short-form of the Eysenck Personality Inventory: a study of 12,898 twin pairs. Behav. Genet. 1980;10:153–162. doi: 10.1007/BF01066265. [DOI] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Prom-Wormley E, Jacobson KC, Lyons MJ, Kremen WS. Adult romantic attachment, negative emotionality, and depressive symptoms in middle aged men: a multivariate genetic analysis. Behav. Genet. 2011;41:488–498. doi: 10.1007/s10519-010-9428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes P, et al. Individual differences in the Behavioral Inhibition System are associated with orbitofrontal cortex and precuneus gray matter volume. Cogn. Affect. Behav. Neurosci. 2012;12:491–498. doi: 10.3758/s13415-012-0099-5. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. 2nd ed. New York: Oxford University Press; 2000. [Google Scholar]
- Hayano F, et al. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin. Neurosci. 2009;63:266–276. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am. J. Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiol. Aging. 2011;32:2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA. Heritability of the Big Five personality dimensions and their facets: a twin study. J. Pers. 1996;64:577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big-Five trait taxonomy: history, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and Research. New York, NY: Guilford Press; 2008. pp. 114–158. [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu. Rev. Clin. Psychol. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kremen WS, et al. Genes, environment and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Res. Hum. Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kremen WS, et al. Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. NeuroImage. 2010a;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, et al. Salivary cortisol and prefrontal cortical thickness in middle-aged men: a twin study. NeuroImage. 2010b;53:1093–1101. doi: 10.1016/j.neuroimage.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Fennema-Notestine C, Eyler LT, Panizzon MS, Chen CH, Franz CE, Dale AM. Genetics of brain structure: contributions from the Vietnam Era Twin Study of Aging. Am. J. Med. Genet. B. 2013;162:751–761. doi: 10.1002/ajmg.b.32162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. J. Pers. 2000;68:967–998. doi: 10.1111/1467-6494.00123. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, Skodol AE. Initial construction of a maladaptive personality trait model and inventory for DSM-5. Psychol. Med. 2012;42:1879–1890. doi: 10.1017/S0033291711002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Kanai R, Bates TC, Rees G. Moral values are associated with individual differences in regional brain volume. J. Cogn. Neurosci. 2012;24:1657–1663. doi: 10.1162/jocn_a_00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Kanai R, Rees G, Bates TC. Neural correlates of the “good life”: eudaimonic well-being is associated with insular cortex volume. Soc. Cogn. Affect. Neurosci. 2013;9:615–618. doi: 10.1093/scan/nst032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC, McCrae RR, Costa PT, Jr, John OP. Heritabilities of common and measure-specific components of the big five personality factors. J. Res. Pers. 1998;32:431–453. [Google Scholar]
- McCrae RR, Costa PT., Jr . A five-factor theory of personality. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. New York: Guilford Press; 1999. pp. 102–138. [Google Scholar]
- Naragon-Gainey K, Gallagher MW, Brown TA. Stable “trait” variance of temperament as a predictor of the temporal course of depression and social phobia. J. Abnorm. Psychol. 2013;122:611–623. doi: 10.1037/a0032997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: NATO ASI SeriesKluwer Academic Press; 1992. [Google Scholar]
- Omura K, Constable RT, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16:1905–1908. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol. Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects Models in S and S-PLUS. Springer; 2000. [Google Scholar]
- Posthuma D, et al. The association between brain volume and intelligence is of genetic origin. Nat. Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Purcell S. Appendix: statistical methods in behavioral genetics. In: Plomin R, DeFries JC, McClearn GE, McGuffin P, editors. Behavioral Genetics. Worth Publishers; 2008. pp. 357–411. [Google Scholar]
- Core Team, R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ( http://www.R-project.org). [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. ( http://www.R-project.org). [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rauch SL, et al. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Riemann R, Angleitner A, Strelau J. Genetic and environmental influences on personality: a study of twins reared together using the self- and peer report NEO-FFI scales. J. Pers. 1997;65:449–475. [Google Scholar]
- Robins LN. Diagnostic grammar and assessment: translating criteria into questions. Psychol. Med. 1989;19:57–68. doi: 10.1017/s0033291700011028. [DOI] [PubMed] [Google Scholar]
- Samuel DB, Widiger TA. A meta-analytic review of the relationships between the five-factor model and DSM-IV-TR personality disorders: a facet level analysis. Clin. Psychol. Rev. 2008;28:1326–1342. doi: 10.1016/j.cpr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: a meta-analytic review. Clin. Psychol. Rev. 2004;23:1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res. Hum. Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits DJ, Boeck PD. From BIS/BAS to the big five. Eur. J. Pers. 2006;20:255–270. [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the Anxiety Disorders. Hillsdale: Erlbaum; 1985. pp. 681–706. [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. The Sage Handbook of Personality Theory and Assessment Personality measurement and testing. II. London: Sage; 2008. pp. 261–292. [Google Scholar]
- Tellegen A, et al. Personality similarity in twins reared apart and together. J. Pers. Soc. Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Cavalleri GL. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annu. Rev. Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Wright CI, et al. Neuroanatomical correlates of extraversion and neuroticism. Cereb. Cortex. 2006;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Yamagata S, et al. Is the genetic structure of human personality universal? A cross-cultural twin study from North America, Europe, and Asia. J. Pers. Soc. Psychol. 2006;90:987–998. doi: 10.1037/0022-3514.90.6.987. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Psychobiology of personality(revised and updated) 2nd edition. New York: Cambridge University Press; 2005. [Google Scholar]