Abstract
Background
We aim to report the incidence of post-intubation hypotension in the critically ill, to report in-hospital mortality and length of stay in those who developed post-intubation hypotension, and to explore possible risk factors associated with post-intubation hypotension.
Material/Methods
Adult (≥18 years) ICU patients who received emergent endotracheal intubation were included. We excluded patients if they were hemodynamically unstable 60 minutes pre-intubation. Post-intubation hypotension was defined as the administration of any vasopressor within 60 minutes following intubation.
Results
Twenty-nine patients developed post-intubation hypotension (29/147, 20%). Post-intubation hypotension was associated with increased in-hospital mortality (11/29, 38% vs. 19/118, 16%) and length of stay (21 [10–37] vs. 12 [7–21] days) on multivariate analysis. Three risk factors for post-intubation hypotension were identified on multivariate analysis: 1) decreasing mean arterial pressure pre-intubation (per 5 mmHg decrease) (p-value=0.04; 95% CI 1.01–1.55); 2) administration of neuromuscular blockers (p-value=0.03; 95% CI 1.12–6.53); and 3) intubation complication (p-value=0.03; 95% CI 1.16–15.57).
Conclusions
Post-intubation hypotension was common in the ICU and was associated with increased in-hospital mortality and length of stay. These patients were more likely to have had lower mean arterial pressure prior to intubation, received neuromuscular blockers, or suffered a complication during intubation.
MeSH Keywords: Hemodynamics, Hospital Mortality, Intensive Care, Intubation, Length of Stay, Risk Factors
Background
Patients admitted to the intensive care unit (ICU) frequently present with acute respiratory failure and/or cardiovascular collapse, and endotracheal intubation is a frequently performed procedure in the critically ill [1]. Not only do these patients present in extremis, but cardiopulmonary reserves are often limited compared to the non-critically ill. Endotracheal intubation performed in a controlled, non-emergent setting, is associated with few complications. However, when carried out within the ICU, where conditions are frequently emergent, the complications increase [2–5]. Complications that may result from this procedure in the critically ill include, but are not limited to, hypoxemia, aspiration, hypotension, and cardiac arrest [2–6]. Information on airway-related complications is fairly robust; however, information on the hemodynamic perturbations is limited. This is of significant relevance, as studies performed in the emergency department setting have demonstrated increased morbidity and mortality [7,8]. Heffner et al. demonstrated that post-intubation hypotension, defined as any systolic blood pressure less than 90 mmHg within 60 minutes of intubation, was associated with higher in-hospital mortality and longer ICU and hospital length of stay [7]. These authors also analyzed risk factors for post-intubation hypotension. On multivariate analysis, pre-intubation shock index, chronic renal disease, intubation for acute respiratory failure, and increasing age were independently associated with the development of post-intubation hypotension [9]. Green et al. performed a chart audit on adult patients requiring emergent intubation over a 16 month period. They defined post-intubation hypotension as systolic blood pressure ≤90 mmHg, a decrease in systolic blood pressure of ≥20% from baseline, a decrease in mean arterial pressure to ≤65 mmHg, or the initiation of any vasopressor during the 30 minutes following intubation. They concluded that increasing age, chronic obstructive pulmonary disease, and pre-emergent endotracheal intubation hemodynamic instability were associated with the development of post-intubation hypotension [10].
The above studies, similar to other studies, have evaluated patients presenting prior to ICU admission [7,9,11]. The ICU patient is distinctive from other patient populations. For example, ICU patients comprise a mixed population with both medical and surgical factors having potential influences on patient outcomes. These same patients may have acquired certain diagnoses such as acute renal failure during their ICU which was not present prior to ICU arrival. Thus, risk factors that may be important in other populations may not apply in the critically ill. In addition, several different definitions for post-intubation hypotension have been reported in the literature. The authors of the current paper performed a prior study evaluating six definitions for post-intubation hypotension. The six surrogates evaluated during the 60 minute post-intubation period were: any systolic blood pressure ≤90 mmHg; any mean arterial pressure ≤65 mmHg; reduction in median systolic blood pressure of ≥20%; any vasopressor administration; any non-sinus rhythm and; and fluid administration of ≥30 ml/kg. We previously reported that, of the six definitions, only the requirement of vasopressors was associated with increased in-hospital and 90-day mortality, as well as increased ICU and hospital length of stay [12]. Importantly, we performed this study in a population of medical and surgical ICU patients.
Thus, our primary aim was to determine the incidence of post-intubation hypotension and its effect on in-hospital mortality and length of stay. Our secondary aim was to determine risk factors for the development post-intubation hypotension, defined as the requirement of any vasopressor within 60 minutes post-intubation, given the association with mortality and length of stay in our prior study.
Material and Methods
All patients analyzed in the current study gave prior research authorization for the use of their medical records. This study was approved by the institutional review board.
Study design
Retrospective cohort study of critical care patients admitted to a medical and surgical ICU during a two-year period.
Study population
The study population was obtained retrospectively from a medical and surgical ICU at Mayo Clinic Rochester, Minnesota. The surgical intensive care unit is a heterogenous population of surgical patients, with the exception of transplant patients. Data was abstracted from both units during the period of 01/01/2010 to 12/31/2011. The electronic medical record was reviewed and data was collected from the initial ICU admission versus repeated ICU admissions, as evidence suggests higher mortality with repeated ICU admissions [13,14]. Moreover, the two-year time period was chosen to limit variation in the intubation practice, which may confound the relationship between the risk factor analyzed and post-intubation hypotension. Thus, the total cohort consisted of 6714 consecutive patients admitted to the two intensive care units during the above time period. The cohort was further reduced to 2684 adult patients (≥18 years) who received invasive mechanical ventilation during their first ICU stay, excluding 101 patients who did not provide research authorization for the use of their medical records.
For abstraction of the relevant data, we developed and validated two electronic search algorithms. This form of chart review has become increasingly popular to conduct retrospective reviews with studies validating the suitability of using electronic search algorithms for data collection within clinical investigations [15]. Given that we were concerned about those critical care patients who emergently needed invasive endotracheal intubation during their first ICU admission, we utilized two electronic search algorithms to identify if a patient had an emergent endotracheal intubation and when this procedure took place. The electronic search algorithms have been previously published [16,17].
Based on the electronic search algorithms, 484 charts were identified that fulfilled the criteria. For data integrity, all 484 charts were manually reviewed with 151 charts excluded, as the patients had been intubated en-route to the ICU. Thus, our final cohort consisted of 333 patients. Please refer to Figure 1 for a complete flow diagram of the process.
Figure 1.
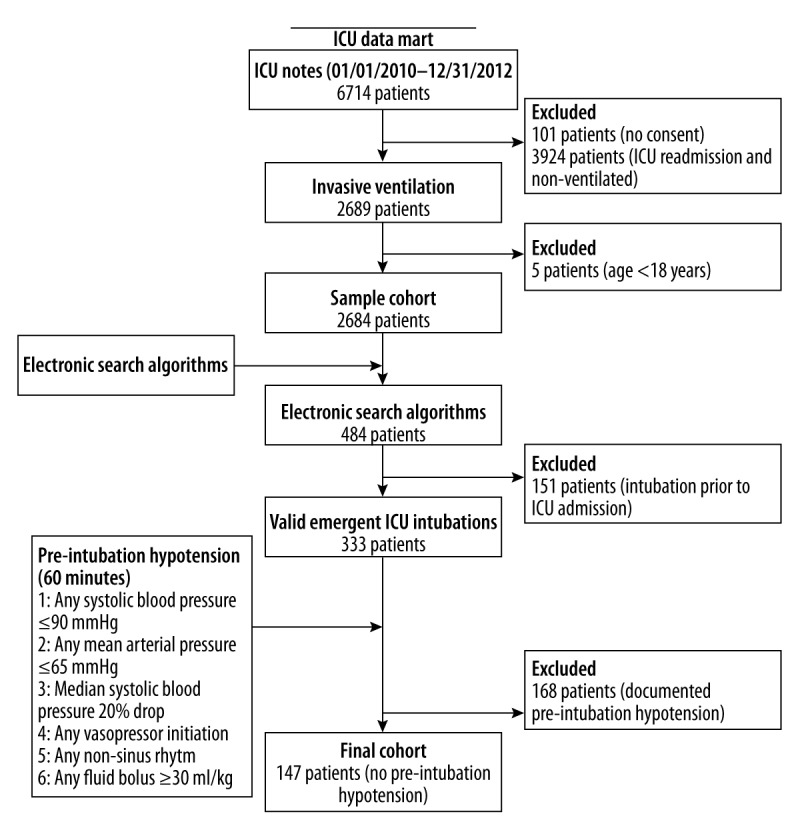
Flow diagram of study patients.
Post-intubation hypotension definition
As there is no consensus definition on post-intubation hypotension, we previously performed a retrospective study within our cohort to arrive at the best definition for post-intubation hypotension that accurately predicted in-hospital and 90-day mortality [12]. In that study, we evaluated six definitions based on literature review and expert opinion. Of the six definitions evaluated, only the requirement of vasopressors post-intubation was associated with in-hospital and 90-day mortality. Therefore, post-intubation hypotension was defined as the requirement of any vasopressor at any time in the 60 minutes following emergent intubation. We excluded patients who met any of the six definitions 60 minutes prior to intubation given that pre-intubation hemodynamic instability is likely to significantly confound any association between putative risk factors and post-intubation hypotension. In addition, the population that is hemodynamically stable pre-intubation is of interest as prevention can be undertaken to prevent hemodynamic instability post-intubation. Hemodynamic measurements (e.g., systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate) were obtained from invasive (arterial) and non-invasive devices. All hemodynamic measurements that were available in the 60 minutes pre- and post-intubation were abstracted. Assessed patient outcomes include ICU and hospital length of stay and ICU and in-hospital mortality. The outcome assessors (N.J.S. and O.D.) were blinded to the status of the groups.
Study protocol
The study protocol used four data abstractors (D.A.D., D.W.B., B.J.S, S.T.) who were blinded to each other. All four data abstractors underwent training prior to study initiation, including database acquisition, electronic medical record instruction, and practice under a supervising physician (R.K.). The data was captured from the Mayo Clinic ICU Data mart. This electronic medical record is reliable and has been previously validated [18]. The four data abstractors followed a standard operating manual for data collection. Data extraction variables included demographic data, co-morbidities, illness severity, and smoking status. Laboratory data consisting of hemoglobin, albumin, arterial blood gas, lactate, and glucose levels were recorded for at least 24 hours before and after emergent intubation. Total fluid volume 24 hours pre- and post-intubation was recorded. The use of non-invasive ventilation was recorded in the 60 minutes pre-intubation and ventilator settings were recorded in the 60 minutes following emergent intubation. Medications used for emergent intubation, the indication for emergent intubation, and complications (hypoxemia, esophageal intubation, vomiting/aspiration, and cardiac arrest) of emergent intubation were recorded. Finally, sepsis diagnosis within the preceding 24 hours of emergent intubation was documented. Data not recorded in the electronic medical record were coded as missing and not imputed. All the data was extracted by four abstractors (D.A.D., D.W.B., B.J.S, S.T.) and 10% of the medical records were selected at random and reviewed by the supervising physician (R.K.) for accuracy using selected variables. Inaccuracies were resolved by a third investigator (N.J.S.).
Statistical analysis
The total cohort of 333 patients was reduced to 147 patients after excluding those who met any of the pre-defined definitions for post-intubation hypotension. Thus, the final cohort of 147 patients was stratified by the presence or absence of post-intubation hypotension, defined as any vasopressor requirement at any time in the 60 minutes post-intubation. Baseline characteristics were assessed in terms of age, sex, weight, co-morbidities, total fluid volume, smoking status, and illness severity.
We then preformed a univariate analysis on the data abstracted to identify possible associations between any risk factors and the development of post-intubation hypotension. Variables that were found to be significantly associated with the outcome of interest were then entered into a multivariable logistic regression model. In the multivariate model, we chose to focus on three possible confounders; age, Sequential Organ Failure Assessment (SOFA) score on day one of ICU admission, and Acute Physiology and Chronic Health Evaluation (APACHE III) score 24 hours from ICU admission. We recognize that there are other possible confounders, but we did not want to over-fit our final model given the number of subjects in the stratified groups. Moreover, severity scores such as SOFA and APACHE include a multitude of other variables to accurately refect the patient’s severity of illness. Age, SOFA, and APACHE III score were entered as continuous variables in the model as a satisfactory linear model was demonstrated by plotting the estimated logits of the response variable (post-intubation hypotension) versus each of the predictor variables (age, SOFA, and APACHE III score). Multicollinearity was assessed by examining the variance inflation factor, with all variance inflation factors being less than 5.0. Model fit was calculated using Harrell’s c-index [19]. The c-index for the models ranged between 0.67–0.70. Odds ratios are presented with 95% confidence intervals. All reported p-values are two-tailed, and a value ≤0.05 was considered statistically significant. Continuous measurements are expressed as mean ± standard deviation (SD) or median and interquartile (IQR) where appropriate, and were compared for statistical differences using paired t-tests or Mann Whitney U tests. Categorical variables are reported as counts and percentages and were tested for significance using Chi-square or Fisher’s exact tests when applicable. We used SAS version 9.3 and JMP version 9.0 (SAS Institute Inc, Cary, NC) statistical packages for all calculations.
Results
The final cohort consisted of 147 patients who were in need of emergent endotracheal intubation during their first ICU admission after excluding 186 patients who met any of the aforementioned exclusion criteria (Figure 1). The demographic and clinical characteristics of total study subjects as well as the subsets of hemodynamically stable (unexposed) and unstable (exposed) patients in the post-intubation period are shown in Table 1.
Table 1.
Sample characteristics grouped by hemodynamic status post-intubation*.
| Variables | Total N=147 | Stable N=118 | Unstable N=29 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) ±SD | 61.42±15.21 | 60.22±15.96 | 66.28±10.60 | 0.05** |
| Weight (kilograms) ±SD | 80.62±22.03 | 81.56±22.46 | 76.79±20.10 | 0.30 |
| Sex (male) N (%) | 81 (55) | 68 (55) | 13 (45) | 0.21 |
| SOFA (day 1) ± SD | 7.61±3.42 | 7.53±3.30 | 7.93±3.94 | 0.75 |
| APACHE III (24 hours from admit) ±SD | 81.79±21.79 | 80.59±21.32 | 86.66±23.35 | 0.18 |
| Comorbidities – current and past | ||||
| Congestive heart failure N (%) | 20 (14) | 15 (13) | 5 (17) | 0.52 |
| Ischemic heart disease N (%) | 35 (24) | 25 (21) | 10 (34) | 0.13 |
| Chronic obstructive lung disease N (%) | 31 (21) | 26 (22) | 5 (17) | 0.57 |
| AKI – emergent intubation N (%) | 69 (47) | 57 (48) | 12 (41) | 0.50 |
| Dialysis/CRRT – emergent intubation N (%) | 7 (5) | 6 (5) | 1 (3) | 1.00 |
| Pneumonia – emergent intubation N (%) | 57 (39) | 45 (38) | 12 (41) | 0.75 |
| End-stage renal disease N (%) | 7 (5) | 6 (5) | 1 (3) | 1.00 |
| Cirrhosis N (%) | 11 (7) | 11 (9) | 0 (0) | 0.12 |
| Stroke N (%) | 12 (8) | 10 (8) | 2 (7) | 1.00 |
| Diabetes mellitus 2 N (%) | 29 (20) | 27 (23) | 2 (7) | 0.07 |
| Intubation reason | ||||
| Airway protection N (%) | 74 (50) | 63 (53) | 11 (38) | 0.14 |
| Acute respiratory failure N (%) | 114 (78) | 88 (75) | 26 (90) | 0.09 |
| Altered mental status N (%) | 26 (18) | 22 (19) | 4 (14) | 0.60 |
| Cardiac arrest N (%) | 2 (1) | 1 (1) | 1 (3) | 0.37 |
| Induction agents | ||||
| Ketamine N (%) | 7 (5) | 4 (3) | 3 (10) | 0.14 |
| Etomidate N (%) | 89 (61) | 72 (61) | 17 (59) | 0.81 |
| Propofol N (%) | 52 (35) | 40 (34) | 12 (41) | 0.45 |
| Midazolam N (%) | 107 (73) | 83 (70) | 24 (83) | 0.18 |
| Fentanyl N (%) | 117 (80) | 92 (78) | 25 (86) | 0.44 |
| Neuromuscular blocker N (%) | 67 (46) | 49 (42) | 18 (62) | 0.05** |
| Sepsis N (%) | 45 (31) | 41 (35) | 4 (14) | 0.04** |
| Intubation events | ||||
| Intubation complication (yes) N (%) | 12 (8) | 7 (6) | 5 (17) | 0.05** |
| Airway difficulty (>1 attempt) N (%) | 15 (10) | 12 (10) | 3 (10) | 1.00 |
| NIV – emergent intubation N (%) | 88 (60) | 74 (63) | 14 (48) | 0.19 |
| Hemodynamic assessment | ||||
| Pre-intubation SBP (IQR) | 120 (109–137) | 110 (100–131) | 0.09 | |
| Pre-intubation MAP (IQR) | 79 (71–89) | 73 (68–81) | 0.05** | |
| Pre-intubation SI (IQR) | 0.92 (0.78–1.12) | 0.99 (0.81–1.22) | 0.36 | |
| Laboratory assessment | ||||
| Pre-intubation lactate level (IQR) | 1.6 (1.1–2.3) | 1.6 (1.1–2.5) | 1.9 (0.8–2.1) | 0.93 |
| Pre-intubation hemoglobin level (IQR) | 9.9 (8.7–11.6) | 9.8 (8.5–11.6) | 10.4 (9.6–11) | 0.60 |
| Pre-intubation albumin level (IQR) | 3 (2.5–3.3) | 3.1 (2.7–3.4) | 2.5 (2.5) | 0.42 |
| Pre-intubation bicarbonate level (IQR) | 24.5 (20–28.5) | 24 (19.5–29) | 25 (21.4–27.3) | 0.86 |
| Pre-intubation leukocyte count (IQR) | 12.6 (9–17.3) | 12 (8.7–16.6) | 15 (10.6–18.6) | 0.07 |
| Ventilatory parameters | ||||
| Post-intubation MAP ±SD | 14.28±4.94 | 14.11±5.02 | 15±4.61 | 0.40 |
| Post-intubation TV ±SD | 532.20±215.80 | 538.54±227.47 | 505.12±157.29 | 0.48 |
| Post-intubation PEEP ±SD | 8.16±3.59 | 8.09±3.75 | 8.46±2.83 | 0.63 |
| Total crystalloid volume (ml) | ||||
| Pre-intubation (24 hours) ±SD | 1878±2181 | 1900±2187 | 1791±2193 | 0.63 |
| Post-intubation (24 hours) ±SD | 3326±2756 | 3129±2678 | 4128±2968 | 0.09 |
| Total colloid volume (ml) | ||||
| Pre-intubation (24 hours) ±SD | 160±448 | 172±467 | 110±362 | 0.40 |
| Post-intubation (24 hours) ±SD | 252±505 | 256 ±545 | 234±299 | 0.84 |
| ICU Metric | ||||
| Mechanical ventilation (days) ±SD | 4.87±4.81 | 4.54±4.53 | 6.20±5.71 | 0.10 |
SD – standard deviation; N – number; SOFA – sequential organ failure assessment; APACE – acute physiology and chronic health evaluation; AKI – acute kidney injury; CRRT – continuous renal replacement therapy; NIV – non-invasive ventilation; SBP – systolic blood pressure; IQR – interquartile range; MAP – mean arterial pressure; SI – shock index; MAP – mean airway pressure; TV – tidal volume; PEEP – positive end-expiratory pressure; ml – milliliters; ICU – intensive care unit.
Surrogate marker of hemodynamic status: no vasopressor 60 minutes post-intubation (stable) vs. any vasopressor within 60 minutes following intubation (unstable);
indicates significance at p-value ≤0.05.
The mean age of the study sample was 61.4 years and 45% were female. The average APACHE III score 24 hours from admission to the ICU was 81.7 with average SOFA score on day 1 of ICU admission of 7.6. Overall, etomidate was used in 61% of intubations with midazolam and fentanyl used in 73% and 80% of emergent intubations respectively. Other commonly used medications included propofol (35%), ketamine (5%), and neuromuscular blockers (52%) (Table 1).
The incidence of post-intubation hypotension was 20% (29 of 147 patients), utilizing our strict definition of any vasopressor administration in the 60 minute post-intubation period. We stratified the groups into those that developed post-intubation hypotension and those that remained hemodynamically stable. Univariate analyses were performed on several variables to identify possible risk factors for the development of post-intubation hypotension. On univariate analyses, increasing age (per 10 year increase) (OR 1.33, 95% CI 1.00–1.79), decreasing mean arterial pressure pre-intubation (per 5mmHg decrease) (OR 1.23, 95% CI 1.00–1.52), neuromuscular blocker administration (OR 2.30, 95% CI 1.00–5.31), and presence of complications during emergent intubation (OR 3.30, 95% CI 1.00–11.30) were associated with the development of post-intubation hypotension (Table 2). Additional medications used to facilitate emergent intubation including propofol, etomidate, ketamine, midazolam, and fentanyl were not associated with the development of post-intubation hypotension (Table 1). Those patients who presented with the diagnosis of sepsis according to the 2008 sepsis guidelines had a decreased incidence of post-intubation hypotension (OR 0.30, 95% CI 0.01–0.92) (Table 2). We did explore whether certain laboratory values such as lactate level, hemoglobin level, bicarbonate level, leukocyte count, arterial blood gas measurements, and albumin level were associated with post-intubation hypotension, but none were significant with the outcome of interest. We also did not find any association between post-intubation hypotension and ventilatory parameters utilized (Table 1).
Table 2.
Risk factors and protective factors associated with the development of post-intubation hypotension on univariable analysis.
| Variable | OR | 95% C.I. | p-value |
|---|---|---|---|
| Age (per 10 year increase) | 1.33 | 1.00–1.79 | 0.05 |
| Mean arterial pressure (per 5 mmHg decrease) | 1.23 | 1.00–1.52 | 0.05 |
| Neuromuscular blocker | 2.30 | 1.00–5.31 | 0.05 |
| Intubation complication | 3.30 | 1.00–11.30 | 0.05 |
| Sepsis diagnosis* | 0.30 | 0.01–0.92 | 0.04 |
OR – odds ratio; CI – confidence interval.
Indicates protective effect.
Multivariate analysis was then performed on the above risk factors. After adjusting for age and illness severity (SOFA and APACHE III score), decreasing mean arterial pressure pre-intubation (per 5 mmHg decrease), neuromuscular blocker administration, and intubation complication were associated with the development of post-intubation hypotension (Table 3). Sepsis diagnosis remained protective against post-intubation hypotension.
Table 3.
Risk factors and protective factors associated with post-intubation hypotension on multivariable analysis after adjusting for age and illness severity (SOFA and APACHE III score).
| Variable | OR | 95% C.I. | p-value |
|---|---|---|---|
| Mean arterial pressure (per 5 mmHg decrease) | 1.25 | 1.01–1.55 | 0.04 |
| Neuromuscular blocker | 2.70 | 1.12–6.53 | 0.03 |
| Intubation complication | 4.25 | 1.16–15.57 | 0.03 |
| Sepsis diagnosis* | 0.24 | 0.08–0.76 | 0.02 |
OR – odds ratio; CI – confidence interval.
Indicates protective effect.
The overall in-hospital mortality rate for our cohort was 20.4% and was higher in patients who developed post-intubation hypotension (37.9%) compared to those who did not (16.1%) (p-value=0.01). Furthermore, patients who developed post-intubation hypotension had an increased median length of stay (21 [10–37] days versus 12 [7–21] days, p-value=0.02) (Table 4).
Table 4.
Post-intubation hypotension with hospital mortality and length of stay after adjusting for age and illness severity (SOFA and APACHE III score).
| Variable | Stable (118) | Unstable (29) | p-value |
|---|---|---|---|
| Hospital mortality | |||
| N (%) | 19 (16) | 11 (38) | 0.01 |
| Hospital length of stay (days) | |||
| [IQR] | 12 [7–21] | 21 [10–37] | 0.02 |
IQR – interquartile range.
Discussion
Our data indicate that post-intubation hypotension is common (20%) in critically ill patients who underwent intubation. Those who developed post-intubation hypotension had a lower mean arterial pressure 60 minutes prior to intubation than those who did not develop post-intubation hypotension. These patients were also more likely to have received neuromuscular blockers or develop a complication during the emergent intubation. In addition, they were more likely to be of higher age. On the other hand, those that were diagnosed with sepsis during their ICU stay had a decreased incidence of post-intubation hypotension. The above associations remained significant even after adjustments for age and illness severity using SOFA and APACHE III score. Furthermore, post-intubation hypotension, using our strict definition, was associated with increased hospital mortality and length of stay.
Patients admitted to the intensive care unit often have acute respiratory failure and/or cardiovascular collapse. Furthermore, physiologic reserve is frequently limited in this population due to previous insults or the current disease process. In turn, they are more vulnerable than non-critically ill patients to procedures performed in the intensive care unit, such as endotracheal intubation. Despite the importance, its impact on patient outcomes has not been well described in the literature, and the reported incidence of post-intubation hypotension varies considerably from 0 to 44% [10,11,13,20]. For example, Lin and colleagues found that 28.6% of patients intubated in the emergency department developed post-intubation hypotension and other studies performed in non-emergency department settings found a range between 9.6% and 38.6% [13,20–22]. Recently, a systematic review conducted by Green and colleagues indicated a summary estimate for the incidence of post-intubation hypotension as 11%, but the results were significantly limited by study heterogeneity [11]. Moreover, studies that did evaluate outcomes associated with post-intubation hypotension suffered from a lack of a standard definition for post-intubation hypotension [23–28]. Thus, this may account for the widely dispersed incidence of post-intubation hypotension. We found an incidence of post-intubation hypotension in our study that was congruent with other studies.
Evidence dictates medications used for emergent intubation may contribute to post-intubation hypotension [29,30]. We did find that the use of a neuromuscular blocker (largely succinylcholine and rocuronium in the current study) was associated with post-intubation hypotension. However, we did not find that other medications used to facilitate emergent intubation were associated with subsequent hemodynamic instability. The finding of neuromuscular blocking agents contributing to post-intubation hypotension is in contrast to the finding of Green et al. [10]. They found that the use of neuromuscular blockers was protective for post-intubation hypotension. The use of a neuromuscular blocker could be hypothesized to lead to post-intubation hypotension, as muscle relaxants are quaternary ammonium compounds and thus, possess histamine-releasing properties (vecuronium, rocuronium, etc.). When large doses of certain muscle relaxants are injected rapidly by the intravenous route, some degree of erythema of the face, neck, and upper torso may develop, possibly together with a brief fall in arterial pressure and a slight-to-moderate rise in heart rate [31]. In addition, neuromuscular blockers, along with antibiotics, are frequently cited as likely culprits of anaphylaxis during endotracheal intubation [32]. One cannot determine whether the association observed in our study is causative or rather related to an attribute of those patients who were selected to receive neuromuscular blockade, thus further research is warranted.
We found that a lower mean arterial pressure 60 minutes prior to emergent intubation was also associated with an increased risk of developing post-intubation hypotension. This finding is similar to the finding of Green et al. [10]. Patients who have less physiologic reserve, such as those with lower mean arterial pressure prior to emergent intubation and those with advanced age (which we also found in our study), would likely be at increased risk to develop post-intubation hypotension with the introduction of positive intrathoracic pressure. As such, preload may not be optimized in this group of patients. We also demonstrated that patients who developed a complication during emergent intubation had an increased risk of post-intubation hypotension. Evidence suggests that complications arising during emergent intubation may lead to hemodynamic instability [22,33]. Complications noted in the current study included hypoxemia, esophageal intubation, vomiting/aspiration, and cardiac arrest.
Interestingly, we found that patients who were diagnosed with sepsis during current ICU admission had a lower incidence of post-intubation hypotension. Our data demonstrated that patients who were diagnosed with sepsis had a higher total volume of intravenous fluid administered 24 hours prior to emergent intubation. At our institution, patients meeting the definition of sepsis have sepsis activation alerts that lead to optimal fluid resuscitation based on current evidence. Thus, we postulate that patients recognized as having sepsis according to the notification system may have received optimal fluid resuscitation as compared to those who were not recognized as having sepsis, resulting in a lower incidence of post-intubation hypotension. Further speculation may be that the physiology demonstrated in septic patients (i.e., high output/low systemic vascular resistance) results in a reduced incidence of post-intubation hypotension.
We found that post-intubation hypotension was associated with increased in-hospital mortality and hospital length of stay. Patients who developed hemodynamic instability had an in-hospital mortality of 37.9% and median hospital length of stay of 21 days, whereas those that remained hemodynamically stable had an in-hospital mortality of 16.1% and median hospital length of stay of 12 days. Our findings are consistent with recent studies, which demonstrate that hemodynamic instability post-intubation is associated with increased morbidity and mortality. In addition, these studies have revealed a higher hospital length of stay as compared to those that remained hemodynamically stable post-intubation [7,8,12].
Our study should be interpreted with caution, as there are several important limitations, including all those inherit to a retrospective study. The first and major concern was the sample size. We conducted the current study over a two-year period to limit the variation in intubation practice. By conducting the study over a larger time frame, we may have found different results. For example, with a bigger study size, we may have found an association with induction medications (e.g., propofol) and post-intubation hypotension. A second limitation is the definition we chose for post-intubation hypotension. If we used other definitions found in the literature, we may have identified other risk factors for the development of post-intubation hypotension. However, we performed a previous study in which we evaluated several definitions, based on literature review and expert opinion, and found that any vasopressor requirement within 60 minutes following intubation was the only predictor of short and long-term mortality as well as other important patient outcomes. Furthermore, this definition represents a treatment for hypotension and as such, the hypotension must be severe and sustained to warrant vasoactive administration. Finally, the administration of vasoactive medications within the critically ill follows guidelines set forth by the society that dictate when to begin vasoactive agents, such as for patients presenting with sepsis who are still hypotensive (mean arterial pressure <65 mmHg) after fluid administration [34]. Thus, we felt it was necessary to evaluate potential risk factors for requiring vasopressors post-intubation. Other limitations include data representative of a single academic center, lack of standardized vital sign recording, lack of provider type performing intubation and duration of attempt(s), lack of echocardiographic data for volume assessment, and the possibility of missed intubations. The study was conducted through the use of a database used in our ICU’s to capture important physiologic and pharmacologic data. The database is reliable and has been validated in other studies. Therefore, we feel we captured the majority of intubations occurring in the two ICU’s within our time frame.
Conclusions
Our study demonstrated that critically ill patients undergoing emergent intubation have a higher than expected rate of post-intubation hypotension and that these patients were more likely to be of higher age, have a lower mean arterial pressure prior to intubation, have received neuromuscular blockers during intubation, or have suffered a complication during the intubation. Furthermore, post-intubation hypotension was associated with increased in-hospital mortality and length of stay. Interestingly, sepsis diagnosis conferred a protective effect against post-intubation hypotension. Future studies are needed to confirm these associations.
Abbreviations
- APACHE
acute physiology and chronic health evaluation
- ICU
intensive care unit
- SOFA
sequential organ failure assessment
Footnotes
Disclosures
All authors declare no conflicts of interest and/or financial disclosures.
Source of support: This work was supported by the Division of Critical Care Medicine with no direct financial support
References
- 1.Divatia JV, Khan PU, Myatra SN. Tracheal intubations in the ICU: Life saving or life threatening? Indian J Anaesth. 2011;55:470–75. doi: 10.4103/0019-5049.89872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan RA, Posner KL, et al. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology. 1990;72:828–33. doi: 10.1097/00000542-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Cheney FW, Posner KL, Caplan RA. Adverse respiratory events infrequently leading to malpractice suits. A closed claims analysis. Anesthesiology. 1991;75:932–39. doi: 10.1097/00000542-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Domino KB, Posner KL, Caplan RA, Cheney FW. Airway injury during anesthesia: a closed claims analysis. Anesthesiology. 1999;91:1703–11. doi: 10.1097/00000542-199912000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: the US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–8. doi: 10.1097/ACO.0b013e32832dba50. [DOI] [PubMed] [Google Scholar]
- 6.Mort TC. Complications of emergency tracheal intubation: immediate airway-related consequences: part II. J Intensive Care Med. 2007;22:208–15. doi: 10.1177/0885066607301359. [DOI] [PubMed] [Google Scholar]
- 7.Heffner AC, Swords D, Kline JA, Jones AE. The frequency and significance of postintubation hypotension during emergency airway management. J Crit Care. 2012;27:417e9–13. doi: 10.1016/j.jcrc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Smischney NJ, Ricter BD, Hoeft CC, et al. Association of post-intubation hemodyanmic instability in an adult icu with in-hospital mortality and icu length of stay. Am J Respir Crit Care Med. 2014;189:A4558. [Google Scholar]
- 9.Heffner AC, Swords DS, Nussbaum ML, et al. Predictors of the complication of postintubation hypotension during emergency airway management. J Crit Care. 2012;27:587–93. doi: 10.1016/j.jcrc.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Green RS, Edwards J, Sabri E, Fergusson D. Evaluation of the incidence, risk factors, and impact on patient outcomes of postintubation hemodynamic instability. CJEM. 2012;14:74–82. doi: 10.2310/8000.2012.110548. [DOI] [PubMed] [Google Scholar]
- 11.Green R, Hutton B, Lorette J, et al. Incidence of postintubation hemodynamic instability associated with emergent intubations performed outside the operating room: a systematic review. CJEM. 2014;16:69–79. doi: 10.2310/8000.2013.131004. [DOI] [PubMed] [Google Scholar]
- 12.Smischney NJ, Demirci O, Ricter BD, et al. Vasopressor use as a surrogate for post-intubation hemodynamic instability is associated with in-hospital and 90-day mortality. BMC Res Notes. 2015;8:445. doi: 10.1186/s13104-015-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaber S, Amraoui J, Lefrant JY, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34:2355–61. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg AL, Watts C. Patient readmitted to the ICUs*: a systematic review of risk factors and outcomes. Chest. 2000;118:492–502. doi: 10.1378/chest.118.2.492. [DOI] [PubMed] [Google Scholar]
- 15.Benson M, Junger A, Fuchs C, et al. Use of an anesthesia information management system (AIMS) to evaluate the physiologic effects of hypnotic agents used to induce anesthesia. J Clin Monit Comput. 2000;16:183–90. doi: 10.1023/a:1009937510028. [DOI] [PubMed] [Google Scholar]
- 16.Smischney NJ, Velagapudi VM, Onigkeit JA, et al. Retrospective derivation and validation of a search algorithm to identify emergent endotracheal intubations in the intensive care unit. Appl Clin Inform. 2013;4:419–27. doi: 10.4338/ACI-2013-05-RA-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smischney NJ, Velagapudi VM, Onigkeit JA, et al. Retrospective derivation and validation of a search algorithm to identify mechanical ventilation initiation in the intensive care unit. BMC Med Inform Decis Mak. 2014;14:55. doi: 10.1186/1472-6947-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herasevich V, Pickering BW, Dong Y, et al. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85:247–54. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–46. [PubMed] [Google Scholar]
- 20.Lin CC, Chen KF, Shih CP, et al. The prognostic factors of hypotension after rapid sequence intubation. Am J Emerg Med. 2008;26:845–51. doi: 10.1016/j.ajem.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Griesdale DE, Bosma TL, Kurth T, et al. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34:1835–42. doi: 10.1007/s00134-008-1205-6. [DOI] [PubMed] [Google Scholar]
- 22.Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–13. doi: 10.1213/01.ANE.0000122825.04923.15. [DOI] [PubMed] [Google Scholar]
- 23.Tayal VS, Riggs RW, Marx JA, et al. Rapid-sequence intubation at an emergency medicine residency: success rate and adverse events during a two-year period. Acad Emerg Med. 1999;6:31–37. doi: 10.1111/j.1553-2712.1999.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 24.Tam AY, Lau FL. A prospective study of tracheal intubation in an emergency department in Hong Kong. Eur J Emerg Med. 2001;8:305–10. doi: 10.1097/00063110-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Marvez E, Weiss SJ, Houry DE, Ernst AA. Predicting adverse outcomes in a diagnosis-based protocol system for rapid sequence intubation. Am J Emerg Med. 2003;21:23–29. doi: 10.1053/ajem.2003.50002. [DOI] [PubMed] [Google Scholar]
- 26.Wong E, Fong YT, Ho KK. Emergency airway management – experience of a tertiary hospital in South-East Asia. Resuscitation. 2004;61:349–55. doi: 10.1016/j.resuscitation.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Wong E, Fong YT. Trauma airway experience by emergency physicians. Eur J Emerg Med. 2003;10:209–12. doi: 10.1097/00063110-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Graham CA, Beard D, Oglesby AJ, et al. Rapid sequence intubation in Scottish urban emergency departments. Emerg Med J. 2003;20:3–5. doi: 10.1136/emj.20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergence department: an observational cohort study. Acad Emerg Med. 2006;13:378–83. doi: 10.1197/j.aem.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 30.Sivilotti ML, Ducharme J. Randomized, double-blind study on sedatives and hemodynamics during rapid-sequence intubation in the emergency department: the SHRED study. Ann Emerg Med. 1998;31:313–24. doi: 10.1016/S0196-0644(98)70341-5. [DOI] [PubMed] [Google Scholar]
- 31.Miller RD. Miller’s Anesthesia. 8th edition. Philadelphia: Elsevier Saunders; 2015. [Google Scholar]
- 32.Mertes PM, Karila C, Demoly P, et al. [What is the reality of anaphylactoid reactions during anaesthesia? Classification, prevalence, clinical features, drugs involved and morbidity and mortality]. Ann Fr Anesth Reanim. 2011;30:223–39. doi: 10.1016/j.annfar.2011.01.002. [in French] [DOI] [PubMed] [Google Scholar]
- 33.Simpson GD, Ross MJ, Mckeown DW, Ray DC. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108:792–99. doi: 10.1093/bja/aer504. [DOI] [PubMed] [Google Scholar]
- 34.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]


